Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A term newborn infant on the day of birth has a hemoglobin (Hgb) of 11.8 g/dL. Is that value low or is it within the expected (normal) reference range?
Expected values, also called “reference ranges,” for Hgb and hematocrit on the day of birth are a function of gestational age, increasing gradually through the second and third trimesters. Studies with very large sample sizes of neonates on the day of birth reveal no differences in Hgb or hematocrit associated with the infants’ sex. Reference ranges for blood Hgb concentrations are shown as Figure 12-1 . The fifth percentile value (the lowest expected limit) at term is 14 g/dL. Thus the value of 11.8 g/dL in this patient is low. 1
1 Christensen RD, Henry E, Jopling J, et al. The CBC: reference ranges for neonates. Semin Perinatol 2009;33:3–11.
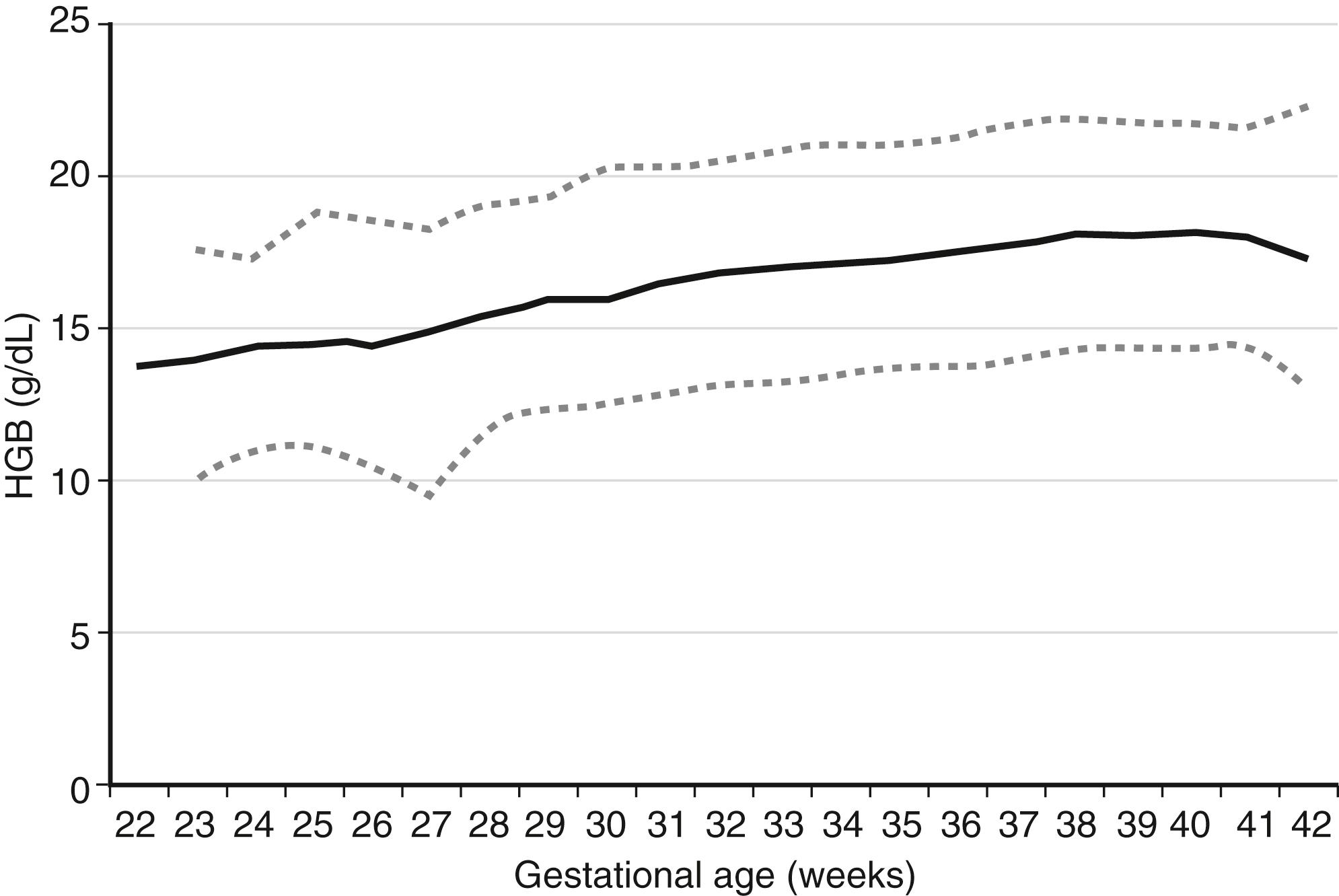
A term newborn infant on the day of birth has an Hgb of 24 g/dL. Is that value high, or is it within the expected (normal) reference range?
The 95th percentile reference range at term is 22.5 g/dL (see Figure 12-1 ). The value of 24 g/dL in this patient is therefore high.
Are Hgb and hematocrit values of newborn infants higher when obtained from capillary blood than when obtained from venous or arterial blood?
Yes. Values from capillary beds (heel stick) are generally about 15% higher and also more variable than venous or arterial values. This observation is due in part to the changing peripheral perfusion in the hours after birth. Poorly perfused heels seem to have capillary pooling or sludging of red blood cells (RBCs) that result in higher values. This phenomenon of higher Hgb level in capillary as opposed to central blood sources is not so significant in older children and adults. If the Hgb value of 24 g/dL in the patient in Question 2 was drawn from a heel stick, you would want to repeat it using a central vascular determination before you decide whether the neonate is truly polycythemic.
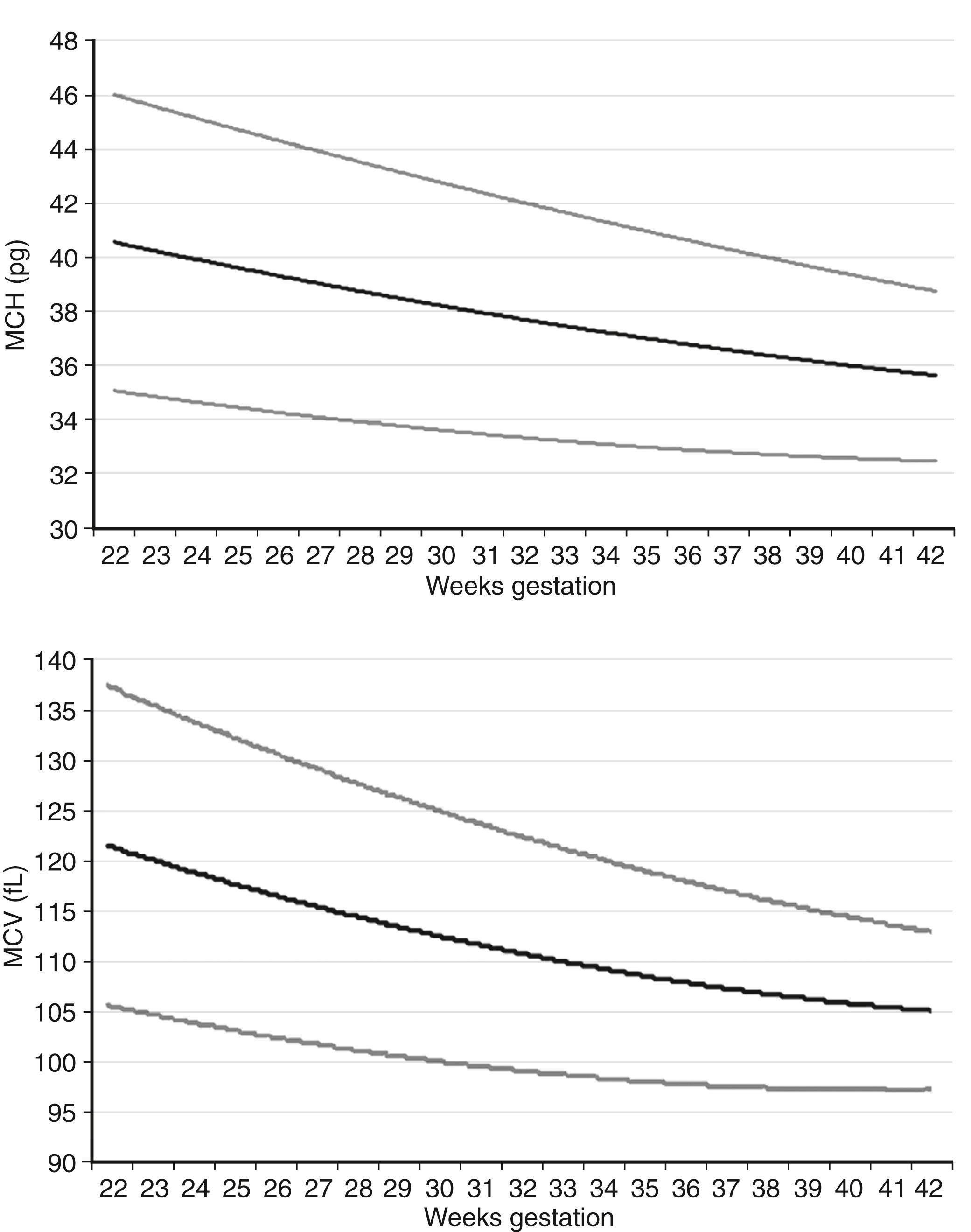
Which one of the following RBC measurements in a fetus or newborn infant does not normally diminish gradually with maturation during the second and third trimesters of pregnancy?
MCV (mean corpuscular volume) fL
MCH (mean corpuscular Hgb) pg
MCHC (mean corpuscular Hgb concentration) g/dL
The MCV and the MCH both diminish gradually through the second and third trimesters, as shown in Figure 12-2 . The MCHC, however, does not change during this period but remains in the range of 31 to 34 g/dL. MCHC values greater than 36 g/dL should alert you to the possibility of hereditary spherocytosis or pyropoikilocytosis, two conditions that generally present with a low MCV and a high MCHC. They commonly also demonstrate hyperbilirubinemia and sometimes a diminishing Hgb concentration. If the MCV is consistently greater than 36 g/dL you should assess the morphology of the RBCs, and you will need to ask whether other family members have abnormally shaped RBCs and have had anemia, neonatal jaundice, or early cholelethiasis (bilirubin stones).
The complete blood count (CBC) of a term neonate is reported to show greater than 100 nucleated red blood cells (NRBCs) per 100 white blood cells (WBCs). Is this value high, or is it within the expected (normal) reference range?
NRBCs can be reported as the number of NRBCs per 100 WBCs or as NRBCs per μL. The latter provides a more accurate accounting because of changing WBC counts over the first several days. The former is more commonly used in clinical practice. Reference ranges for NRBCs per 100 WBCs are shown in Figure 12-3 . For term infants on the day of birth, the 95th percentile (highest expected limit) is 15 per 100 WBCs. Therefore the value of 100 in this patient is abnormally high.
A newborn infant at 28 weeks’ gestation has no NRBCs per 100 WBCs. Is that value low, or is it within the expected (normal) reference range?
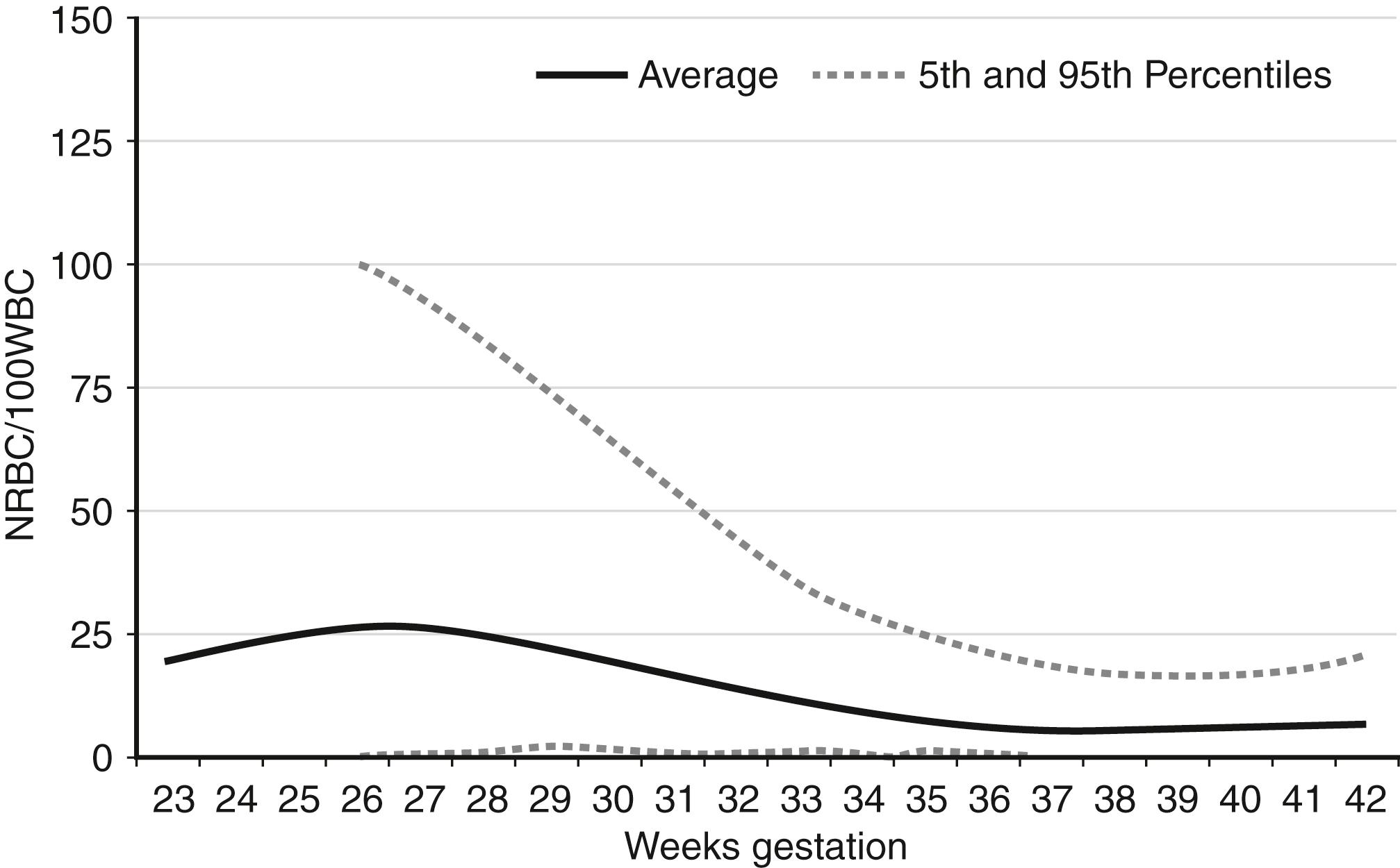
A value of zero (0) NRBCs per 100 WBCs is always within the reference range, regardless of gestational age. Thus the value of 0 in this patient is within the expected (normal) range.
You are asked to evaluate the result of a Hgb electrophoresis from a state metabolic screen, drawn on a nontransfused, extremely-low-birth-weight (less than 1 kg) neonate. The report reads as follows: 85% Hgb F, 5% Hgb A, and 10% Hgb Barts. What is your interpretation?
Hgb is a tetramer of globin chains, usually of two distinct types, bound to a heme moiety. Adult hemoglobin (Hgb A) consists of two alpha chains and two beta chains, whereas fetal hemoglobin (Hgb F) consists of two alpha chains and two gamma chains ( Figure 12-4 ).
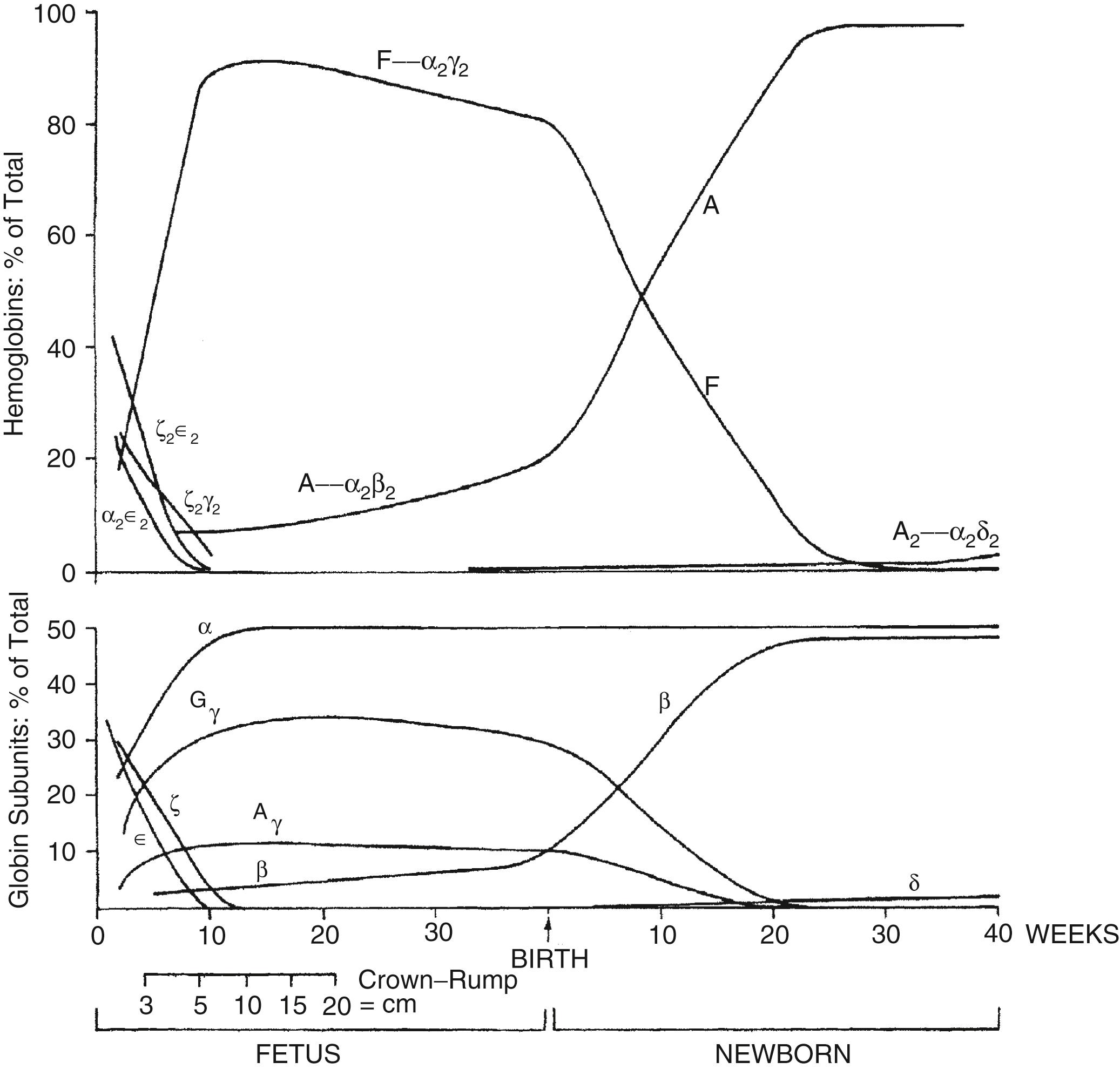
Embryonic Hgbs are present in the first 8 weeks after conception and consist of Hgb Gower 1 (zeta 2, epsilon 2), Hgb Gower 2 (alpha 2, epsilon 2), and Hgb Portland (zeta 2, gamma 2). Hgb Barts consists of four gamma chains and occurs in the absence or deficiency of alpha chains. Thus 10% of the Hgb observed as Barts is abnormal and suggests a deficiency of at least one, and probably two, of the four alpha chain genes. Deletion of one alpha gene results in a phenotypically normal individual, and deletion of two can result in mild microcytic anemia with the presence of Barts Hgb during the fetal and early newborn period. Deletion of three genes gives rise to Hgb H disease; deletion of all four gives rise to a lethal syndrome of hydrops fetalis with all or most of the Hgb being Barts, and no Hgb A or F or alpha 2 (because there are no alpha chains). 2
2 Kemper AR, Knapp AA, Metterville DR, et al.Weighing the evidence for newborn screening for Hemoglobin H disease. J Pediatr 2011;158:780–3.
How does Hgb F differ from Hgb A?
Hgb F binds oxygen more avidly. The oxyhemoglobin dissociation curve for Hgb F is shifted to the left of the adult curve. The higher affinity for oxygen facilitates transfer of oxygen from maternal Hgb A but also results in decreased oxygen release to the fetal tissues. The latter situation is not a disadvantage, however, because fetal tissues use oxygen primarily for growth; metabolic functions are mostly handled by the mother.
Factors that shift the oxyhemoglobin dissociation curve to the right (increasing oxygen delivery to tissues) include increased temperature, increased partial pressure of carbon dioxide in the blood (PaCO 2 ), increased RBC 2,3-diphosphoglycerate content, and decreased pH.
A term neonate has a total serum bilirubin (TSB) level of 18.8 mg/dL measured at 48 hours after birth as part of a pre–hospital-discharge bilirubin screening program. A CBC indicates that the Hgb is 11.2 g/dL, the MCV is 88 fL, and the MCHC is 38.2 g/dL. Which of the four values (bilirubin, Hgb, MCV, MCHC) is normal for age?
None of the four is normal. The bilirubin and the MCHC are high, and the Hgb and the MCV are low.
Which of the following would be appropriate diagnostic and management steps at this point?
Initiate intensive phototherapy.
Take a careful family history of neonatal jaundice and anemia, chronic jaundice and anemia, and gallstones (bilirubin cholelethiasis) at an early age.
Determine the maternal and neonatal blood type and the direct antiglobulin test (Coombs test).
Review the blood film, specifically looking for erythrocyte morphologic abnormalities.
Obtain a reticulocyte count, urine analysis (looking for free Hgb), and serum haptoglobin.
All the preceding steps may be helpful in the diagnosis and management of this case. Consider the following:
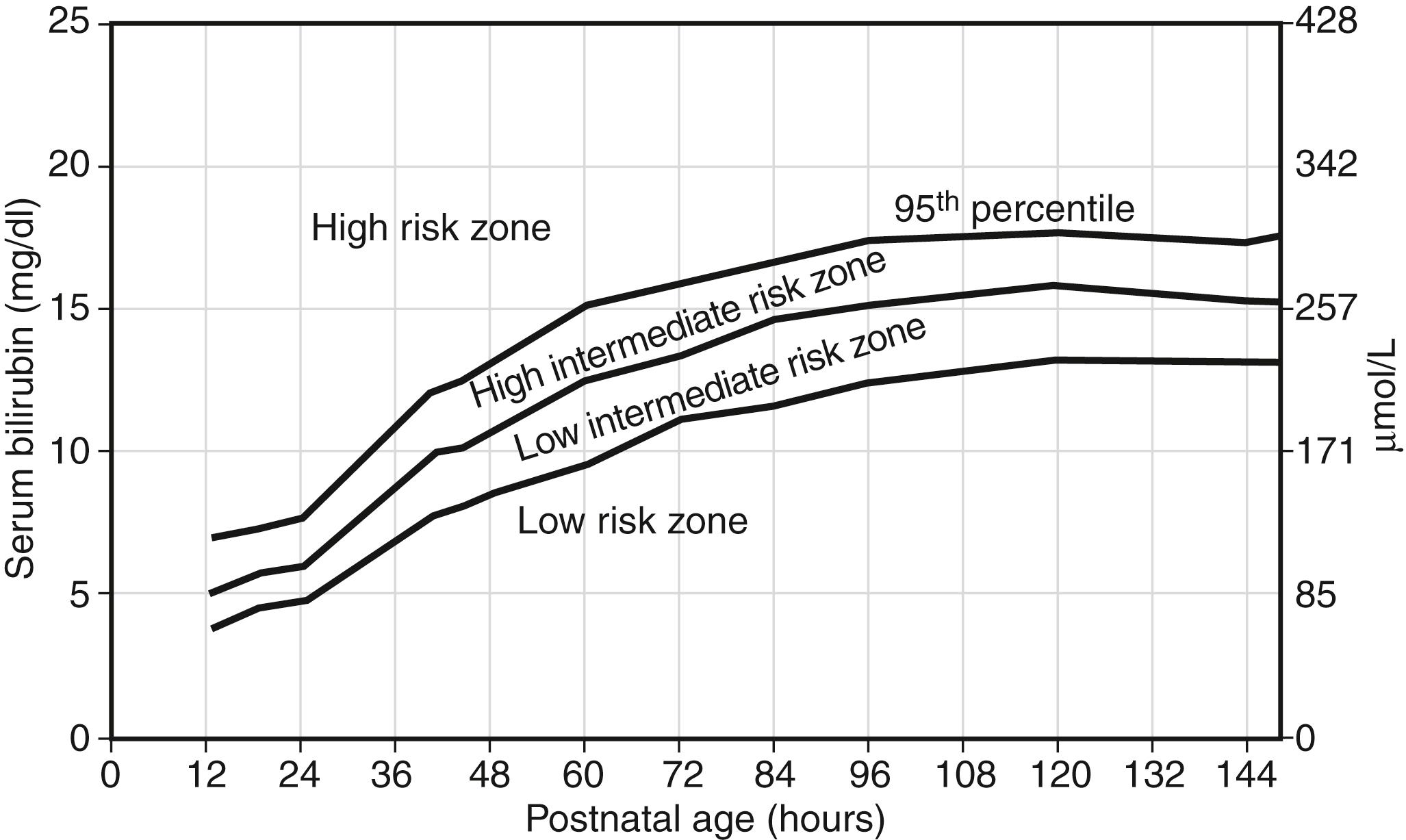
Intensive phototherapy and careful follow-up of the TSB are needed because the TSB plots well into the “high risk” zone ( Figure 12-5 ).
The family history might reveal that the father or mother has hereditary spherocytosis (HS). Approximately two thirds of cases of HS are inherited in an autosomal dominant fashion. HS in this neonate would be compatible with the laboratory findings given. Between 40% and 50% of neonates with HS have a mutation in ANK1 (at 8p11.2) encoding the RBC cytoskeletal protein component ankyrin 1. Between 20% and 35% have a mutation in SLC4A1 (at 17q21) encoding band 3, and 15% to 30% have a mutation in SPTB (at 14q23-24.1) encoding beta-spectrin.
Alternatively, the family history might reveal that either father or mother has hereditary elliptocytosis (see the discussion later in this chapter explaining how this could explain this neonate’s findings). On the other hand, the family history might be completely unrevealing because about one third of HS cases in neonates are either de novo mutations or autosomal recessive varieties. The latter includes mutations in SPR1 (1q22-23) encoding alpha-spectrin and EPB42 (at 15q15-21) encoding protein 4.2. The latter mutation is more likely among neonates of Japanese descent.
Type and Coombs testing should be done when the TSB falls in the “high risk” zone. The MCHC can be high in ABO hemolytic disease associated with spherocytes, but it generally does not exceed 36.5 fL. The value greater than 38 in this case suggests that HS is more likely.
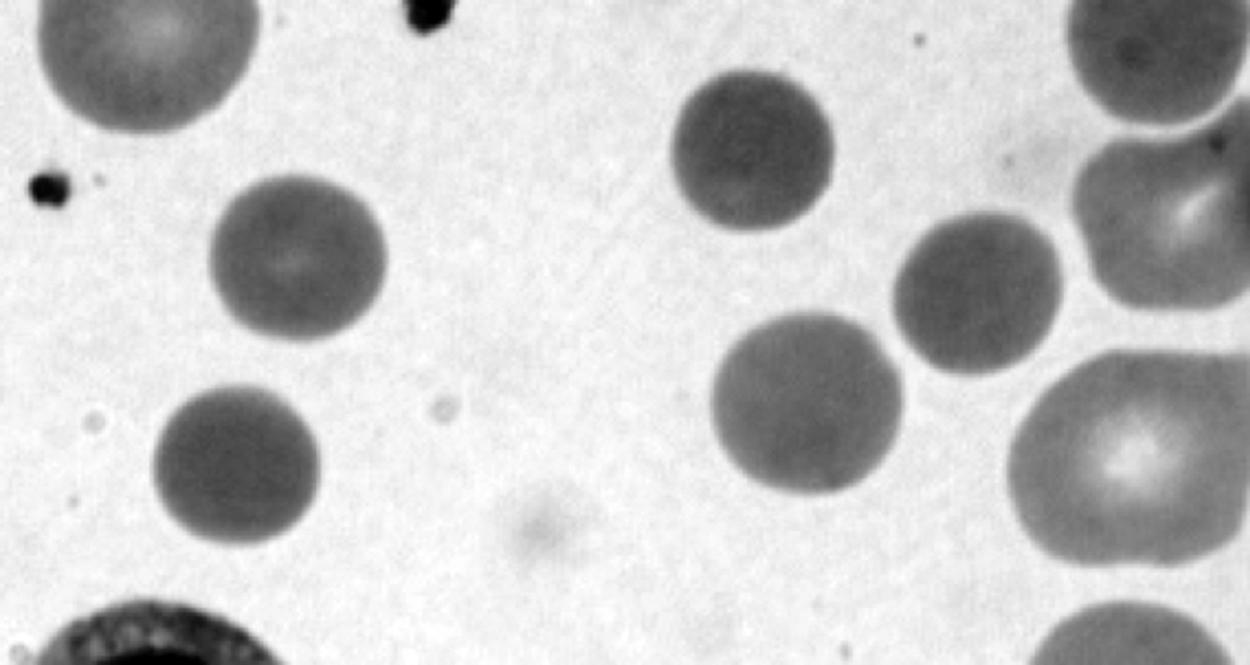
It is appropriate to examine the blood film for the presence of spherocytes or other morphologic abnormalities. Although microspherocytes can be seen in ABO hemolytic disease (sometimes presenting a dilemma between HS and ABO hemolytic disease in neonates with Coombs-positive jaundice), the high MCHC in this case suggests that HS is more likely. Figure 12-6 shows an example of a blood smear of a neonate with HS.
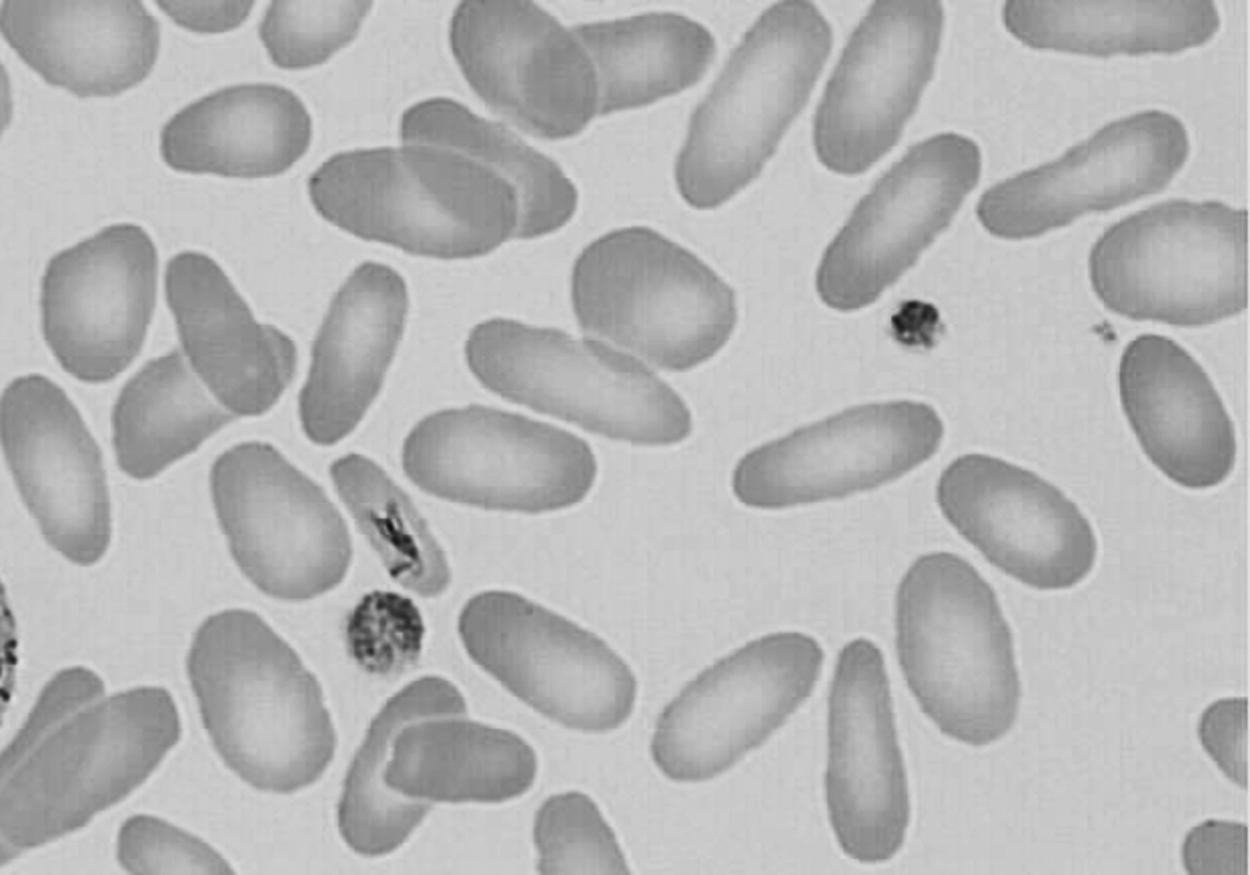
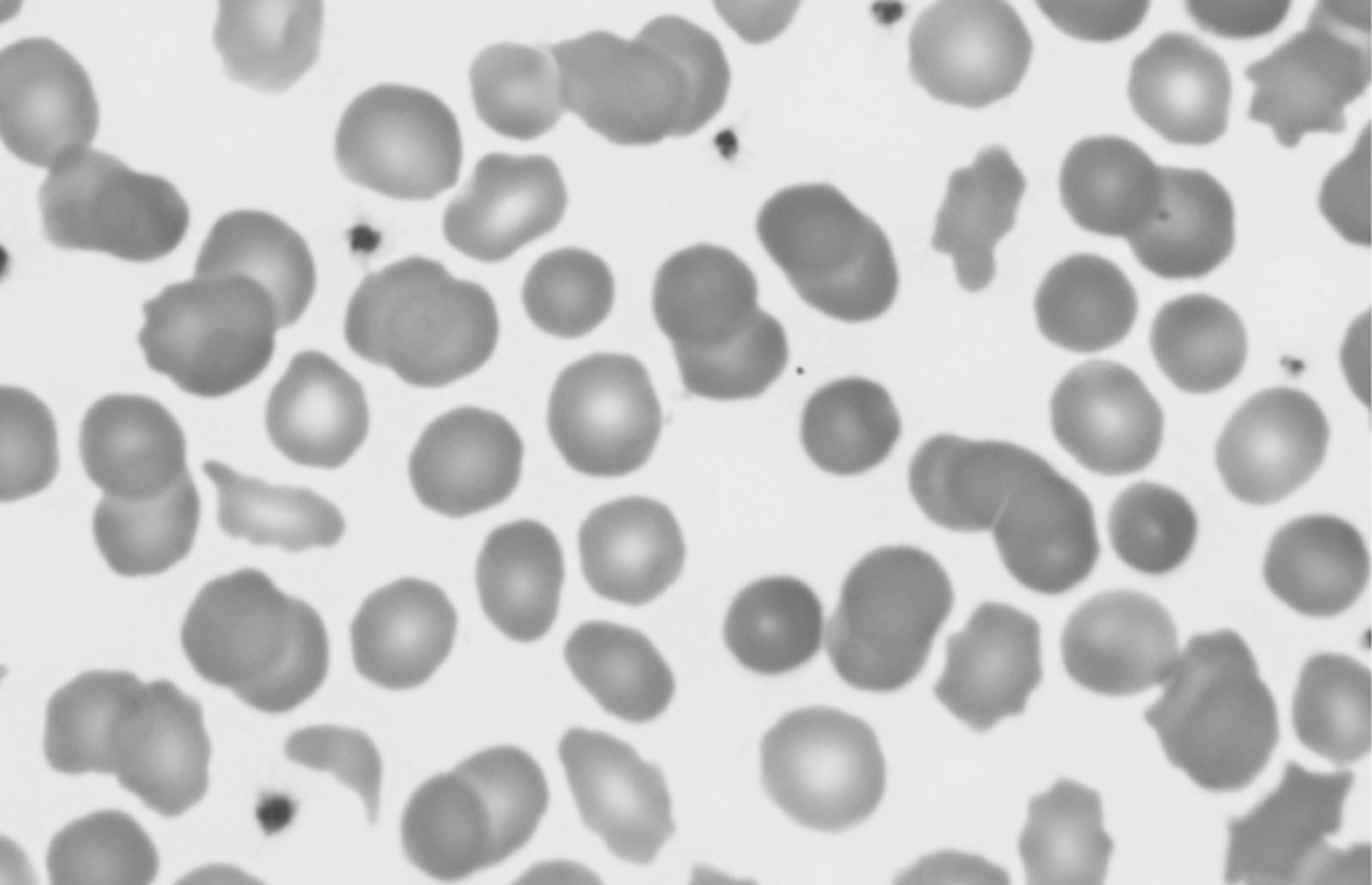
Hereditary elliptocytosis (HE) in a neonate does not generally result in significant hemolytic jaundice and anemia, and the RBC indices are not generally abnormal. However, a related condition called pyropoikilocytosis does indeed present similarly to this case, including early jaundice, anemia, low MCV, and high MCHC. Pyropoikilocytosis generally occurs when a father or mother has HE, consisting of an alpha-spectrin deficiency, and the other spouse has an asymptomatic alpha-spectrin defect. As a result, the neonate has a genetic condition similar to autosomal recessive inheritance as a compound heterozygote. Figure 12-7 shows an example of a blood film of HE, and Figure 12-8 shows an example of neonatal pyropoikiolocytosis. 3
3 Cohen RS, Wong RJ, Stevenson DK. Understanding neonatal jaundice: a perspective on causation. Pediatr Neonatol 2010;51:143–8.
Following a long labor of a term primipara, vacuum extraction is successfully accomplished. A capillary blood gas reading obtained within a few minutes of delivery was normal, including an Hgb count of 16 g/dL. Over the next hour the site of the vacuum attachment to the crown of the head becomes progressively larger and more fluctuant. A repeat Hgb count is still 16 g/dL. One clinician suggests that this finding might represent a subgaleal hemorrhage, but another states that the stable Hgb level is more likely to represent caput succedaneum. Which of the following would be appropriate diagnostic and management decisions?
Order continuous heart rate and respiratory rate monitoring with frequent blood pressure measurements, looking for any signs of compensated hypovolemia.
Obtain a CBC (at least a Hgb and hematocrit) now and again in about 2 hours.
Obtain a blood type and cross match in case an early transfusion is needed.
Wrap the head very tightly with an elastic bandage to prevent further head swelling.
Consider imaging the head to determine whether the swelling is consistent with blood in the subgaleal space.
The initially stable Hgb level does not exclude the diagnosis of a subgaleal hemorrhage. Hemorrhage itself does not lower the Hgb and hematocrit. The fall occurs only when extravascular fluid moves into the vascular space as a physiologic response to hypovolemia.
Although much less common than a caput, a subgaleal hemorrhage can be life-threatening and therefore demands aggressive monitoring and support. Head wrapping has been attempted in the past as a potential method for tamponade, but in general this approach has not been successful because it tends to increase the intracranial pressure.
With signs of hypovolemia, transfusion support may be warranted. Coagulopathy can occur, generally secondary to shock and disseminated intravascular coagulopathy (DIC), and can worsen the hemorrhage. Portable cranial ultrasound will generally confirm the presence of a subgaleal hemorrhage. Computed tomography or magnetic resonance imaging will provide more accurate and detailed information, but these are usually not needed to make the diagnosis of a subgaleal hemorrhage.
All neonatal subgaleal hemorrhages follow vacuum extraction delivery (true or false).
False. Most neonatal subgaleal hemorrhages do indeed follow vacuum extraction, but some follow forceps delivery and some occur with nonoperative delivery. However, in a series from Intermountain Healthcare, we found that every neonate with a subgaleal hemorrhage that required one or more RBC transfusions was delivered by either vacuum or forceps extraction.
All neonates who had a “spontaneous” subgaleal hemorrhage (not delivered by vacuum or forceps extraction) lacked signs of shock, had no transfusions, and generally had a good outcome. Thus vacuum delivery is the most significant risk factor for developing a neonatal subgaleal hemorrhage. 4
4 Reid J. Neonatal subgaleal hemorrhage. Neonatal Netw 2007;26(4):219–27.
A subgaleal hemorrhage following vacuum extraction delivery is rare, occurring in fewer than 1 percent of all vacuum deliveries (true or false).
True. In a recent report from Taiwan, one in 218 vacuum deliveries developed a subgaleal hemorrhage. In a study from Intermountain Healthcare, a subgaleal hemorrhage was diagnosed in one in 598 vacuum deliveries. A subgaleal hemorrhage is therefore rare, even after a vacuum delivery, but because of the vigilance needed for proper diagnosis and management, the possibility of a subgaleal hemorrhage should be considered after any operative delivery in which scalp fluctuance is observed.
If a subgaleal hemorrhage is diagnosed, the expected mortality rate is about 25% (true or false).
False. Some publications describing cases from the 1980s and earlier did indeed report a mortality rate this high, but more recent series suggest the mortality rate is 5% to 10%. Regardless, this injury is devastating. Vigilance and aggressive management are likely responsible for the observed improvement in outcome.
Hemolytic disease of the fetus and newborn (HDFN) can occur when a woman has immunoglobulin G antibodies directed against paternal RBC antigens inherited by the fetus. What is a typical clinical presentation for a case of HDFN?
Neonatal hemolytic jaundice is typical, particularly manifesting in the following ways:
Hyperbilirubinemia typically results in an elevated cord blood TSB; also, a TSB in the “high risk” zone within 24 or 48 hours of birth is possible.
Reticulocytosis: After day of life one or two, the reticulocyte count generally falls to near zero percent. However, in most cases of HDFN the reticulocyte count remains elevated, sometimes higher than 10%.
Hemolysis: Evidence includes a falling Hgb, free Hgb in the urine, and an absent serum haptoglobin concentration.
Erythroblastosis: Most cases other than anti-D have a normal or only a slightly elevated NRBC count. Severe cases of HDFN can have marked anemia, erythroblastosis, and hydrops fetalis. 5
5 Wennberg RP, Ahlfors CE, Aravkin AY. Guidelines for neonatal hyperbilirubinemia: an evidence based quagmire. Curr Pharm Des 2009;15:2939–45.
HDFN arising from maternal anti-Kell (Kell 1 ) antibody can present in a manner very different from the typical presentation described previously. What is that presentation, and why is that variety of HDFN different from the rest?
HDFN resulting from maternal anti-Kell antibody presents with fetal or neonatal anemia but no reticulocytosis, no evidence of hemolysis, and no hyperbilirubinemia. These cases present with hyporegenerative anemia. The explanation is that the Kell antigen is expressed on erythroid progenitor cells, whereas most other blood group antigens are not expressed until the cells clonally mature. As a consequence of maternal anti-Kell antibody binding to fetal erythroid progenitors, fetal RBC production is reduced and hyporegenerative anemia results. The KEL gene (7q33) encodes a 93 kilodalton transmembrane zinc-dependent endopeptidase that is responsible for cleaving endothelin-3. The Kell protein has recently been designated CD238.
In developed countries ABO incompatibility is now the leading cause of HDFN (true or false).
True. Women lacking the A and the B erythrocyte antigens often have anti-A and anti-B antibodies even before pregnancy. In the case of women with blood type O, their anti-A and anti-B antibodies are sometimes of the immunoglobulin G type and therefore can cross the placenta and bind to fetal antigens. Unlike the situation observed with maternal anti-D, in neonates with ABO hemolytic disease the principal problem is usually jaundice. Anemia, erythroblastosis, and hydrops are all very rare. The ABO locus is on chromosome 9 and has three main allelic forms: A, B, and O. The A and B alleles encode glycosyltransferases. The O allele differs from the A allele by deletion of only one nucleotide—guanine at position 261. This deletion causes a frame shift and results in premature termination of translation of the mRNA. 6
6 Geaghan SM. Diagnostic laboratory technologies for the fetus and neonate with isoimmunization. Semin Perinatol 2011;35(3):148–54.
What is the H antigen, and what does it have to do with the ABO blood type?
The H antigen is the precursor to the ABO blood group antigens. The H locus is on chromosome 19 and encodes the H antigen, which is expressed on the RBC surface. The H antigen is then modified by the A or the B antigen to produce the final A, B, or O antigen. Very rarely an individual lacks the H antigen because of a mutation in the H gene. This results in type O blood, but because the precursor molecule (the H antigen) is also missing, even type O blood cannot be transfused because the individual recognizes the H antigen in the type O blood as foreign. This unusual O blood type is called Bombay blood group and occurs in approximately four per million people, except in parts of India where it may be as common as 1 in 10,000. Neonates who are type O on the basis of Bombay can hemolyze if transfused with type O blood.
A mother about to deliver at 29 weeks’ gestation requests that the obstetrician and the neonatal team do everything possible to avoid an RBC transfusion in the neonate. What options are available to help the family with this request?
Delay clamping of the umbilical cord, or cord “stripping” or “milking.” These maneuvers, roughly equivalent in terms of the volume of fetal blood transferred from the placenta to the fetus, can be expected to result in an Hgb concentration of about 2 g/dL. Ask the obstetrician to consider these approaches.
Draw all laboratory tests on NICU admission (e.g., blood culture, CBC, state metabolic screen) using fetal blood in the placenta after placental delivery, thereby removing no blood from the neonate initially.
Carefully consider the need for all blood tests you order during the first several days to weeks, with the understanding that many early RBC transfusions in very-low-birth-weight infants are generally needed on the basis of anemia that results from phlebotomy for laboratory testing.
Consider slightly lowering the Hgb value you consider as a “transfusion trigger.” For instance, if your guidelines call for RBC transfusion at 10 g/dL or lower, consider lowering it to 9 g/dL or lower for this patient.
Consider administering the long-acting erythropoietin analog darbepoetin (Aranesp) once in the first few days and again 1 week later. Use 10 μg/kg as a unit dose.
Let the parents know that despite your best efforts to help them with their request, the baby’s best interests might force you to administer an RBC transfusion if the baby would be critically compromised otherwise. Explain all the steps that you are taking to avoid transfusing the infant. 7
7 McPherson RJ, Juul SE. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr Opin Pediatr 2010;22:139–45.
A twin-twin transfusion is expected on the basis of discordant-sized monochorionic twins. Fetal ultrasonography indicates the likelihood of anemia in the smaller twin because of the middle cerebral artery blood flow. It appears that the larger twin has pleural fluid and ascites, although these are subtle findings. You are anticipating that the smaller twin will be anemic and the larger twin may be polycythemic, but what other hematologic differences do you anticipate?
The donor (anemic, smaller) twin is more likely to have a hyporegenerative neutropenia, similar to that seen in neonates born after pregnancy-induced hypertension. This situation is likely to present with no left shift (a normal immature-to-total neutrophil ratio) and a duration of only about 2 or 3 days. Similarly, the donor twin is more likely to have a moderately low platelet count with a normal mean platelet volume (MPV). The pathogenesis of these findings is not known with certainty but likely relates to the accelerated erythropoietic effort in the anemic twin, with a concomitant temporary reduction in platelet and neutrophil production. Also, the anemic twin will usually have a higher NRBC count, generally above the reference range for age (see Figure 12-3 ). 8
8 Mosquera C, Miller RS, Simpson LL. Twin-twin transfusion syndrome. Semin Perinatol 2012;36:182–9.
RBC transfusion can be life-saving for neonates with acute hemorrhage or severe anemia. However, before ordering any RBC transfusion, the clinician must assess the potential risks and potential benefits. What are some of the risks associated with RBC transfusion in the NICU?
Typical transfusion reactions (i.e., those commonly reported for adult recipients) are only rarely observed in transfused neonates. These include febrile nonhemolytic reactions, urticarial (allergic) reactions, hypothermia, circulatory overload, hypotensive reactions, citrate toxicity (e.g., peripheral paresthesia, tingling, buzzing, cramps, nausea, vomiting), and acute hemolysis resulting from undetected incompatibility. Transfusion-transmitted diseases include bacterial contamination, which is considerably more common than the hepatitis and other viruses transmitted in past decades, before development and implementation of modern hemovigilance techniques and procedures.
Adverse associations with transfusions that are unique to neonates include transfusion-associated necrotizing enterocolitis (generally very-low-birth-weight neonates 3 to 4 weeks old receiving a “late” transfusion) and severe intraventricular hemorrhage in extremely-low-birth-weight neonates after an “early” transfusion. Transfusion-related acute lung injury (TRALI) reactions involve acute onset of (or acute worsening of) respiratory distress after transfusion. All plasma-containing blood products have been implicated in TRALI reactions. TRALI is now among the three leading causes of transfusion-related fatalities, along with ABO incompatibility and bacterial contamination, but it is rarely reported (perhaps because it is rarely recognized) in neonatal transfusion recipients. 9 10
9 Strauss RG. How I transfuse red blood cells and platelets to infants with the anemia and thrombocytopenia of prematurity. Transfusion 2008;48:209–17.
10 Strauss RG. Anaemia of prematurity: pathophysiology and treatment. Blood Rev 2010;24:221–5.
After a double-volume exchange transfusion for extreme hyperbilirubinemia in a term neonate, you obtain a CBC. The exchange was performed with packed RBC reconstituted with plasma to a hematocrit concentration of approximately 60%. Before the exchange transfusion a CBC revealed a hematocrit concentration of 30%, an Hgb of 10 g/dL, a platelet count of 230,000/μL, and an absolute neutrophil count of 2000/μL. What significant differences do you anticipate finding in the CBC after the exchange?
Several differences can be anticipated. Awareness of these differences prevents confusion when you compare the CBCs before and after the exchange.
The hematocrit and Hgb will be higher than before the exchange. If they are not higher, you might want to check the hematocrit and Hgb in the remaining unused reconstituted unit to ensure that the product you received approximated what you ordered.
The erythrocyte indices, particularly the MCV and MCH, will fall because adult donor erythrocytes have partly replaced erythrocytes of the neonate. The MCHC will likely be about the same because this measurement is generally in the same range in neonates and adults.
The platelet count will be considerably lower after the exchange because of the lack of platelets in the reconstituted donor unit. It is not uncommon to find a platelet count below 100,000/μL after an exchange transfusion. The count will not likely fall to a level requiring a platelet transfusion, however, unless you must repeat the double-volume exchange transfusion within a few hours. Indeed, if a repeat exchange transfusion is needed soon after the first, be aware that the platelet count after the second exchange might fall to exceedingly low levels. Because there is no large marrow ready reserve of platelets, the platelet count will not rebound rapidly (within hours) of the exchange.
The neutrophil count, already on the low side (2000/μL) before the exchange will be lower still afterward. Similar to the anticipated fall in platelet count, the reconstituted donor unit will lack neutrophils, and the count will fall. It would be expected that the post–exchange transfusion neutrophil count would be below 1000/μL in this patient.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here