Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The editors gratefully acknowledge contributions by Dr. Anne F. Reilly, Dr. Greg A. Holländer, and Dr. Anders Fasth that were retained from previous editions of Pediatric Secrets .
Bone marrow failure is manifested by pancytopenia or, at times, by cytopenia of a single cell type. It can be acquired (acquired aplastic anemia) or inherited/genetic (e.g., Fanconi anemia, severe congenital neutropenia, Diamond-Blackfan anemia, amegakaryocytic thrombocytopenia, thrombocytopenia-absent radius, dyskeratosis congenita).
Sieff CA. Introduction to acquired and inherited bone marrow failure. Hematol Oncol Clin North Am. 2018;32(4):569–590.
Chirnomas SD, Kupfer GM. The inherited bone marrow failure syndromes. Pediatr Clin North Am. 2013;60(6):1291–1310.
After careful exclusion of the known causes listed here, 80% of cases remain classified as idiopathic . A variety of associated conditions include the following:
Radiation
Immune diseases: eosinophilic fasciitis; hypogammaglobulinemia
Drugs and chemicals
Regular : Cytotoxic (as in treatment for malignancy), benzene
Idiosyncratic : Chloramphenicol, anti-inflammatory drugs, antiepileptics, gold, nifedipine
Viruses : Epstein-Barr virus (EBV), cytomegalovirus (CMV), hepatitis (primarily B), parvovirus (in immunocompromised hosts), HIV
Thymoma
Pregnancy
Paroxysmal nocturnal hemoglobinuria
Preleukemia
Shimamura A, Guinana EC. Acquired aplastic anemia. In Nathan DG, Orkin SD, Ginsburg D, Look AT, eds. Nathan and Oski’s Hematology of Infancy and Childhood . 6th ed. Philadelphia, PA: W.B. Saunders; 2003:257.
Severe disease includes a hypocellular bone marrow biopsy (< 30% of the normal hematopoietic cell density for age) and decreases in at least two out of three peripheral blood counts : neutrophil count < 500 cells/mm 3 , platelet count < 20,000 cells/mm 3 , or reticulocyte count < 1% after correction for the hematocrit. Categorization has important prognostic and therapeutic implications.
In the absence of definitive treatment, < 20% of children with severe acquired aplastic anemia survive for > 2 years. When bone marrow transplantation is performed using a human leukocyte antigen (HLA)–identical sibling donor, the 2-year survival rate exceeds 90%. The usual approach to the newly diagnosed child with severe acquired aplastic anemia is to perform bone marrow transplantation if there is an HLA-identical sibling to serve as the donor. About 80% of children with severe aplastic anemia do not have a sibling donor for bone marrow transplantation. These children receive medical therapy, usually the combination of antithymocyte globulin, cyclosporine, and hematopoietic growth factors, such as granulocyte colony-stimulating factor (GCSF). Two-year response rates are 30% to 70%, and survival rates exceed 90% in children. Matched unrelated bone marrow transplants have historically only been considered in patients who fail medical therapy; however, they may be considered earlier in patients with severe aplastic anemia who have features that indicate a potential poor response to medical therapy.
Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20(9):67.
Scheinberg P, Wu CO, Nunez O, et al. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr. 2008;153(6):814.
Fanconi anemia , or constitutional aplastic anemia, is a genetic disorder in which numerous physical abnormalities are often present at birth, and aplastic anemia occurs around the age of 5 years. The more common physical abnormalities include hyperpigmentation, anomalies of the thumb and radius, small size, microcephaly, and renal anomalies (e.g., absent, duplicated, or pelvic horseshoe kidneys). Patients with Fanconi anemia are also susceptible to leukemia and epithelial carcinomas.
Chromosomal breakage analysis , for example, with diepoxybutane or mitomycin C, can be used to make the diagnosis, and molecular diagnosis can confirm the diagnosis and be used to test relatives. In studies of peripheral blood lymphocytes, a high percentage of patients with Fanconi anemia will have chromosomal breaks, gaps, or rearrangements. Many genes causing the Fanconi anemia syndrome are critical for DNA repair, explaining patients’ predisposition to cancers and sensitivity to chemotherapy. Molecular diagnosis has assumed increasing importance, as studies linking genotype and phenotype can be predictive.
Triemsta J, Pham A, Rhodes L, et al. A review of Fanconi anemia for the practicing pediatrician. Pediatr Ann. 2015;44(10):444–445, 448, 450.
De Rocco D, Bottega R, Cappelli E, et al. Molecular analysis of Fanconi anemia: the experience of the Bone Marrow Failure Study Group of the Italian Association of Pediatric Onco-Hematology. Haematology. 2014;99(6):1022–1031.
Walden H, Deans AJ. The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Annu Rev Biophys. 2014;43:257–278.
Transient erythroblastopenia of childhood (TEC) and Diamond-Blackfan anemia . Both are disorders of red blood cell (RBC) production that occur during early childhood. Both disorders are characterized by a low hemoglobin level and an inappropriately low reticulocyte count. The bone marrows of patients with these conditions may be indistinguishable, showing reduced or absent erythroid activity in both cases.
TEC is a self-limited disorder , whereas Diamond-Blackfan syndrome usually requires lifelong treatment .
Age of presentation : Although there is an overlap in the age of presentation, Diamond-Blackfan syndrome commonly causes anemia during the first 6 months of life, whereas TEC occurs more frequently after the age of 1 year.
Red cells: The red cells in patients with Diamond-Blackfan syndrome have fetal characteristics that are useful for distinguishing this disorder from TEC, including increased mean cell volume, elevated level of hemoglobin F, and presence of i antigen.
Adenosine deaminase: The level of adenosine deaminase may be elevated in patients with Diamond-Blackfan syndrome but normal in children with TEC.
Mutations: Twenty-five percent of white patients with Diamond-Blackfan anemia have been found to have mutations in the gene for ribosomal protein S19, and molecular diagnosis for these mutations is very helpful when positive. Recently additional gene mutations have been identified in Diamond-Blackfan anemia. These also affect ribosomal proteins. In total, about three-fourths of Diamond-Blackfan patients can be identified by mutational analysis.
Bartels M, Bierings M. How I manage children with Diamond–Blackfan anemia. Br J Haematol. 2019;184(2):123–133.
Viachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anemia: results of an international clinical conference. Br J Hematol. 2008;142(6):859–876.
Kostmann syndrome is severe congenital neutropenia. At birth, or shortly thereafter, very severe neutropenia (absolute neutrophil count of 0 to 200/mm 3 ) is noted, often at the time of significant bacterial infection (e.g., deep skin abscess, pneumonia, sepsis). Even with antibiotic treatment, there is a high mortality during infancy unless GCSF therapy is used to elevate the neutrophil count. Some recipients of GCSF have survived the infection risk but have developed myelodysplastic syndrome or acute myeloid leukemia. Therefore individualized judgment and monitoring are essential in GCSF treatment of severe congenital neutropenia. An alternative treatment is bone marrow transplantation from an HLA-identical sibling donor. Patients with Kostmann syndrome may have mutations in ELANE or HAX1 genes.
Donadieu J, Beaupain B, Fenneteau O, Bellanné-Chantelot C. Congenital neutropenia in the era of genomics: classification, diagnosis and natural history. Br J Hematol. 2017;179(4):557–574.
Shwachman-Diamond syndrome is characterized by exocrine pancreatic dysfunction (causing steatorrhea), skeletal abnormalities (e.g., metaphyseal dysostosis, which are defects in normal cartilage ossification), growth retardation, and bone marrow insufficiency leading to neutropenia. It may initially be misdiagnosed as cystic fibrosis (CF) because of overlapping symptoms, but can be distinguished because sweat chloride levels are normal and mutations in the CF gene are lacking. Genetic testing for mutations in the SBDS (Schwachman-Bodian-Diamond syndrome) gene is diagnostic.
Nelson AS, Myers KC. Diagnosis, treatment and molecular pathology of Shwachman-Diamond syndrome . Hematol Oncol Clin North Am. 2018;32(4):687–700.
Burroughs L, Woolfrey A, Shimamura A. Shwachman-Diamond syndrome: a review of the clinical presentation, molecular pathogenesis, diagnosis, and treatment. Hematol Oncol Clin North Am. 2009;23(2):233–248.
Platelet problems : Although there can be considerable overlap, in general, platelet problems result in petechiae, especially on dependent parts of the body and mucosal surfaces. Additional manifestations of platelet disorders include epistaxis, hematuria, menorrhagia, and gastrointestinal (GI) hemorrhages.
Coagulation factor deficiencies or platelet problems : Ecchymoses are suspicious for coagulation factor deficiencies or platelet problems when they occur in unusual areas, are out of proportion with the extent of described trauma (also seen in child abuse), or are present in different stages of healing. Delayed bleeding from old wounds and extensive hemorrhage (particularly into joint spaces or after immunizations) are also suggestive of coagulation protein disorders.
Disseminated intravascular coagulation (DIC) : Bleeding from multiple sites in an ill patient is worrisome for DIC. If a patient has tolerated tonsillectomy and/or adenoidectomy or extraction of multiple wisdom teeth without major hemorrhage, a significant inherited bleeding disorder is unlikely.
Rodriguez V, Warad D. Pediatric coagulation disorders. Pediatr Rev. 2016;37(7):279–291.
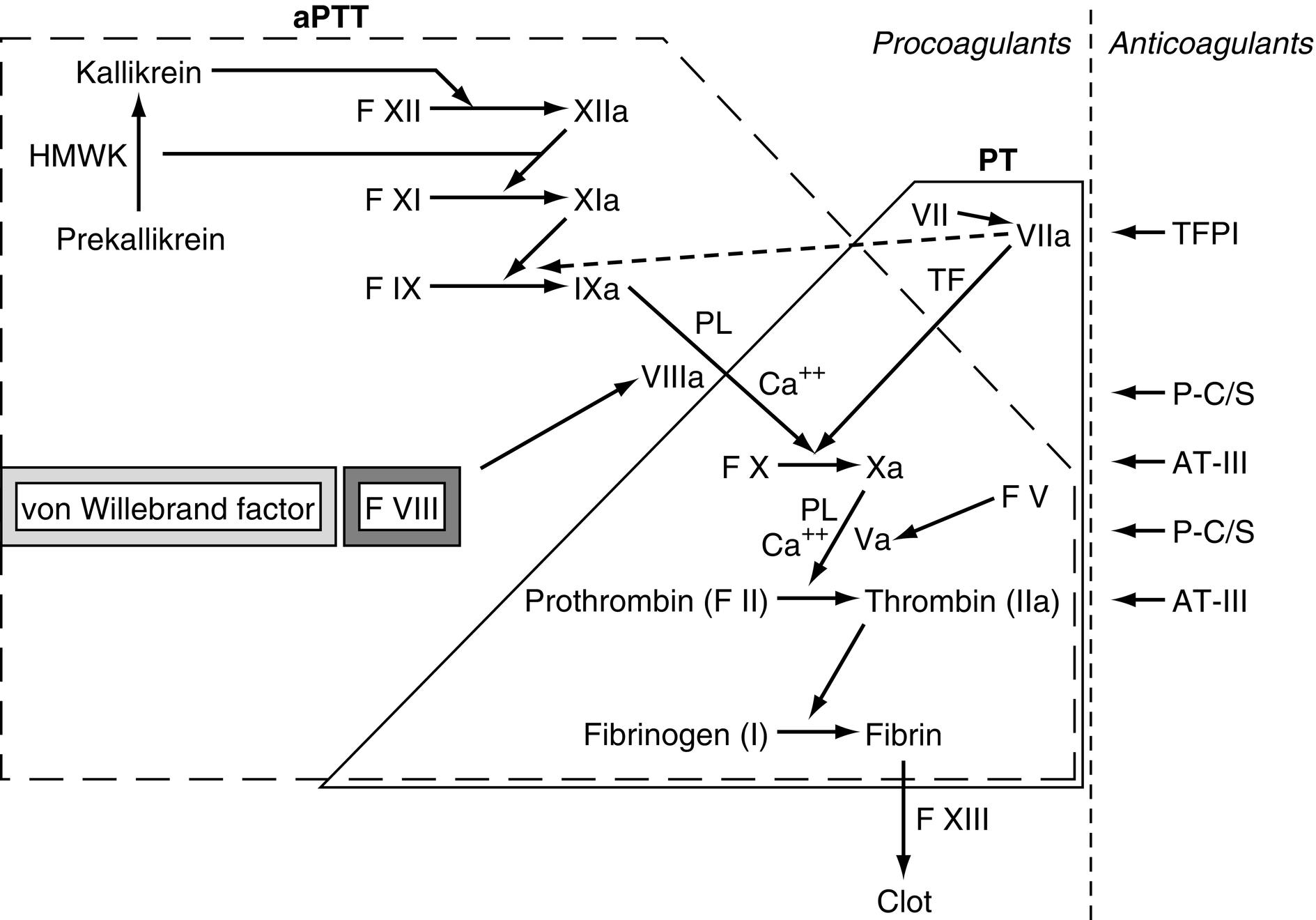
See Fig. 9.1 .
See Table 9.1 .
| Scenario | Common and Important Causes | Comments |
|---|---|---|
| Prolonged PT | Vitamin K deficiency Liver disease Warfarin Factor VII deficiency Disseminated intravascular coagulation (DIC) |
Isolated PT elevation is a sensitive marker early in DIC development |
| Prolonged aPTT | Von Willebrand disease Hemophilia (factor VIII, IX, or XI deficiency) Heparin Antiphospholipid antibodies (associated with minor infections or, rarely, autoimmune or thromboembolic disease) |
Rare deficiencies of factor XII, congenital abnormalities of the receptor for vitamin B 12 –intrinsic factor complex Gastric mucosal defects that interfere with the secretion of intrinsic factor or phosphokinase may also elevate aPTT but are not clinically significant Half of children with prolonged aPTT do not have a bleeding disorder |
| Prolonged PT and aPTT | Heparin Warfarin Liver disease DIC |
Fibrinogen measurement can help distinguish among liver disease and DIC (decrease in fibrinogen) and vitamin K (no decrease in fibrinogen) |
The INR , introduced in an attempt to standardize the PT, results from a calculation in which an individual patient’s PT test value is divided by the laboratory’s pooled normal plasma standard PT, then raised to an exponent applicable to each individual PT-initiating reagent available. Its utility is in monitoring Coumadin (warfarin) use, in that the reported value has clinical utility, regardless of which laboratory performed the PT test. The INR for individuals with normal coagulation proteins not receiving Coumadin therapy is 1.0 (± ≈ 0.1 to 0.2 based on that laboratory’s upper and lower range). For those receiving Coumadin therapy, the desired INR varies with the condition being treated, but it is often 2.0 to 3.0.
TEG is a whole-blood test of coagulation. Blood is rotated gently, and the timing to clot initiation ( R eaction time), speed of clot formation ( K inetic time), thrombin burst (α angle, which is the slope of the line between R and K, a measurement of the rate of clot formation), clot strength ( Maximal A mplitude, or MA ), and clot lysis time are measured. These measurements can assess platelet function, clot strength, and fibrinolysis in a way that other testing cannot. It is used often in surgery or in trauma settings to direct blood product use.
Carter BG, Carland E, Monagle P, et al. Impact of thromboelastography in paediatric intensive care. Anaesth Intensive Care. 2017;45(5):589–599.
von Willebrand disease: This is the most common coagulopathy, and it is autosomal dominant in the majority of cases. Frequency is estimated to be between 1 in 100 and 1 in 500.
Factor VIII deficiency (hemophilia A) and factor IX deficiency (hemophilia B): These conditions are inherited in an X-linked pattern so that females are carriers and males are affected. Inquiry about affected maternal male first cousins or uncles is appropriate. In general, heterozygotes for clotting factor deficiencies are not clinically affected. Factor VIII deficiency is more common (1 in 5000) than factor IX deficiency, affecting 80% to 85% of all patients with clinically diagnosed factor deficiency.
Rodriguez V, Warad D. Pediatric coagulation disorders. Pediatr Rev. 2016;37(7):279–291.
The abnormal factor VIII gene responsible for hemophilia A exhibits marked heterogeneity, and up to one-third of cases (either the immediate-carrier mother or the son himself) may have developed a spontaneous mutation . Molecular diagnosis of the most common mutation in severe factor VIII deficiency—a gene inversion in the distal portion of the gene in the affected male, the mother, and maternal relatives—may help the physician with understanding the family history.
Severe: < 1% factor VIII or IX activity; spontaneous bleeding common; bleeding often involves joints, soft tissue, brain (intracranial hemorrhages in neonates), postcircumcision; most common type (50% to 70% of cases)
Moderate: 1% to 5% factor VIII or IX activity; bleeding after minor trauma, but not usually spontaneous; may involve joints and soft tissue, but less commonly central nervous system (CNS) or postcircumcision; least common type (10% of cases)
Mild: 6% to 30% factor VIII or IX activity; bleeding only after major trauma or surgery; joint and soft tissue involvement, but uncommon after circumcision; more common than moderate type (30% to 40% of cases)
Sharathkumar AA, Pipe SW. Bleeding disorders. Pediatr Rev. 2008;29(4):121–129.
National Hemophilia Foundation. www.hemophilia.org . Accessed January 10, 2020.
Never forget anatomic or surgical technical causes and corrections for hemorrhage. As a result, primary measures are local measures (“push on it, put a stitch or staple in it”), supplemented occasionally with licensed topical prothrombotic agents. Replacement of the deficient blood component(s) is also important, but pharmacologic measures such as desmopressin acetate (DDAVP, which increases von Willebrand factor), antifibrinolytics such as epsilon aminocaproic acid and tranexamic acid (which stabilize clots), and topical hemostatic preparations can be useful.
The following guidelines are applicable to patients with moderate (1% to 5% of normal factor levels) to severe (< 1% of normal) hemophilia:
For minor hemorrhages (e.g., small muscle or oral), factor levels should be increased to 20% to 30% of normal.
For major bleeding episodes (e.g., hip bleeds, intracranial hemorrhage, bleeding around the airway), factor levels should be raised 70% to 100%, and repeat dosing should be strongly considered under close medical supervision.
Recombinant factor VIII and factor IX concentrates are the treatments of choice. Each unit of factor VIII or factor IX is equivalent to the activity of 1 mL of normal plasma. With the recombinant products, a dose of 1 unit/kg should increase the factor VIII level by 1.5% to 2% and the factor IX level by 1%. Calculations can be made as follows:
For example: If you have a 28-kg patient with a head bleed and severe factor VIII deficiency that you wish to correct 100%, your goal dose is 100 × 28 × 0.5 = 1400 units.
Another example: If you have a 50-kg patient with a minor bleed and severe factor IX deficiency that you wish to correct 30%, your goal dose is 30 × 50 = 1500 units.
Always round up to the nearest vial size so that there is no wastage of recombinant factor.
Of note, if there is an antibody inhibitor of the replacement factor, correction will not be achieved. Under these circumstances, alternative therapies are needed, such as porcine factor VIII, factor VIII inhibitor bypassing activity complexes, or recombinant factor VIIa.
Josephson N. The hemophilias and their clinical management. Hematology Am Soc Hematol Educ Program. 2013;2013:261–267.
In a study of boys with severe hemophilia A who were given regular recombinant factor VIII infusions up to 6 years of age, prophylaxis prevented joint damage and decreased the frequency of joint and other hemorrhages. Prophylaxis works. However, the cost is between $200,000 and $300,000 annually. Emicizumab, a recombinant humanized monoclonal antibody, has been approved by the Food and Drug Administration (FDA) since 2017 for prophylaxis in patients with hemophilia A without inhibitors and since 2018 for patients with inhibitors. Other therapies involving recombinant bioengineering are in development. How to reconcile the benefits and costs of effective expensive therapies remains a challenge for the health care system.
Weyand AC, Pipe SW. New therapies for hemophilia. Blood. 2019;133(5):389–398.
Acharya SS. Advances in hemophilia and the role of current and emerging prophylaxis. Am J Manag Care. 2016;22(5 Suppl):s116–s125.
Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544.
The half-lives for the first doses of factors VIII and IX are 6 to 8 hours and 4 to 6 hours, respectively. With subsequent doses, factor VIII has a half-life of 8 to 12 hours, whereas factor IX has a half-life of 18 to 24 hours. Thus, for serious bleeding, the second dose of factor VIII should be given 6 to 8 hours after the first, whereas the second dose of factor IX should be given 4 to 6 hours after the first. Subsequent doses are usually given every 12 hours for factor VIII replacement and every 24 hours for factor IX replacement, but the measurement of actual factor levels may be necessary to guide therapy in life-threatening situations.
Gill JC. Transfusion principles for congenital coagulation disorders. In Hoffman R, Benz EJ, Shattil SJ, et al, eds. Hematology: Basic Principles and Practice . 3rd ed. New York: Churchill Livingstone; 2000:2282.
Both long-acting recombinant factor IX and long-acting recombinant factor VIII are approved in the United States. They significantly affect the frequency of dosing for factor, especially because each is used for prophylaxis. The half-lives are extended by fusion with the Fc moiety of immunoglobulin G (IgG), which prevents lysosomal degradation of the factor. Other mechanisms to prevent degradation and prolong the half-life of factors VIII and IX, including PEGylation (the process of covalent attachment of polyethylene glycol [PEG] polymer chains to the recombinant factors) and albumin fusion, are also available. Gene therapy also holds future promise for long-term cure.
Shapiro A. Long-lasting recombinant factor VIII proteins for hemophilia A. Hematology Am Soc Hematol Edu Program. 2013;2013:37–43.
Factor VII deficiency. PT measures the function of the common pathway factors (including X, V, II, and fibrinogen) and the extrinsic pathway (tissue factor and factor VII). The aPTT measures the common pathway plus the function of the intrinsic pathway (including factors XII, XI, IX, and VIII). Isolated factor VII deficiency selectively elevates the PT. Other causes of elevated PT (e.g., liver disease, vitamin K deficiency, Coumadin toxicity) are not selective for lowering factor VII activity.
More commonly called factor XI deficiency, this is an uncommon type of hemophilia (< 5% of total hemophilia patients). Unlike the X-linked nature of hemophilias A and B, it is an autosomal recessive disease that occurs most frequently in Ashkenazi Jews. Plasma levels of factor XI are less predictive of clinical bleeding.
Asakai R, Chung DW, Davie EW, et al. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325(3):153–158.
In 1952, investigators in England noted that when blood from one group of hemophiliacs was added to the blood of another group of hemophiliacs, the clotting time was shortened. This provided the basis for the discovery of plasma substances in addition to what was then called “antihemophilic globulin” (and now called factor VIII), which is responsible for normal clotting. The name was derived because the first patient examined in detail with the unusual clotting deficiency (later designated as factor IX) was a boy named Stephen Christmas. The publication of the landmark article in fact occurred during the last week of December in 1952.
Biggs R, Douglas AS, Macfarlane RG, et al. Christmas disease: a condition previously mistaken for haemophilia. Br Med J. 1952;2(4799):1378–1382.
X-linked recessive disorder
Hemophilia A: Factor VIII abnormalities (80% to 85% of total cases)
Hemophilia B: Factor IX abnormalities
Severity based on factor levels: Severe (< 1%), moderate (1% to 5%), mild (5% to 30%)
Common initial presentation: Bleeding after circumcision
Synthesized in megakaryocytes and endothelial cells, vWF is a large multimeric protein that binds to collagen at points of endothelial injury. It serves as a bridge between damaged endothelium and adhering platelets, and it facilitates platelet attachment. It also serves as a carrier protein for factor VIII in circulation; it minimizes the clearance of factor VIII from plasma and accelerates its cellular synthesis.
von Willebrand disease is actually a group of disorders caused by qualitative or quantitative abnormalities in vWF. Coagulation abnormalities in children with severe disease can include a prolonged bleeding time, prolonged PTT, decreased factor VIII coagulant activity, decreased factor VIII antigen, and decreased ability of patient plasma to induce aggregation of normal platelets in the presence of ristocetin (the so-called “ristocetin cofactor activity”).
Sharma R, Flood VH. Advances in diagnosis and treatment of Von Willebrand disease. Blood. 2017;130(22):2386–2391.
Quantification of vWF antigen
Measurement of vWF function (either ristocetin-based platelet aggregation test, known as ristocetin cofactor assay ) or vWF collagen-binding assay
Factor VIII clotting activity
Screening tests for bleeding disorders (such as aPTT and bleeding time) can be normal in mild disease. Stress, pregnancy, or medications (e.g., oral contraceptives) can falsely elevate vWF levels in a patient. Once a diagnosis of vWF deficiency is suspected, vWF multimer analysis or genetic testing may assist in defining the subtype of vWF deficiency.
Cooper S, Takemoto C. Von Willebrand disease. Pediatr Rev. 2014;35(3):136–137.
vWF activity. vWF will bind to the glycoprotein IB receptor on platelets in the presence of the antibiotic ristocetin. A patient’s plasma is serially diluted and mixed with platelets. The presence of vWF allows for platelet agglutination, which can then be quantified on the basis of the dilutions.
Treatment depends on the variant of von Willebrand disease that is identified:
If protein is normal but diminished in quantity , DDAVP is given to stimulate endogenous release. DDAVP is now available for intravenous use and for intranasal use (Stimate). It is important to test von Willebrand disease patients for the safety and efficacy of either form of DDAVP before clinical use. It is also important to distinguish the form of intranasal DDAVP used for vWF therapy from that used for enuresis management.
If protein is abnormal but bleeding is mild, desmopressin may also be of value.
If protein is abnormal but bleeding is severe, licensed vWF concentrates may be administered. Plasma-derived but highly purified products (trade names include Alphanate, Humate-P and Wilate) provide both vWF and factor VIII. The ristocetin cofactor activity is quantitated for each vial, which allows for more precise use. A recombinant product (trade name Vonvendi) is also approved for adults.
Sharma R, Flood VH. Advances in the diagnosis and treatment of von Willebrand disease. Blood. 2017;130(22):2386–2391.
Up to 20% may have a bleeding disorder, particularly von Willebrand disease. The American College of Obstetrics and Gynecology recommends screening for any patient < 18 years with menorrhagia.
Graham RA, Davis JA, Corrales-Medina FF. The adolescent with menorrhagia: diagnostic approach to a suspected bleeding disorder. Pediatr Rev. 2018;39(12):588–600.
Kulp JL, Mwangi CN, Loveless M. Screening for coagulation disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2008;21(1):27.
DDAVP is a synthetic analog of vasopressin, the antidiuretic hormone. Within 1 to 2 hours of its administration (either intravenous, subcutaneous, or intranasal), plasma vWF levels increase by two-fold to eight-fold. DDAVP appears to act by causing the release of vWF from the endothelial cells. Factor VIII levels also increase in part because of the increased stabilization of vWF/factor VIII complex by DDAVP, which lessens proteolytic degradation. As a caution, DDAVP administration in the setting of von Willebrand disease type IIB may cause a dangerous drop in platelet count due to increased vWF binding and platelet clearance.
This is controversial. Studies have shown that the frequency of abnormal screening testing is low, around 2%. Most of the abnormalities identified were transient and unlikely to be clinically significant. In the absence of symptoms and a negative family history, the diagnosis of a bleeding disorder is unlikely. However, others argue that screening should be used despite the small yield to avoid missing an undiagnosed bleeding disorder.
Nieto RM, De Leon LE, Diaz DT, et al. Routine preoperative laboratory testing in elective cardiothoracic surgery is largely unnecessary. J Thorac Cardiovasc Surg. 2017;153(3):678–685.
Shah MD, O’Riordan MA, Alexander SW. Evaluation of prolonged aPTT values in the pediatric population. Clin Pediatr. 2006;45(4):347–353.
Burk CD, Miller L, Handler SD, et al. Preoperative history and coagulation screening in children undergoing tonsillectomy. Pediatrics. 1992;89(4 Pt 2):691.
Vitamin K is essential for the γ-carboxylation of both procoagulants (including factors II, VII, IX, and X) and anticoagulants (proteins C and S). γ-carboxylation occurs in the liver and converts the proteins to their functional forms. Vitamin K is obtained in three ways: (1) as dietary fat-soluble K1 (phytonadione) from leafy vegetables and fruits, (2) as K2 (menaquinone) from synthesis by intestinal bacteria, and (3) as water-soluble K3 (menadione) from commercial synthesis.
Malabsorptive intestinal disorders (e.g., cystic fibrosis, Crohn disease, short-bowel syndrome)
Prolonged antibiotic therapy (this diminishes intestinal bacteria)
Prolonged hyperalimentation without supplementation
Malnutrition
Chronic hepatic disorders (hepatitis, α 1 -antitrypsin deficiency) can diminish both the absorption of fat-soluble vitamin K (as a result of diminished bile salt production) and the use of vitamin K in factor conversion
Drugs that can disrupt vitamin K include phenobarbital, phenytoin, rifampin, and Coumadin
Factors II, V, VII, IX, and X are made in the liver, and all of these factors (except factor V) are vitamin K dependent. Therefore the measurement of factor V is a useful test to distinguish liver disease from vitamin K deficiency because this factor is reduced in the former and normal in the latter disorder. Factor VIII is reduced in patients with DIC because of the consumptive process, but this factor is normal or increased in patients with liver disease and vitamin K deficiency. Therefore the factor VIII level is a good test to distinguish DIC from the other two disorders ( Table 9.2 ).
| Factor V | Factor VII | Factor VIII | |
|---|---|---|---|
| Liver disease | Low | Low | Normal or increased |
| Vitamin K deficiency | Normal | Low | Normal |
| Disseminated intravascular coagulation | Low | Low | Low |
DIC is an acquired syndrome that is precipitated by a variety of diseases and characterized by diffuse fibrin deposition in the microvasculature, consumption of coagulation factors, and endogenous generation of thrombin and plasmin. The process is uncontrolled, and the result can be significant microthrombus formation with ischemic injury to multiple organ systems.
See Table 9.3 .
| Test | Usual Results |
|---|---|
| Prothrombin time; activated partial thromboplastin time | Prolonged |
| Fibrinogen | < 100 mg/dL ⁎ |
| Platelet count | Low |
| D-Dimer | > 2 μg/mL |
| Factors II, V, and VIII | Usually low ⁎ |
⁎ These results may be normal, however, especially in patients with mild disseminated intravascular coagulation because synthesis increases with accelerated consumption.
DIC occurs most commonly in the context of bacterial sepsis and hypotension. The best treatment is reversal of the underlying cause through treatment of the infection and appropriate fluid and pressor management. If bleeding is severe or if hemorrhage is occurring in a life-threatening location, platelets and fresh frozen plasma (FFP) should be given to make up for the loss of these elements, which is occurring from consumption. Heparin has not been proven to be effective for increasing survival in patients with sepsis and DIC. The replenishment of depleted antithrombin III levels with antithrombin III concentrate may decrease the risk for new thromboses. Supplementation with prothrombin complex concentrates (aPCCs), recombinant protein C, and thrombomodulin has been trialed in adults and pediatric patients with DIC but have not shown a clear clinical benefit.
Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102(2):187–193.
Factor V Leiden: This is an abnormal factor V protein that is resistant to the normal antithrombotic effect of activated protein C.
Protein C deficiency: Protein C inactivates factors V and VIII and stimulates fibrinolysis.
Protein S deficiency: Protein S serves as a cofactor for the activity of protein C.
Antithrombin III deficiency: Antithrombin III is involved in the inhibition of thrombin; factor X; and, to a lesser extent, factor IX.
Prothrombin variation: Mutation at gene position 20210 increases prothrombin levels possibly through decreased messenger RNA (mRNA) degradation.
Hyperhomocysteinemia: Can be the result of a variant of the MTHFR gene, but the level of homocysteine needs to be elevated for the variant to play a role. Those with predisposition to hyperhomocysteinemia due to thermolabile MTHFR variants benefit from folate supplementation, sometimes with vitamins B 6 and B 12 in addition.
Antiphospholipid antibodies: These are passed from mother to infant prenatally. They can also be acquired, often in adolescence, in the presence of systemic autoimmune diseases such as systemic lupus erythematosus (SLE).
Yang JY, Chan AK. Pediatric thrombophilia. Pediatr Clin North Am. 2013;60(6):1443–1462.
Factor V Leiden, protein C deficiency, protein S deficiency, and antithrombin III deficiency are all inherited in an autosomal dominant pattern. Factor V Leiden is transmitted with incomplete penetrance. The factor V Leiden mutation is present in 3% to 6% of Caucasian children, and evidence indicates that some of these heterozygous individuals are at increased risk for venous thrombosis, especially when combined with other risk factors. Nearly 200 pathogenic mutations in the PROC gene have been described for protein C deficiency. Protein S deficiency is due to mutations in the PROS1 gene. Mutations in the SERPINC1 gene are responsible for antithrombin III abnormalities.
In young patients with a spontaneous DVT (not line-associated), one main concern is an inherited thrombophilia. Adolescents with unprovoked DVTs may have an inherited condition; however, they may also have additional modifiable risk factors that predispose them to DVTs, such as the use of estrogen-containing birth control pills, smoking, driving/sitting for prolonged periods, excessive repetitive motions, and pregnancy. Autoimmune phenomena, including antiphospholipid antibody syndrome, also are increased in frequency in adolescents and should be evaluated.
May-Thurner syndrome is an anatomic variant where the left common iliac vein is compressed by the right common iliac artery, causing venous outflow tract obstruction and predisposing patients to DVTs in the left lower extremity.
Paget-Schroetter disease is a form of upper extremity DVT in the axillary or subclavian veins due to extrinsic compression or repetitive injury as the subclavian vein passes by the junction of the first rib and the clavicle. This is also called “effort thrombosis,” as athletes (particularly pitchers) and violin players are susceptible.
LMWH is the sulfated oligosaccharide heparin, derived from natural sources such as beef lung and pig intestine, that has been subjected to heparinase treatment to reduce the average molecular weight. Dosing and bioavailability are standardized, with less frequent or no monitoring of the anti–factor Xa activity, depending on clinical circumstances. LMWH still works by binding antithrombin to enhance its anti–factor IIa and anti–factor Xa activities.
Pentasaccharide (Fondaparinux) is a synthetic five-sugar agent that binds antithrombin and primarily inhibits factor Xa. It has a longer half-life and reduced monitoring advantages over heparin, but currently no antidote is available clinically.
Radulescu VC. Anticoagulation therapy in children. Semin Thrombo Hemost. 2017;43(8):877–885.
Direct thrombin inhibitors (DTIs) are anticoagulant drugs that block the enzymatic activity of thrombin, the final enzyme of the clotting cascade, which cleaves fibrinogen to fibrin. Unlike heparin, which enhances the activity of antithrombin as a mechanism of action, DTIs bind directly to thrombin. DTIs can be given parenterally or orally, such as dabigatran, and are usually cleared by the liver. Use in children is reserved for conditions in which heparin is contraindicated, such as heparin-induced thrombocytopenia (HIT).
Direct factor Xa inhibitors affect coagulation earlier in the cascade by preventing factor Xa from cleaving prothrombin to thrombin. These are oral agents and include apixaban (Eliquis) and rivaroxaban (Xarelto). In adults, they are effective in treatment and secondary prevention of thrombosis and rarely need monitoring.
von Vajna E, Alam R, So T-Y. Current clinical trials on the use of direct oral anticoagulants in the pediatric population. Cardiol Ther. 2016;5(1):19–41.
IgG is the only isotype that is transferred across the placenta. All IgG subclasses cross the placenta, and their relative concentrations in the cord serum are comparable with those of the maternal serum. Transfer of IgG can first be detected as early as 8 weeks of gestation, and levels rise steadily between 18 and 22 weeks. By 30 weeks, the serum concentrations of IgG are about 50% of those observed in neonates born at term. IgG concentrations comparable with those of the mother are achieved by 34 weeks of gestation, and values at term can be higher by about 10% compared with maternal serum levels as a result of the active transport across the placenta.
IgG levels in a full-term baby are equal or higher (5% to 10%) than maternal levels as a result of active placental transport. With an IgG half-life of 21 days, this transported maternal IgG reaches a nadir after 3 to 5 months. As the infant begins to make IgG, the level begins to rise slowly; it is 60% of the adult level at 1 year of age, and it achieves the adult level by 6 to 10 years of age.
Immunoglobulin M (IgM) concentrations are normally very low at birth, and 75% of normal adult concentrations are usually achieved by about 1 year of age.
Immunoglobulin A (IgA) is the last Ig produced and approaches 20% of adult value by 1 year; however, full adult levels are not reached until adolescence. Because delays in the production of IgA are not unusual, the diagnosis of IgA deficiency is difficult to make with certainty in a child who is younger than 2 years.
Immunoglobulin D (IgD) and immunoglobulin E (IgE), both of which are present in low concentrations in the newborn, reach 10% to 40% of adult concentrations by 1 year of age.
The fetus is in a sterilel or semisterile environment and is not exposed to foreign antigens.
The active transport of maternal IgG across the placenta may suppress fetal antibody synthesis.
Fetal and neonatal monocyte-macrophages may not process foreign antigens normally.
The thymus is the primary lymphoid organ for the production and generation of T cells bearing the α/β T-cell antigen receptor. The thymus is responsible for the central selection of the T-cell repertoire, which allows for the establishment of tolerance toward self-antigens and responsiveness to nonself (i.e., foreign) antigens.
At birth, the thymus is at two-thirds of its mature weight, and it reaches its peak mass at about 10 years of age. Subsequently, thymic size declines, but substantial function (as measured by the output of new T cells) persists into very late adulthood (70 to 80 years of age).
There is a diminished neutrophil storage in the neonate, and the cells display a reduced adhesion and migration capacity in response to chemotactic stimuli. By contrast, the efficiency for the ingestion and killing of bacteria is normal for these cells. Under suboptimal conditions, however, these effector functions may be diminished, and neutrophils from sick and stressed neonates can display a decreased microbicidal activity.
The Coulter counter, which is the most commonly used automated electronic cell counter, uses the impedance principle. A precise volume of blood passes through a narrow aperture and impedes an electrically charged field, and each “blip” is counted as a cell. The larger the RBC, the greater the electric displacement. In a separate chamber, the same volume is hemolyzed and colorimetrically analyzed to determine hemoglobin concentration.
Measured values
RBC count
Mean corpuscular volume (MCV)
Hemoglobin (Hb)
Calculated values
Mean corpuscular hemoglobin (MCH, measured in pg/cell) = (10 × [Hb / RBC])
Mean corpuscular hemoglobin concentration (MCHC, measured in g/dL) = (100 × [Hb / Hct])
Hematocrit (Hct, given as a percentage) = (RBC × [MCV / 10])
RBC distribution width (RDW) = coefficient of variation in RBC size
Microcytic: iron deficiency, thalassemias, sideroblastic anemia
Normocytic: autoimmune hemolytic anemia (AIHA), hemoglobinopathies, enzyme deficiencies, membrane disorders, anemia of chronic inflammation
Macrocytic: disorders of B 12 and folic acid metabolism, bone marrow failure
70 + (age in years). This number (in mm 3 ) approximates the lower limit of MCV in children < 12 years old, below which microcytosis is present. After the age of 12 years, the lower limit for normal MCV is 82 fL. The MCV is higher in newborn infants and varies inversely with gestational age. In a newborn infant, the lower limit of MCV is 94 fL.
Increased serum erythrocyte lactate dehydrogenase: More commonly seen in patients with hemolytic diseases, it can be greatly elevated in patients with ineffective erythropoiesis (e.g., megaloblastic anemia).
Decreased serum haptoglobin: When RBCs lyse, serum haptoglobin binds the released hemoglobin and is excreted. However, up to 2% of the population has congenitally absent haptoglobin.
Hyperbilirubinemia (indirect): This is usually increased with RBC lysis. However, it may also be elevated in patients with ineffective erythropoiesis (e.g., megaloblastic anemia). Additionally, 3% to 7% of the Caucasian population has Gilbert disease, which can cause intermittent indirect hyperbilirubinemia in the setting of physiologic stresses (fasting, illness, vigorous exercise) due to a defect in the hepatic processing of bilirubin.
Because the reticulocyte count is expressed as a percentage of total RBCs, it must be adjusted for the degree of anemia with the following formula: reticulocyte % × (patient Hct / normal Hct) = corrected reticulocyte count. For example, a very anemic 10-year-old patient with a hematocrit level of 7% (in contrast with an expected normal hematocrit of 36%) and a reticulocyte count of 5% has a corrected reticulocyte count of 1.0%: 5% × (7%/36%) = 1%. This is not appropriately elevated, as might be seen in patients with severe iron deficiency. The key concept is the appropriateness of the reticulocyte response to anemia. The corrected “retic count” should be elevated if the bone marrow is working properly and has all the right nutrients for making RBCs, including iron, folate, and vitamin B 12 .
As a rule, the reticulocyte count is elevated in conditions of shortened RBC survival (e.g., hemoglobinopathies, membrane disorders, immune hemolysis) and decreased in anemias that are characterized by impaired RBC production (e.g., iron deficiency, aplastic anemia). The reticulocyte count may be unexpectedly low in a setting of shortened RBC survival in the following conditions:
Aplastic or hypoplastic crisis is occurring at the same time, as is seen in patients with human parvovirus B19 infection.
An autoantibody in immune-mediated hemolysis reacting with antigens that are present on reticulocytes leads to increased clearance of these cells.
In patients in chronic states of hemolysis, the marrow may become unresponsive as a result of micronutrient deficiency (e.g., iron, folate) or because of a reduction in erythropoietin production, as is seen in patients with chronic renal failure.
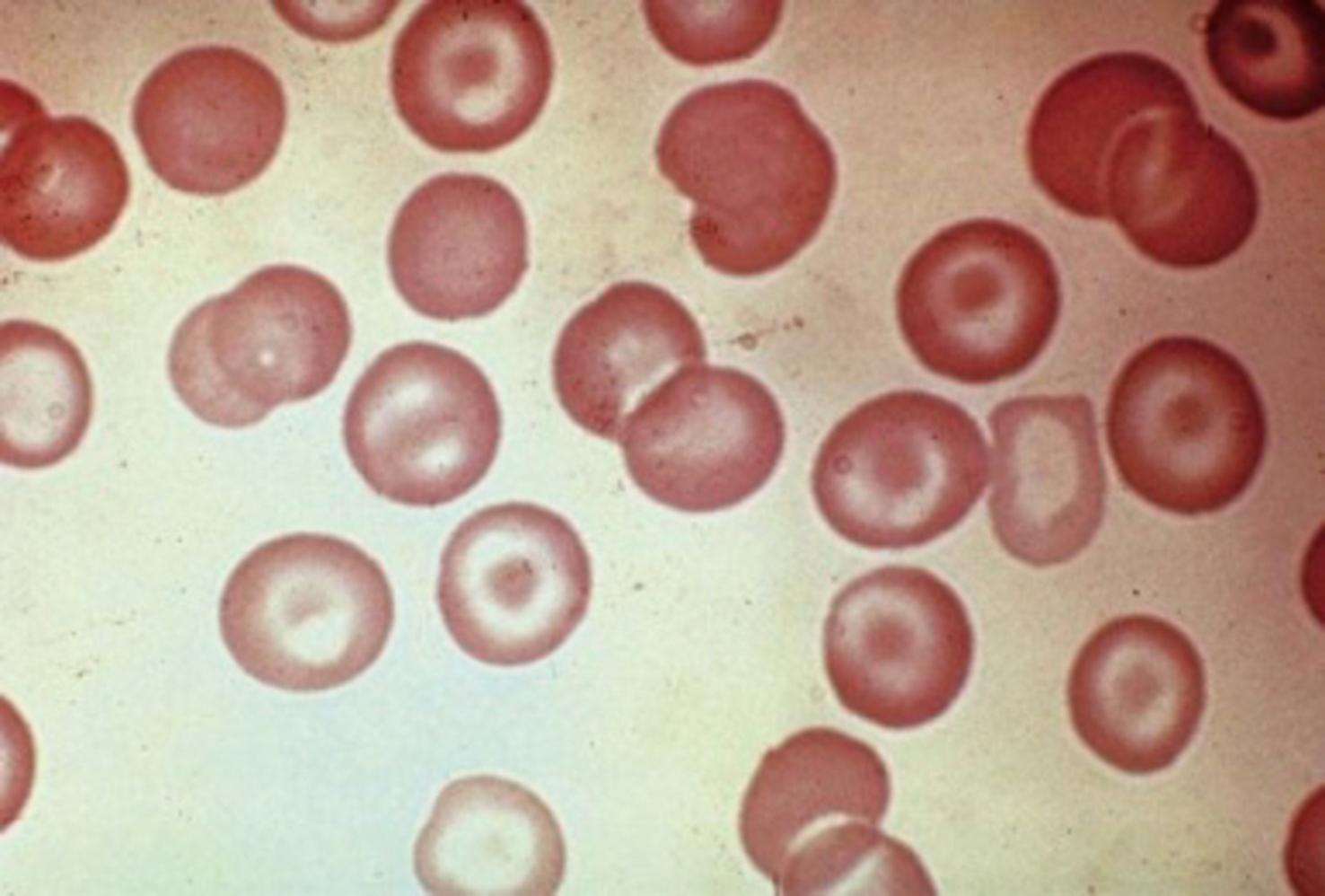
RBC targets on a peripheral smear are caused by excessive membrane relative to the amount of hemoglobin. Therefore target cells ( Fig. 9.2 ) are found when the membrane is increased (e.g., in patients with liver disease) or when the intracellular hemoglobin is diminished in smaller RBC volumes (e.g., in patients with iron deficiency or thalassemia trait). Target cells may also be found in patients with certain hemoglobinopathies (e.g., hemoglobins C and SC). In these instances, the target cells are caused by aggregation of the abnormal hemoglobin.
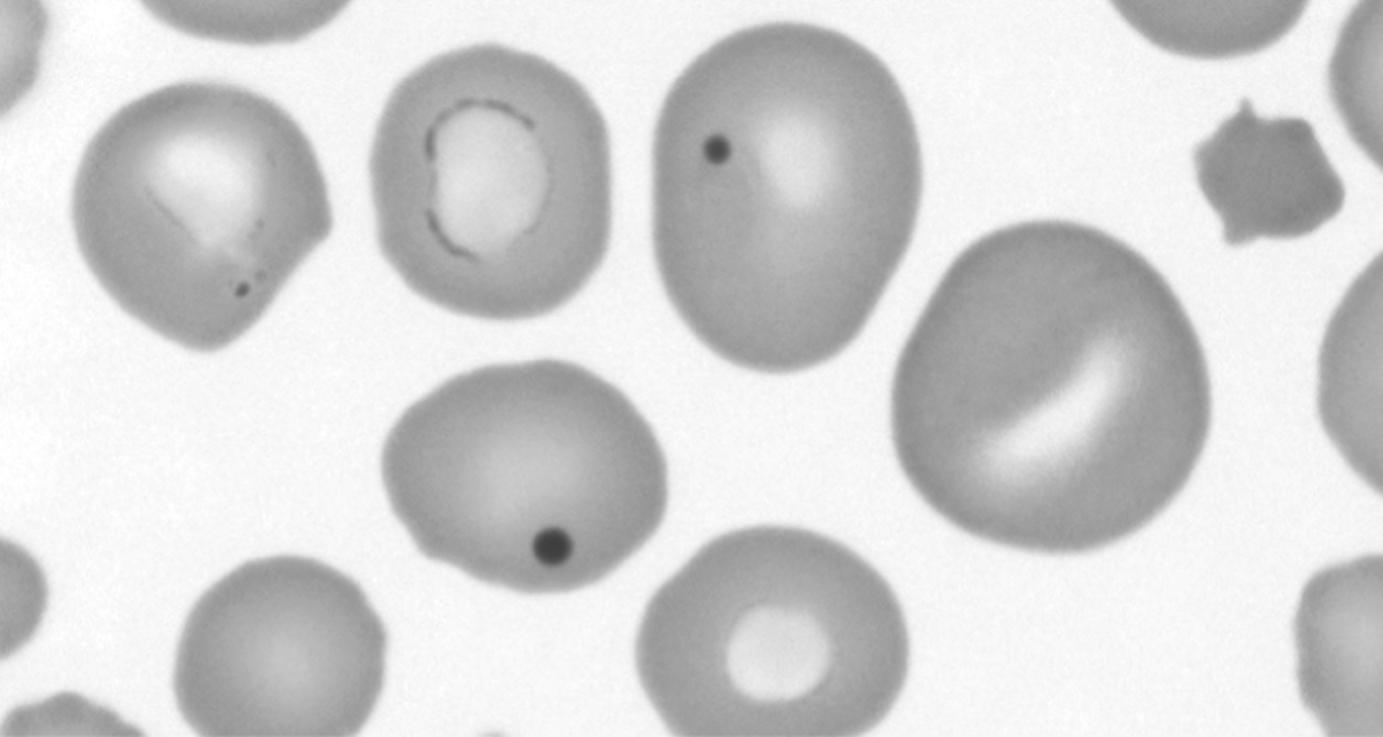
Howell-Jolly bodies are nuclear remnants that are found in the RBCs of patients with reduced or absent splenic function (e.g., sickle cell disease, heterotaxy) and in patients with megaloblastic anemias. They are occasionally present in the RBCs of premature infants. These remnants are part of the process of normal RBC maturation but are typically removed by a normal spleen. Howell-Jolly bodies are dense, dark, and perfectly round, and their characteristic appearance makes them easily distinguishable from other RBC inclusions and from platelets overlying RBCs ( Fig. 9.3 ).
Heinz bodies represent precipitated denatured hemoglobin in the RBC. Heinz bodies occur when the hemoglobin is intrinsically unstable (e.g., hemoglobin Koln) or when the enzymes that normally protect hemoglobin from oxidative denaturation are abnormal or deficient (e.g., glucose-6-phosphate-dehydrogenase [G6PD] deficiency). These inclusions are not visible with a routine Wright-Giemsa stain but can be readily seen with methyl violet or brilliant cresyl blue stains.
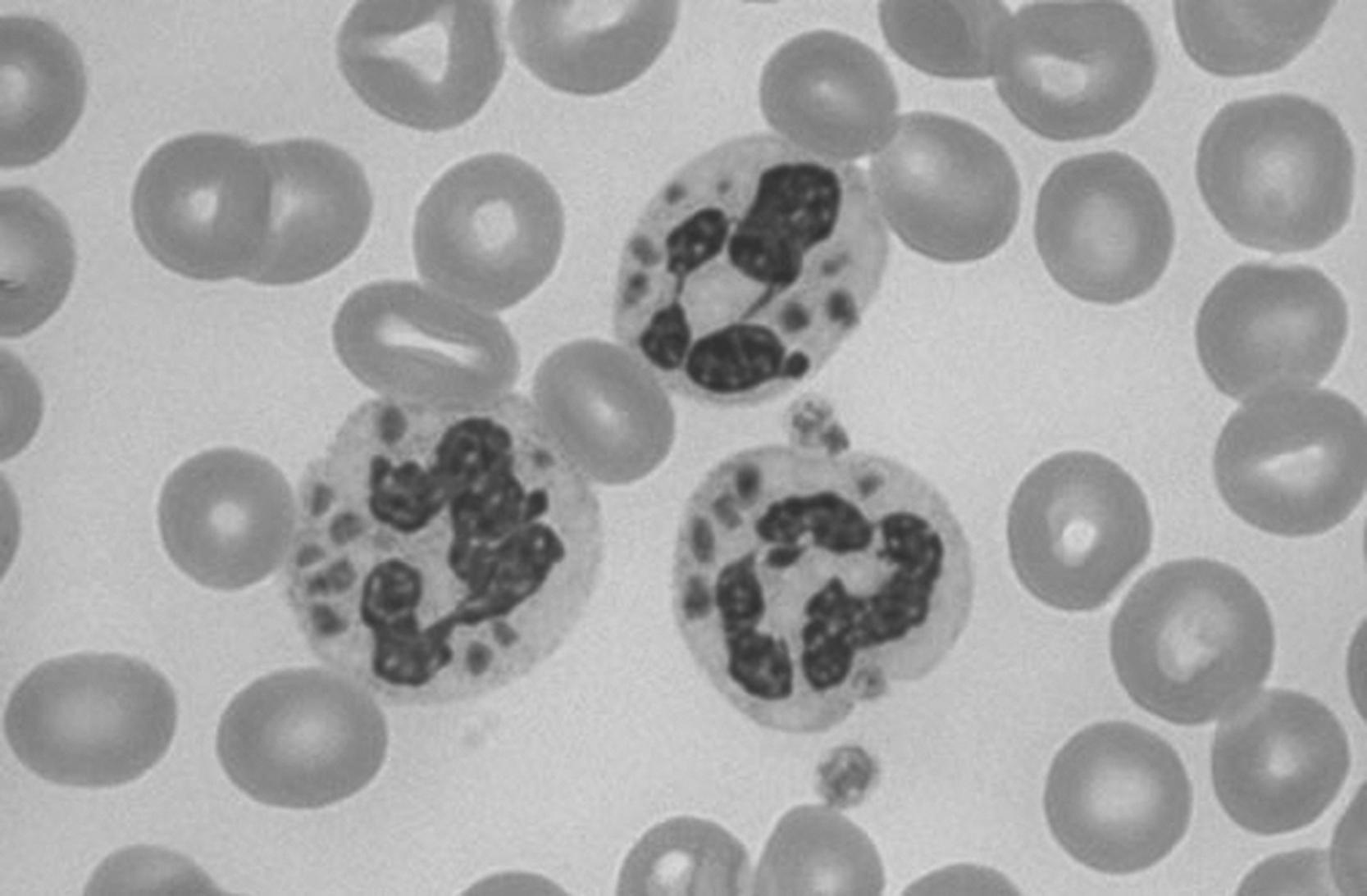
Atypical lymphocytes ( Fig. 9.4 ) are young lymphocytes (not lymphoblasts) that are characterized by an irregular plasma membrane with a large nucleus. Cytoplasm is typically basophilic. On a blood smear, where an atypical lymphocyte abuts an RBC, the shape of the lymphocyte will deform around it. Atypical lymphocytes are seen in a variety of illnesses, most commonly infectious mononucleosis.
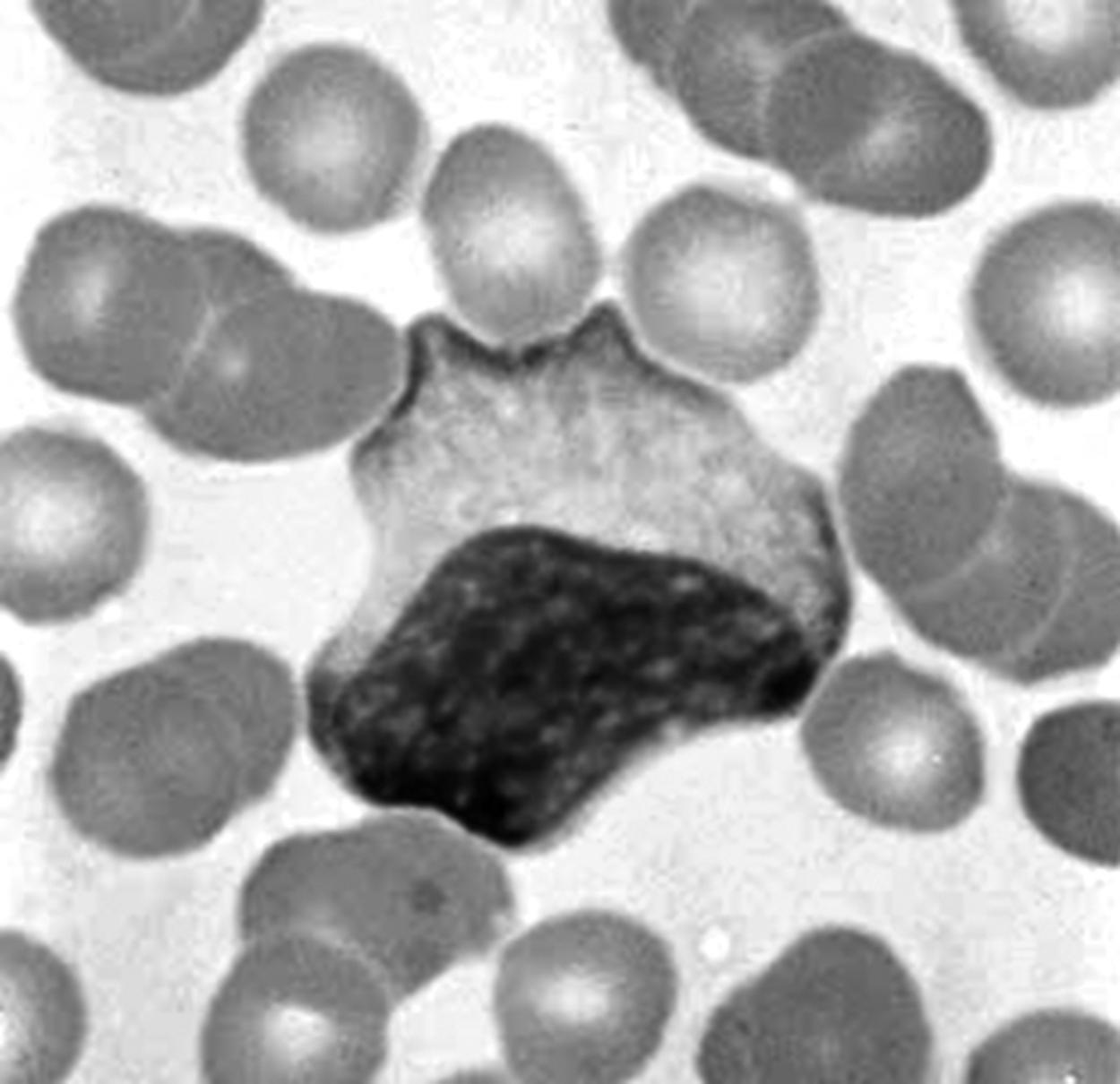
Chédiak-Higashi syndrome. This is an autosomal recessive disease with a defect in phagocytosis due to a mutation of a lysosomal trafficking regulator protein. Microtubules do not form normally, and neutrophils do not respond to chemotactic stimuli. Giant lysosomal granules, which fail to function properly, are evident in a peripheral smear. Associated features include partial albinism, peripheral neuropathy, and a susceptibility to recurrent pyogenic infections.
Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Curr Opin Hematol . 2008;15(1):22–29.
Newborn (full term): 13.0 g/dL
3 months: 9.5 g/dL
1 to 3 years: 11.0 g/dL
4 to 8 years: 11.5 g/dL
8 to 12 years: 11.5 g/dL
12 to 16 years: 12.0 g/dL
Dallman P, Siimes MA. Percentile curves for hemoglobin and red-cell volume in infancy and childhood. J Pediatr. 1979;94(1):26–31.
Physiologic anemia occurs at 8 to 12 weeks in full-term infants and at 6 to 8 weeks in premature infants . Full-term infants may exhibit hemoglobin levels as low as 9 g/dL at this time, and very premature infants may have levels as low as 7 g/dL. The mechanisms responsible for physiologic anemia are not completely understood. RBC survival time is decreased in both premature and full-term infants. Furthermore, the ability to increase endogenous erythropoietin production is somewhat blunted, although the response to exogenous erythropoietin is normal. Lastly, the hemoglobin concentration appears lower because the plasma volume increases with growth during that period of life without a similar increase in the hemoglobin concentration (i.e., there is a dilutional effect).
Chronic infection and other inflammatory states impair the release of iron from reticuloendothelial cells, thereby decreasing the amount that is available for RBC production. The lack of mobilizable iron may result from the action of proinflammatory cytokines (e.g., interleukin-1 [IL-1], tumor necrosis factor [TNF]-α). Giving additional iron under these circumstances further increases reticuloendothelial iron stores and does little to help the anemia.
Acute infection may cause anemia through a variety of mechanisms, including bone marrow suppression, shortened RBC life span, RBC fragmentation, and immune-mediated RBC destruction.
Key question: Is the anemia the cause of the splenomegaly, or is the splenomegaly the cause of the anemia?
Anemia-causing splenomegaly
Membrane disorders
Hemoglobinopathies
Enzyme abnormalities
Immune hemolytic anemia
Splenomegaly-causing anemia
Cirrhotic liver disease
Cavernous transformation of portal vessels
Storage diseases
Persistent viral infections
A leukemoid reaction usually refers to a WBC count of > 50,000/mm 3 and an accompanying shift to the left (i.e., the differential count shows an increase in immature cells). Causes include bacterial sepsis, pertussis, tuberculosis, congenital syphilis, congenital or acquired toxoplasmosis, and erythroblastosis fetalis.
Eosinophilia, which is usually defined as more than 10% eosinophils or an absolute eosinophil count of 1000/mm 3 or greater, is most commonly seen in three atopic conditions: atopic dermatitis , allergic rhinitis , and asthma .
Visceral larval migrans (toxocariasis)
Other parasitic disease (trichinosis, hookworm, ascariasis, strongyloidiasis)
Eosinophilic leukemia
Hodgkin disease
Drug hypersensitivity
Idiopathic hypereosinophilic syndrome
Methemoglobinemia should always be considered when a patient presents symptoms of cyanosis without demonstrable respiratory or cardiac disease. Methemoglobin is produced by the oxidation of ferrous iron in hemoglobin into ferric iron. Methemoglobin cannot transport oxygen. Normally, it constitutes < 2% of circulating hemoglobin. Oxidant toxins (e.g., antimalarial drugs, nitrates in food or well water) can dramatically increase the concentration. Patients with cyanosis as a result of methemoglobinemia can have normal oxygen saturation as measured by pulse oximetry because the oximeter operates by measuring only hemoglobin that is available for saturation.
In an acute situation in which levels of methemoglobin are > 30%, treatment consists of 1 to 2 mg/kg of 1% methylene blue administered intravenously over 5 minutes and repeated in 1 hour if levels have not fallen to normal. Failure to respond to therapy should raise the possibility of G6PD deficiency, which prevents the conversion of methylene blue to the metabolite that is active in the treatment of methemoglobinemia. In these cases, hyperbaric oxygen therapy or exchange transfusion may be necessary.
Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth . 2014;28(4):1043–1047.
Antioxidant defense mechanisms (e.g., soluble cytochrome b 5 and nicotinamide adenine dinucleotide hydrogen (NADH)–dependent cytochrome b 5 reductase) are 40% lower in infants than in teenagers.
An infant’s intestinal pH is relatively alkaline compared with that of older children. If nitrates are ingested (e.g., from fertilizer-contaminated well water), this higher pH more readily allows bacterial conversion of nitrate to nitrite, which is a potent oxidant.
Infants are more susceptible to various oxidant exposures: nitrate reductase from foods such as undercooked spinach, menadione (vitamin K 3 ) for the prevention of neonatal hemorrhage, over-the-counter teething preparations with benzocaine, and metoclopramide for gastroesophageal reflux.
Bunn HF. Human hemoglobins: normal and abnormal. In Nathan DG, Orkin SH, eds. Nathan and Oski’s Hematology of Infancy and Childhood. 5th ed. Philadelphia, PA: W.B. Saunders; 1998:729.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here