Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Severe burn injury causes a myriad of hematologic perturbations. Burn excision as well as substantial in-hospital phlebotomy causes severe blood loss anemia and even hemorrhagic shock, requiring substantial transfusion. Large-scale fluid resuscitation and shock cause a significant coagulopathy. Surgical extirpation can similarly cause a significant dilutional coagulopathy requiring transfusion. Hematopoiesis, the generation of new blood cells, is directed away from red blood cell (RBC) production toward myeloid blood cell production by the hyperinflammatory cytokine cascade. The hyperinflammatory nature of burn injury further makes the patient hypercoagulable, generally requiring significant anticoagulation or thromboprophlyactic therapy. Providing total burn care requires knowledge of hematology, hemostasis, thromboprophylaxis, and transfusion medicine to overcome the substantial pathophysiology experienced by these patients.
Anemia is the condition occurring when the RBC concentration, or hemoglobin (Hb) concentration, falls below normal laboratory limits for healthy adults. These normative values do not correlate with sufficient oxygen delivery. Weiskopf and Feiner classically demonstrated normal oxygen delivery despite dramatic acute anemias while examining Hb concentrations as low as 5 g/dL in euvolemic subjects. Anemia is common in burn patients, especially in those with severe thermal injuries. Burn patients suffer from anemia due to acute surgical blood loss and the anemia of critical illness. This can occur in patients with as little as 10% total body surface area (TBSA) burned. A mild reduction of RBCs mass is of little clinical significance ; when the concentration is significantly reduced, or total blood volume loss is beyond 30%, it becomes clinically relevant, leading to impaired end-organ perfusion and oxygenation. The initial 2 weeks post-burn feature anemia mainly resulting from blood loss through the burn wound, dilution due to resuscitation, and surgical blood loss from repeated débridement to prepare the wound bed with well-vascularized tissue. Subsequently, anemia is characterized as the anemia of critical illness and develops from inadequate nutrition, stunted erythropoiesis, or phlebotomy and dressing changes. Specifically, bone marrow dysfunction leading to dampened erythropoiesis has been explored in autopsy studies and in the mouse model of burn injury in vitro. In an autopsy study of patients who died from myocardial infarction, sepsis, or burns, the bone marrow of burn patients contained significantly fewer erythroblasts compared to the other groups.
Acute blood loss results in at least 2% blood volume loss per percent body surface area excised; thus during major burn excisions, there is significant blood loss often requiring massive transfusions (MTs). MT is defined as the transfusion of packed red blood cells (pRBCs) of 10 units or more within 24 hours of admission. While acute surgical blood loss is obvious and prominent during the treatment phase, burn patients suffer a prolonged anemia of critical illness during their recoveries, which is insidious. The anemia of critical illness is the inability of RBC production to meet RBC demand and losses during critical illness. More than 50% of transfusions during a burn patient's hospital course may be caused by the anemia of critical illness. It has been equated to an acute form of the anemia of chronic disease and anemia of inflammation in conjunction with nutritional deficiencies. Acute blood loss anemia is controlled with surgical technique, while the anemia of critical illness can be restrained with reduced phlebotomy, decreased blood loss with dressing changes, improved nutrition, and resolving the critical illness by covering the patient with skin, thereby abating his disease. Prevention of anemia is accomplished through alteration of its sources, acute blood loss, and the anemia of critical illness, while the mainstay of treatment for both types of anemia is the transfusion of pRBCs.
Controlling blood loss and hemorrhage during burn care is important to prevent episodes of hemorrhagic shock and limit total transfusion need. While Barbosa and Rowel demonstrated that a 6-h RBC transfusion requirement is one of the mortality predictors in MT trauma patients, it is unclear if these findings extend to controlled blood loss during a burn extirpation. In these cases, a skilled anesthesiologist can match the transfusion rate and blood product mix to the bleeding rate, thus preventing hemorrhagic shock, maintaining euvolemia, and avoiding coagulopathy while the surgeon removes burned and diseased tissue and engrafts the patient.
As with burn-susceptible infections, preventing burn-derived anemia from developing is more optimal than treating the anemia or subsequent sequela. Several methods have been established to mitigate acute surgical blood loss, including epinephrine tumescence, thrombin-soaked dressings, and tourniquet use. Additionally, excision with electrocautery at a fascial or subcutaneous level can limit blood loss significantly in large, full-thickness burns. Regardless of excision methodology employed, many studies found the injection of dilute epinephrine into the subdermal space promoted vasoconstriction and reduced blood loss during surgical management of the wound. In a pediatric trial, epinephrine tumescence alone decreased blood loss from 3.5% to 5% to 0.98% of total blood volume per percent of body excised and grafted. Importantly sufficient time must be allotted for the epinephrine to take effect, with an ideal interval of 25 minutes. Epinephrine tumescence has no significant hemodynamic consequences, nor does it alter wound healing. A recent study confirmed that subcutaneous epinephrine injection had no adverse effect on perfusion, pain, or scar quality versus the saline-administered control group. Of interest lately has been tumescent infiltration of lidocaine and epinephrine by clysis. Gumus showed that this technique resulted in more facile excision with diminished blood loss. Similarly, clysis has been demonstrated to reduce the need for blood transfusions. The use of thrombin-soaked pads for additional hemostasis support postoperatively has been studied in the context of epinephrine tumescence and provides supplementary hemostasis by reducing unnecessary ooze from the dressing site. New silicone gel dressings also significantly reduced the amount of blood loss per percent excised and the amount of blood transfused. We employ nonadherent dressings (Telfa) soaked with epinephrine intraoperatively. This facilitates hemostasis and does not restart the bleeding upon removal of the pads, as often occurs with epinephrine-soaked laparotomy pads that avulse the clots they helped induce on the wound surface when they are removed. Finally the use of an extremity tourniquet during excision and débridement can decrease acute surgical blood loss without compromising graft adherence. Kragh et al. found tourniquet use efficacious in both adults and children. In combination, all three techniques (epinephrine tumescence, thrombin-soaked dressings, and tourniquet use) can reduce intraoperative transfusion from 3.3 to 0.1 units per operative case with 96% graft take and total units transfused from 15.7 to 7.9 units per patient. However recent analysis indicates that utilizing the epinephrine-tumescent technique obviates the need for tourniquets. Of note, administration of topical bovine thrombin must be avoided in burn patients with prior exposure because they have been shown to develop coagulation derangements and severe bleeding complications from an acquired factor V deficiency. Mullins et al. reported safe and effective hemostasis through recombinant human thrombin applied as a spray. Due to rampant overestimations of operative transfusion needs and the expenses incurred by blood typing and cross-matching, a preoperative estimate of 1.78 units of pRBCs per 1000 cm 2 of burn wound excised best utilizes blood bank resources.
In addition to anemia, thermal injuries are associated with systemic coagulopathy, and the hemostatic changes seen in patients with severe burns appear similar to those in patients with other major traumas. The severity of the burn correlates with the extent of hemostatic changes ; typically only severely burned patients (≥30% TBSA) develop extensive coagulopathy. However, no consensus currently exists on the definition of coagulopathy in burn injuries. Despite being documented since the 1970s, the physiology of coagulopathy in burns is still ill-defined. However, coagulopathy of thermal trauma does present with characteristics common to sepsis-induced coagulopathy: decreased levels of antithrombin and other native anticoagulants, elevated levels of activated factor VII, fibrinogen degradation products, plasminogen activator inhibitor-1 (PAI-1), and thrombin-antithrombin complex (TAT). The three types often discussed in burn- and trauma-related literature are trauma-induced coagulopathy (TIC), disseminated intravascular coagulation (DIC), and acute traumatic coagulopathy (ATC). Acute coagulopathy of trauma shock has largely been subsumed under TIC. Caused by trauma, TIC is characterized by coagulation activation, hyperfibrinolysis, and consumption coagulation. The main pathophysiology of TIC is DIC . Specifically DIC presents as the fibrinolytic phenotype in the early, acute phase of trauma and burn , whereas the thrombolytic phenotype is associated with sepsis-induced DIC, which presents later in the clinical course of burn pathophysiology. As defined by the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis, DIC is an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization originating from and causing damage to the microvasculature, potentially leading to multisystem organ dysfunction (MOD). DIC is characterized not by the significant bleeding, transfusion requirements, and fourfold higher mortality associated with TIC, but by excessive thrombosis, unchecked inflammation and MOD, insufficient anticoagulation mechanisms, and increased fibrinolysis. Indeed, early coagulopathy is associated with increased incidence of ventilator-associated events among burn patients. MTs are administered more frequently in patients with DIC. ATC is associated with the depletion of fibrinogen, platelet dysfunction, and activation of protein C. This particular coagulopathy is not secondary to other conditions, such as hypothermia, and markers for it can be discerned within 30 minutes of the inciting thermal event. In the pediatric population, ATC is defined by an international normalized ratio (INR) of 1.3 or greater, and those who present with fibrinolytic shutdown are more likely to develop deep vein thrombosis (DVT), especially because prophylactic anticoagulant administration is not recommended in the pediatric population due to their lower rates of venous thromboembolism (VTE). Coagulopathy seen in burns is mediated by preexisting conditions (e.g., age and comorbidities) as well as environmental (e.g., ambient temperature) and therapeutic factors (e.g., pre-hospital fluid administration).
As demonstrated in multiple studies, the timing and onset of coagulopathy correspond with the severity of the burn, , and TIC may present without initial hypercoagulopathy. Few burn patients present with coagulopathy at admission, but a large number develop it within a day post-burn. In fact, at admission, no statistically significant differences were observed between nonsurvivors and survivors in coagulation and fibrinolysis markers. The inciting causes of coagulopathy in burned patients include tissue hypoperfusion from fluid resuscitation, systemic inflammatory response syndrome (SIRS), blood loss from surgical excision, hypothermia, endothelial damage, consumption and/or dilution of coagulation factors, and acidemia. Tejiram et al. effectively reviewed the myriad of changes seen in the clotting dynamics of burn patients and related more subtle factor changes as possible markers of mortality than partial thrombin time (PTT) and INR, which were normal in their cohort. Classically, hypothermia, acidosis, and coagulopathy are collectively known as the lethal triad, corresponding to MTs and mortality in burn and trauma patients.
Prevention of hypothermia in the operative theater is completely within the purview of the operative team and the operating room in the same way that the temperature of a steak is completely within the purview of the chef and the oven. As a manifestation of the First Law of Thermodynamics, a patient cannot cool beyond the temperature of the operating room. The clinical practice guideline in many burn centers is to maintain the ambient temperature of the ICU and operating rooms at 86–104°F (30–40°C) as a component of standard of care in the treatment of burn patients. Maintaining sufficient heat in the operating room to prevent hypothermia is essential. In patients undergoing surgery, inadvertent perioperative hypothermia resulted from 50% to 90% of the cases. Singer et al. determined hypothermia to be associated with high mortality, although it was much more commonly found in patients with large burns. In fact, it has been shown that hypothermia, the condition where the core temperature falls below 36°C (96.8°F), is not associated with external factors at the time of the burn, but correlates to burn severity and patient physiological status. Burn patients are most vulnerable during excisional surgery due to operative heat loss concurrent with evaporative water loss or massive fluid therapy.
Acidosis is an ever-present threat for the recovering burn patient. Lactic acidosis from shock states occurs transiently during recovery and surgery and exacerbates bleeding, as does hypercarbia. Uremia causes an anion gap acidosis and reduces platelet aggregation, which can be reversed with desmopressin. Hyperchloremia of normal saline administration is an effect of hemodilution or a decrease in renal excretion of H + . While administration of natural saline is associated with hyperchloremia-induced metabolic acidosis, Ringer's lactate, the other crystalloid solution typically used, correlates with metabolic alkalosis.
Diagnosis of coagulopathy depends on detection. The INR measured upon admission is the basis for the diagnosis of acute traumatic coagulopathy. Initial Hb levels may mask bleeding, and repeated measurements are necessary to use Hb levels diagnostically as a marker for bleeding. Notably, low initial Hb is considered an indicator for hemorrhage-associated coagulopathy. However, assessing specific coagulation markers has proved both time-consuming and expensive, and typical laboratory tests, such as PTT, are limited in diagnostic value. The markers of the various coagulopathies seen in burns present similarly to the coagulopathy seen in patients with sepsis and severe trauma. Among the coagulopathic changes are increased levels markers of thrombin activation, activated factor VII (FVIIa), thrombin-antithrombin complex (TAT) and inhibited fibrinolysis with increased levels of PAI-1, as well as decreased levels in proteins C and S, fibrinogen, and antithrombin. In the first day post-burn, patients with severe burns show marked decreases in fibrinolysis as well as platelet activity. Lavrentieva et al. demonstrated that, in the early post-burn phase (day 3 to day 7 post-burn), survivors could be distinguished from nonsurvivors by the levels of natural coagulation inhibitors (i.e., proteins C and S and antithrombin), fibrinolytic factors (i.e., PAI-1 and tissue-plasminogen activator), and TAT, a marker of thrombin generation, but not at admission when both groups presented with statistically similar levels in all parameters. While van Haren et al. also found that severely burned patients become hypercoagulable following admission despite pharmacologic thromboprophylaxis, they determined that thromboelastography (TEG) was a more reliable indicator of coagulopathy than were coagulation markers. Interpretation of TEG data is reviewed in Fig. 22.1 . Furthermore, TEG provides diagnostic answers in a much shorter time than standard laboratory testing, resulting in faster goal-directed therapies. The majority of burn centers worldwide used standard coagulation tests, but the move to using TEG is warranted. With the advent a portable fibrinogen analyzer, fibrinogen levels can be measured in mere minutes. Low levels of fibrinogen have been shown to be predictors for MTs ; Hayakawa et al. demonstrated that fibrinogen reached critical diagnostic levels (150 mg/dL) sooner than other coagulation parameters, making it a key factor in determining blood product needs as well as being a coagulation indicator. More recently, other studies showed higher levels of fibrinogen (211 mg/dL and 190 mg/dL) functioned as useful predictors of MT.
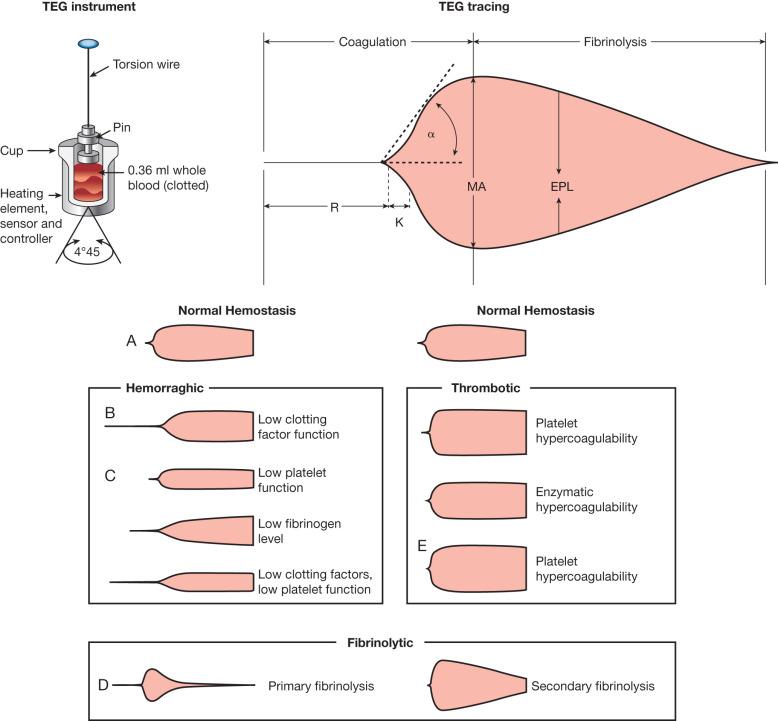
Not only is there limited consensus on the diagnosis of the type of coagulation associated with burns, there is no agreement on the therapies used to respond to the condition when it presents. It is accepted that the activation of fibrinolysis is inadequate to offset the excessive fibrinogen formation in the early post-burn phase in patients severely burned. Hemostasis depends on fibrinogen for clot formation and platelet aggregation, and the depletion of fibrinogen correlates with poor outcomes. While fibrinogen is the coagulation protein with the highest plasma concentration, plasma transfusions do not correct the fibrinogen depletion seen in TIC and ATC unless massive volumes are infused. Current European Trauma Guidelines suggest the administration of fibrinogen concentrate or cryoprecipitate if a trauma patient presents with both significant bleeding and thromboelastometric signs of functional fibrinogen deficit or a fibrinogen concentration (FIB) of less than 1.5–2 g/L. Topical hemostatic agents such as thrombin spray rely on plasma FIB to effect hemostasis in operative wounds.
Limiting fibrinolysis is another important method to control blood loss during burn excisions. Tranexamic acid (TXA), a synthetic derivative of lysine, has found great utility in treating burn patients. In the landmark CRASH2 randomized controlled trial, Roberts et al. demonstrated a reduction of blood loss and mortality with TXA use in trauma patients. Not only does TXA safely and significantly reduce the mortality rates of trauma patients with, or at risk of, substantial bleeding when administered early in the course of treatment, but its de minimis side-effect profile in conjunction with its low cost and ease of use have made it an integral component of resuscitation protocols worldwide. However, the benefits of TXA are seen only when administered within 3 hours of injury; after that window, it has been shown to increase mortality. Thus it is most efficacious to treat acute traumatic coagulopathy. Fibrinolysis is less likely to be fully activated the sooner patients receive TXA; fibrinolysis continues unceasingly once activated, and only restoration of endogenous antifibrinolytic elements can then abate the activity. Some practitioners feel that the potential risk is too great for use in patients whose bleeding is not life threatening. Although reports exist highlighting the prothrombotic effects resulting from each antifibrinolytic drug, we advocate the use of TXA perioperatively for large burn excisions.
Burn patients have many disparate indications for transfusion. In the setting of active operative hemorrhage, the rapid transfusion of pRBC, plasma, and other products is essential to prevent hemorrhagic shock. In the postoperative period, transfusion is necessary to treat blood loss anemia, coagulopathies, and consumptive thrombocytopenias. As a component of resuscitation, plasma transfusion is often required to treat coagulopathies resulting from consumption and under-production of factors, as well as for volume expansion, as discussed in Chapter 8 on the pathophysiology of burn shock and burn edema and in Chapter 9 on fluid resuscitation and early management. Understanding the vastly disparate indications for transfusion in burn patients allows clarity in the urgency and aggressiveness of transfusion used in these patients with ever-changing status.
The first major indication for transfusion is intraoperative blood loss during burn wound excision. The anesthesia team must monitor overall preload while estimating blood loss to prevent hypovolemia and hemorrhagic shock. Surgical control of hemorrhage is critical, but large blood loss is expected, especially in the setting of large burns. Surgical blood loss has been estimated and measured in a variety of ways, but reports based on surgical and anesthesia team estimates are simple and reliable. Following serial hematocrit value along with hemodynamic markers is a standard method of monitoring blood loss and transfusion success. Various formulae have been developed to calculate the estimated blood loss. Total blood volume is estimated using the patient's weight, preoperative and postoperative Hb, and presumed normal adult Hb of 70 cc/kg. Generally accepted and employed constants are adult men 75 cc/kg, adult women 65 cc/kg, infants 80 cc/kg, term infants 85 cc/kg, and premature neonates 95 cc/kg. Gross initially described the mathematics of the formula in 1983 ; we have derived an equation estimating allowable operative blood loss with which we estimate blood loss for all our operations. Fig. 22.2 reflects the formula we utilize, and, although patient conditions, such as venous capacitance and changes in vascular tone, can significantly alter blood volume of distribution, this formula has proved the best estimator of blood loss in our experience.
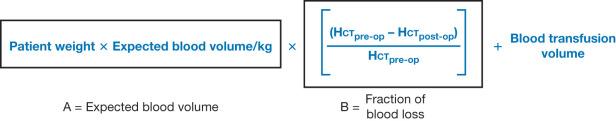
Due to blood type and cross-match expenses and overestimation of operative transfusion needs a preoperative estimate of 1.78 units of pRBCs per 1000 cm 2 of burn wound excised best utilizes blood bank resources. However, even since publication of the prior edition of this book, studies show 25% of patients received cross-match orders exceeding national guidelines, with surgeons being most responsible for this overestimation. Interestingly, change in hematocrit, not Hb, is considered the most reliable indicator for continuing blood loss, and, at admission, is the best predictor of 24-hour blood product requirements, as well as being associated with signs of shock and hemorrhage in trauma patients, both adult and pediatric. Additionally multiple studies have found massive blood transfusion in trauma, surgery, and critical care to be a predictor of SIRS, multiple organ failure, and increased infection and mortality, although it is unclear if the need for significant transfusion is a marker for disease severity rather than the transfusion itself being the etiologic cause.
In the setting of active operative hemorrhage, transfusion rates should match the rate of blood loss. Estimation of ongoing rates of bleeding and matching transfusion rates requires careful coordination between the anesthesia and operative teams. MT protocols have been demonstrably life-saving in these cases. In the setting of operative hemorrhage, the standard transfusion ratio is 1 pRBC to 1 flash frozen plasma (FFP). Electrolytes must be monitored to prevent hypocalcemia instigated by the citrate anticoagulant in the blood products. As was demonstrated in the PROPPR study, major trauma centers were unable to deliver adequate amounts of thawed plasma for MT sufficiently quickly, so a burn team anticipating significant blood loss intraoperatively is well advised to order appropriate amounts of blood products prior to initiating the surgery. With modern rapid infusers, such as the Belmont, capable of transfusing warmed, mixed blood products at rates of 500 cc/min, there is little reason for insufficient transfusion rates during a burn extirpation.
In the ICU, blood transfusions are typically unnecessary to treat or prevent hemorrhagic shock; rather, they are warranted for less emergent indications, such as blood loss anemia, coagulopathy, or volume expansion. Traditionally a defined Hb trigger of 10g/dL or hematocrit of 30%, the “10-and-30” rule, has guided transfusion practices. These triggers continue in many centers worldwide. However large-scale works in critical care literature and smaller, retrospective reviews of adult and pediatric burn patients have established the benefits of a lower, more restrictive transfusion trigger, indicating that some patients had been receiving blood to no benefit. The Society of Critical Care Medicine addressed transfusions in the intensive care setting. For critically ill patients, a restrictive strategy was found as efficacious as the conventional liberal one, so in patients with evidence of hemorrhagic shock, hemodynamic instability, or acutely bleeding, an Hb trigger of less than 7 g/dL indicates for transfusion, and this should be given as single units in the absence of acute bleeding. Recent research supports the theory that pRBC transfusions should not rely on the use of standardized triggers, but rather be tailored to the burn patient's blood volume status, acuity of blood loss, and perfusion requirements.
Transfusion needs increase with burn size, as do complications. Each 1% increase in TBSA of burn has a corresponding 6% increase in mortality risk. In a large study of transfusion trends in burn patients, those with 20% or greater TBSA required 13.7 ± 1.1 units, whereas those with 50% or greater TBSA required more than 30 units of pRBCs. Burn patients often receive multiple transfusions; in one study, more than half the transfusions resulted from anemia of critical illness (nonsurgical). While pRBC transfusion rapidly and reliably corrects anemia, it is associated with many of the consequences of bloodborne transmission, including hepatitis B, hepatitis C, and HIV. While the infectious transmission rate has significantly decreased with improved screening methods, it is markedly higher in low- and middle-income countries than in high-income countries like the United States (0.1% and 0.003%, respectively). More importantly, pRBC transfusion is associated with immunomodulation, including increased infectious morbidity, with a 13% increase in risk of developing an infection per unit of blood transfused. Muszynski et al. nicely reviewed the extensive hyperinflammatory and severe immunosuppressive effects of blood transfusions and related these data to critical care outcomes.
Other significant consequences include transfusion-related acute lung injury (TRALI), which is difficult to diagnose in burn patients because simultaneous lung injury from resuscitation or inhalation injury may contribute to TRALI diagnostic criteria, and ABO incompatibility, which can be rapidly fatal. Implementation of restrictive-transfusion strategies in which pRBCs are transfused only for hemodynamic instability or at lower Hb concentrations has reduced overall transfusion and infection rates, benefitting both cost and survival. FFP transfusion is also associated with TRALI in burn patients, and early transfusion of FFP correlates with increased incidence of other deleterious effects post-burn. However by not administering plasma from women with a history of pregnancy, the risk of TRALI from FFP is significantly mitigated, and using pathogen-inactivated plasma reduces the risk of transmitting infectious diseases. The indicators for increased transfusions of FFP and pRBC were high TBSA and use of argatroban anticoagulation.
Except in the setting where MTs are indicated, greater ratios of FFP and platelets to pRBCs correlate to longer ICU stays and higher mortality rates. When high ratios of FFP to pRBC are unable to be transfused, it has been demonstrated that resuscitating patients with a minimum of 1 L crystalloid per unit pRBC leads to improved mortality rates. However, a recent study showed that TBSA burn and patient age independently correlated to mortality, not RBC or plasma transfusions. Blood product resuscitation was not hemostatic, and coagulopathy and thrombocytopenia combined may contribute to intraoperative hemorrhage, which blood product transfusions would be inadequate to correct. Brakenridge and coworkers showed that, despite prior reports of associations between FFP and large-volume crystalloid transfusions with MOD, it was the MT volumes of pRBC that correspond to MOD, not blood products. Blood component ratios failed to predict inflammatory complications, whereas injury severity, sex, and total pRBC volume did. Additionally transfusion of stored pRBC correlates with increased complications due to microparticles released from RBCs able to induce cellular dysfunction. The poor quality of stored erythrocytes has been documented with dynamic microscopy. Furthermore, recent data would suggest different protocols for transfusion in the operating room (acute blood loss) versus in the setting of critical illness (bedside). A series of studies examining different ratios of FFP to pRBC transfusions have yet to demonstrate any difference in transfusion volume for burned pediatric patients. Last, it has been demonstrated that transfusion protocols for adults are not efficacious in children; a new score should be developed for transfusions in the burned pediatric population.
Recently, platelet-rich plasma (PRP), in which the platelet concentration is above baseline in blood plasma, has come under consideration for use in transfusing burn patients because its hemostatic antimicrobial effects have shown promise in wound healing in animal studies. The concentration of growth factors and number of platelets dictate the clinical efficacy of PRP. It is not transfusions of pRBCs but rather of platelets that have recently been correlated to nosocomial infections in the critically ill. Platelet transfusion is not without complications because platelets are stored at room temperature, thereby facilitating higher rates of bacterial contamination than for other blood products. One in 1000–3000 platelet units may be bacterially contaminated; one-sixth of these episodes result in a septic event. In a study of blood bank utilization by a burn unit, 15% of all admitted patients received platelets with either pRBCs or FFP. Given that cryopreserved platelets demonstrate superior hemostatic activity over liquid platelets, studying the efficacy of storing platelets at cold temperatures for burn units is warranted. Last, immediate administration of pRBCs, plasma, and platelets upon admission has been shown to benefit patient outcome.
Commonly coincident with sepsis, thrombocytopenia requiring platelet transfusion is rare in burn patients. Often platelet counts and function are stable unless there is an infectious or septic event. In coagulopathic patients with hemodynamically significant oozing from wound and donor sites, the administration of platelets and recombinant factor VIIa has been shown to improve hemostasis. Of interest, recombinant factor VIIa seems most efficacious at lower temperatures. Prothrombin complex concentrate (PCC) is gaining utility in treating coagulopathies derived from medications such as warfarin or argatroban and now has an expanding role in treating trauma and perioperative coagulopathies. PCC is also gaining use as an adjunct or replacement for FFP to reverse factor-deficient coagulopathies and expedite operative intervention.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here