Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diagnosis and management of hematologic disorders in children in developing countries pose a number of problems that are not encountered in advanced Western societies. Although all the conditions that have been described in this book are seen in these populations, their clinical features may be modified to a varying degree by the coexistence of malnutrition, chronic bacterial infection, or parasitic illness. Furthermore, many of the common killers, particularly in tropical climates, produce their own complicated hematologic manifestations.
It therefore follows that the study of hematologic disease in these populations presents a particular challenge to hematologists. It is often very difficult to define the clinical and pathophysiologic features of a single hematologic disorder in this setting. Sickle cell anemia is a good example. This disorder, as described in Chapter 19 , usually produces a well-defined clinical and hematologic picture in North American or European children. However, the disease may be different in the rural populations of Africa, where its course is generally complicated by diarrheal illness, malnutrition, endemic malaria, and as yet ill-defined climatic effects on the clinical phenotype. It is very difficult to separate the environmental and genetic factors that must underlie the extraordinarily varied severity of this condition in different parts of the world.
The same problems are encountered when attempts are made to define the hematologic manifestations of disorders that are peculiar to tropical climates. For example, despite many years of intensive investigation, it is still not possible to provide a clear picture of the pathophysiologic cause of the anemia of malaria caused by Plasmodium falciparum infection. Although investigators have been unable to provide a clear picture for many reasons, the major difficulty for persons who wish to study this important problem is finding patients with malaria who do not have other diseases that may cloud the clinical picture, such as chronic bacterial infection, human immunodeficiency virus (HIV) infection, malnutrition, hemoglobinopathies, or red blood cell glucose-6-phosphate dehydrogenase (G6PD) deficiency (see Chapter 17 ).
Despite these difficulties, in recent years it has been possible to start to understand the pathogenesis of some of the hematologic manifestations of systemic disease in children in the Third World. In this chapter some of the progress that has been made in this extremely important area of childhood hematology is reviewed.
Numerous surveys have been conducted to determine the prevalence of anemia in tropical populations. However, because of differences in methodology and demographic design, it is very difficult to interpret the results and compare one study with another. Representative data from several countries, using the World Health Organization definition of anemia ( Table 38-1 ), are shown in Table 38-2 . It is clear that in many populations the prevalence of anemia in preschool children is extremely high, and in some locations almost 100% of the population is affected. Although the data shown in Table 38-2 were compiled more than 20 years ago, the situation remains serious. Although the 2010 Global Burden of Disease study investigators described a modest decrease in the global prevalence rates of anemia, they also reported that the burden of anemia, as measured in years lived with disability (YLDs), had increased from 65.8 to 68.3 million YLDs, or 8.8% of the global total of YLDs from all conditions.
| Age | Hemoglobin Concentration (g/dL) |
|---|---|
| Children, 6 mo–6 yr | 11 |
| Children, 6-14 yr | 12 |
| Adult males | 13 |
| Adult females (nonpregnant) | 12 |
| Adult females (pregnant) | 11 |
| Geographic Area | Preschool Children | Nonpregnant Women | Pregnant Women | Adult Males |
|---|---|---|---|---|
| Latin America (7 countries) | – | 17 | 24 | 4 |
| Chile | 35 | – | – | – |
| Nigeria | 63 | 46 | 52 | 36 |
| Northern India | 90 | 84 | 80 | 48 |
| Southern India | 76 | 81 | 88 | 56 |
| Burma | 3-27 | 5-15 | 82 | 1-5 |
| Philippines | 42 | 37 | 72 | 7 |
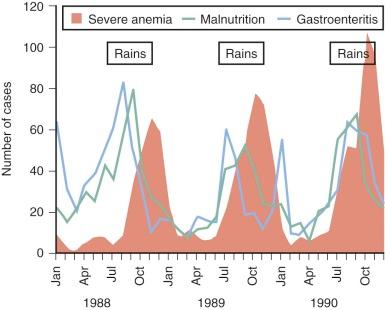
It is equally difficult to determine the relative importance of different causes of anemia in the tropics. Most surveys have concentrated on only one mechanism, such as iron or folate deficiency. However, to get a true picture of the cause of anemia in a particular population, it is necessary to obtain consecutive data over a substantial period. For example, studies in Gambia have shown that mean hemoglobin levels in children vary significantly at different times of the year; anemia is much more common in the wet season when malaria transmission is at its highest ( Fig. 38-1 ). The wet season is also the time when diarrhea and malnutrition are most common because heavy rains after many dry months have profound effects on the community, sanitation measures are disrupted, and food stores are at a low point in the annual cycle. Although these observations emphasize the multifactorial cause of anemia, it is clear that iron deficiency, which affects at least 20% of the world's population, is the major factor and that the numerous other diseases that may exacerbate anemia are often operating in the setting of low stores of iron in the body.
The body's response to infection may also reduce iron stores and iron utilization. The level of hepcidin, the body's main regulator of iron absorption, is modified by proinflammatory mediators such as tumor necrosis factor (TNF) and interleukin (IL)-6, which are elevated in a wide variety of infections. High hepcidin levels stimulated by malaria infection or bacterial infections reduce absorption of iron from the gut and also reduce incorporation of iron into red blood cells. Iron may act as a growth factor for malaria parasites, and thus a raised hepcidin level and a reduction in available iron may form part of a protective innate response to malaria infection. However, this protective response may contribute to functional iron deficiency and anemia in endemic areas.
Iron deficiency and anemia are less common in groups that have persisted as hunter-gatherers, such as the Hadza in Tanzania and the pastoralists who drink blood and eat meat—for example, the Masai in Kenya. On the other hand, absorption of nonheme iron, except from breastmilk, is comparatively restricted, and the content of iron in breastmilk is very low. Therefore iron deficiency is common in communities whose food is predominantly of vegetable origin. The three great staples in these populations are rice, wheat, and maize. Sorghum and millet are also important in some areas of Africa and Asia. Soy and similar legumes are major sources of protein in many countries. The iron content of these diets is generally low and absorption is inhibited by fiber, phytates, phosphates, and polyphenols, which are all found at high levels in these largely vegetarian diets. Loss of iron from chronic hemorrhage resulting from hookworm infection or schistosomiasis further contributes to the high incidence of iron deficiency anemia in the developing world.
The infants of mothers with iron deficiency have low iron stores, their folate status at birth reflects that of their mother, and the folate content of breastmilk is diminished by maternal deficiency and maternal malaria.
In many populations, anemia may be associated with folate deficiency. Again, the reasons for the deficiency are complex and multifactorial. Although intake of folate varies widely among different populations, depending on the way in which food is prepared and the temperature at which it is cooked, it is clear that low intake is not necessarily the result of lack of folate in the diet. Work in Africa suggests that the continuous anorexia associated with recurrent infections such as malaria or tuberculosis is the most important cause of folate deficiency in children. As will be discussed later, postinfective malabsorption is a particularly common cause of folate deficiency, especially in the Indian subcontinent. Folate deficiency may be exacerbated by erythroid hyperplasia associated with chronic malarial infection or hemoglobinopathy.
Although nutritional vitamin B 12 deficiency is uncommon, Indian infants born to mothers with sprue (discussed later) who are fed breastmilk or goat milk containing insufficient vitamin B 12 may be susceptible to megaloblastic anemia with locomotor complications during the early months of life. This syndrome, which is often fatal, appears to be complicated by a marked predisposition to infection.
Although many of the population surveys on the prevalence of anemia in tropical countries have concentrated on one particular cause and intercurrent illness has not been assessed, studies in Africa—in which attempts have been made to assess body iron stores, folate levels, and the presence of intercurrent infection—indicate that chronic recurrent malaria, without other important complicating factors, is the major cause of anemia in these populations.
A major issue is whether iron supplementation is the most effective way to prevent anemia in regions where malaria is highly endemic. For example, a study in Tanzania showed that either iron supplementation or malaria chemoprophylaxis could be used to reduce the incidence of anemia in the first year of life but that the benefits of malaria prophylaxis were outweighed by increased risk for both malaria and anemia after the prophylaxis was stopped, thus suggesting that iron supplementation is the better option. Then again, a reduction in iron levels is part of the natural host defense against infection, and a long-standing debate has ensued about whether supplementation has adverse consequences, particularly on mortality from infectious diseases.
Studies with a large sample size are needed to address this issue, and results of two such studies have recently been published. On Pemba Island, Zanzibar, where malaria is endemic, an initial study found that iron supplementation improved motor and language development, but a subsequent larger study involving 24,000 children found that deaths and hospital admissions were significantly higher in children who received iron and folate supplementation compared with children who did not receive such supplementation. Although the cause of increased deaths in the children receiving supplementation was not precisely defined, malaria appeared to play a major role. In contrast, authors of a study of 25,000 preschool children in southern Nepal found that children who received iron and folic acid had no significant difference in mortality compared with children who did not receive the supplements, although modest protective effects against diarrhea, dysentery, and acute respiratory illness were suggested.
Iannotti and colleagues reviewed 26 randomized controlled trials of preventive oral iron supplementation in children younger than 5 years. Their general conclusion was that iron-deficient children may need to be identified to ensure that iron supplementation programs are effectively targeted to improve hemoglobin levels and cognitive and motor development. Although no effect on morbidity was found in most studies, few had an adequate sample size or study design to resolve this issue. The outcome of a supplementation program may depend greatly on the pattern of endemic disease in the population.
Malaria is the most important parasitic illness that affects humans. The total burden of disease has recently been estimated to be 515 million episodes annually, and malaria is responsible for 18% of all childhood deaths in sub-Saharan Africa (equivalent to 800,000 deaths each year). With increasing drug resistance of the malarial parasite, the problems of both treatment and control are becoming more complex, and this disease remains one of the major unsolved world health problems, although the widespread use of insecticide and bed nets appears to be reducing the incidence of severe malaria in many parts of Africa.
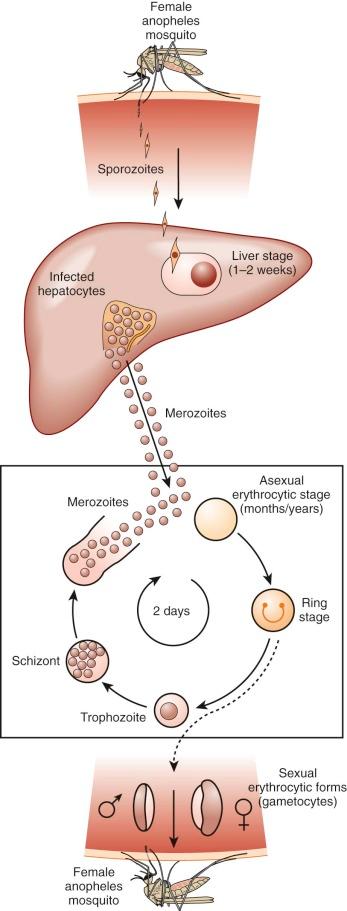
Because of its peculiar life cycle ( Fig. 38-2 ), the malarial parasite is particularly prone to cause hematologic manifestations. During a blood meal, female anopheline mosquitos inject sporozoites that disappear from the circulation after about 1 hour and enter liver parenchymal cells, where they proliferate into thousands of merozoites ( Fig. 38-3 ). The period of development in liver cells varies between species. Merozoites rupture from liver cells, pour into the bloodstream, and invade erythrocytes. Further development of the intraerythrocytic parasite follows one of two pathways: asexual differentiation or differentiation into sexual parasites called gametocytes ( Fig. 38-4 ). The latter continue their development within anopheline mosquitos. Asexual parasites develop from young ring forms through trophozoites to dividing forms called schizonts ( Fig. 38-5 ). Upon rupture of infected erythrocytes, forms called merozoites are released, invade other erythrocytes, and thus continue the erythrocyte cycle. When billions of schizonts rupture simultaneously and release cytokine-inducing toxins, they cause paroxysms of malarial fever.

When a child is infected with malaria for the first time, the result is usually an intermittent febrile illness lasting a few weeks, but complete eradication of malarial parasites from the bloodstream may take months or years. In the phase between cessation of the fever and final resolution of the infection, the child may appear well, but the destruction of red blood cells continues. From a hematologic viewpoint, the major question is how soon will the child be reinfected, because in some communities reinfection occurs almost every day, and immunity to malaria is slowly acquired and never complete. In regions of high transmission, children eventually acquire the ability to maintain a parasite density below the level that causes fever, but chronic or repeated infections cause a state of chronic anemia.
Four species of Plasmodium infect humans— P. falciparum , P. vivax , P. ovale , and P. malariae —and a fifth species, P. knowlesi , which is normally restricted to macaque monkeys, has recently been discovered in the human population in Borneo. Each has particular morphologic and biologic properties and clinical manifestations. P. falciparum is the predominant cause of clinical malaria in Africa and much of Southeast Asia, whereas P. vivax tends to predominate in Central America and the Indian subcontinent, but their distribution overlaps considerably and multiple infections are not uncommon. P. vivax is essentially absent in populations of Central and West Africa because their erythrocytes fail to express the Duffy antigen or IL-8 receptor, to which the merozoites of this species attach during invasion.
Another peculiarity of P. vivax , also shared with P. ovale , is the ability to form hypnozoites, which can remain dormant in cells for months or years. This ability results in relapsing infections that are often associated with mild chronic anemia. However, profound anemia and other grave complications are almost always seen with P. falciparum malaria, and the following sections apply mainly to this particular species.
Life-threatening complications are estimated to occur in about 1% of episodes of P. falciparum infection in African children, and it has been estimated that a child in rural Africa has a 15% lifetime risk of a malaria infection that requires hospital admission as a result of complications. Such complications include profound anemia, cerebral malaria (a syndrome of unarousable coma that is often accompanied by severe convulsions), hypoglycemia, jaundice, renal failure, pulmonary edema, and coagulation abnormalities. Important and as yet largely unexplained differences are seen in the clinical spectrum of severe P. falciparum malaria in different parts of the world. In Southeast Asia, all the aforementioned complications are commonly seen, whereas in Africa, where cerebral malaria and severe malarial anemia together account for about 1 million deaths per year and hypoglycemia is also common, the other complications of P. falciparum malaria are surprisingly rare. Malarial illness affects mostly children in Africa, whereas adults are more commonly affected in some other parts of the world; it has been suggested that much of the variation in clinical symptomatology is related to age. However, this theory does not provide a full explanation. Even among African children regional differences exist, and the impression is growing that higher rates of malarial transmission lead to a greater prevalence of anemia but, paradoxically, a low incidence of cerebral malaria.
The lethality of P. falciparum compared with other species of Plasmodium probably stems from two biologic properties. First, the parasite density that it achieves is typically a hundred times higher than that of other species before its growth is curtailed by host defense mechanisms. Second, mature intraerythrocytic forms of P. falciparum adhere to the endothelium of postcapillary venules and thus sequester in tissues. Because these properties are common to all strains of P. falciparum , the greater puzzle is why lethal complications arise in only a minority of infected persons. Some important clues have emerged recently. Parasite sequestration is mediated by a parasite-derived molecule known as PfEMP1 that is expressed on the surface of a mature infected erythrocyte and binds to a variety of endothelial adhesion molecules, including CD36, intracellular adhesion molecule 1, E-selectin, and vascular cell adhesion molecule. Whether a parasite will bind to a specific endothelial adhesion molecule is determined by its PfEMP1 type, which shows an extraordinary degree of phenotypic variability. Expression of endothelial adhesion molecules varies between organs and is regulated by cytokines released by the host in response to the infection, such as TNF. Another potentially important pathophysiologic mechanism is vascular sludging, which results from the clumping of parasitized erythrocytes, either with platelets (“autoagglutination” or “clumps”) or with unparasitized erythrocytes (“rosettes”). Both phenomena are strain specific and have been associated with the severity of malaria. As usual, the parasite uses diverse adhesive mechanisms; complement receptor–1 is a key binding target for some rosetting parasites, whereas the platelet glycoprotein CD36 and other as yet unknown proteins are used in the formation of autoagglutinates or clumps. Rosetting has been found to be reduced in persons with blood group O who are known to be protected against severe malaria.
Recognition is growing that a significant proportion of cases of severe malaria in the tropics are bacteremic. Sickle cell trait has been found to be protective against bacteremia, which has led to the discovery—using an epidemiologic method known as mendelian randomization—that P. falciparum infection is a strong predisposing factor for bacteremia in Kenyan children. An interesting relationship exists between salmonellosis, malaria, and malarial anemia. It remains open to speculation whether Salmonella infection aggravates malarial anemia, whether anemia is a marker of chronic malarial infections that favor salmonellosis, or whether some other explanation exists for this curious and striking relationship.
The pattern of hematologic changes in malaria varies considerably, depending on the type of patient. During an acute attack of P. falciparum malaria in a nonimmune person, the hematocrit starts to decrease after 1 or 2 days and continues to decrease for about 1 week after antimalarial treatment. Subsequently, a steady increase in the hematocrit usually occurs, although several weeks may pass before the hematologic picture is back to normal. The anemia, which is not usually life threatening, is characterized by both hemolysis and an ineffective marrow response, features that have also been documented in persons with acute P. vivax infection. When profound anemia occurs with acute P. falciparum malaria, it is often associated with multiorgan failure, although it can also occur as an isolated complication.
From the perspective of the health of children who live in the tropics, the most important hematologic problem is seen in a child chronically infected with P. falciparum , whose anemia is debilitating and sometimes fatal. Although this problem most commonly occurs in areas of high malarial transmission, it is a growing problem in regions of lower transmission because of the increase in resistance to antimalarial drugs, which has served to prolong the average duration of infection. Attempts to investigate the problem of chronic malarial anemia are often hampered by the coexistence of iron or folate deficiency and hemoglobinopathies. In a study in which patients with these conditions were carefully excluded, the most striking pathophysiologic findings were hemolysis, hypersplenism, and a suboptimal bone marrow response. In the sections that follow, some of the mechanisms for these components of the anemia of malaria are discussed in more detail.
Normal children living in rural Africa typically have extremely low levels of haptoglobin that increase significantly after institution of malaria prevention programs, thus providing some indication of the burden of chronic hemolysis that malaria causes in many tropical communities. The mechanisms of red blood cell destruction in persons with P. falciparum malaria are complex and not fully understood. Clearly, erythrocytes are destroyed when schizonts rupture and release their progeny, and depending on the state of immunity, a proportion of the parasitized erythrocytes are destroyed by the host before schizont rupture can take place. Both mechanisms can have devastating consequences if the patient is hyperparasitemic (sometimes more than 50% of erythrocytes are infected), but such patients are rare. In most infections, fewer than 1% of erythrocytes are infected, a loss that is important in the context of chronic infection but would not account for the severity of anemia that is commonly observed. Both mathematical modeling and clinical observation suggest that 10 times as many uninfected erythrocytes are removed from the circulation for each infected erythrocyte. Indeed, cross-transfusion experiments clearly indicate that erythrocyte survival is shortened both in the acute phase of malaria and during convalescence.
Some degree of splenomegaly is a normal feature of malarial infection, and the prevalence of splenomegaly in regions of malarial transmission is used as a major indicator of the level of malarial endemicity. The importance of the spleen in host defense against malaria has been demonstrated in experimental systems, and persons whose spleens have been surgically removed are thought to be more susceptible to severe infection (discussed later). The phenomenon of parasitic sequestration, discussed earlier, is thought to have evolved primarily as an immune evasion strategy whereby the mature parasite can avoid passing through the spleen.
Several studies have attempted to define the pathophysiologic changes that occur in the spleen during the acute phase of malaria. In animal models, malaria is accompanied by increased intravascular clearance of infected or rigid heat-treated cells by the spleen, alterations in the splenic microcirculation, and recruitment of myelomonocytes to the spleen and liver. In studies of human malaria it has been found that increased splenic clearance of heated red blood cells occurs during acute attacks. Reduced deformability of uninfected red blood cells is observed in P. falciparum malaria and is a significant predictor of the severity of anemia, consistent with the notion that these cells are being removed by the spleen (discussed later). It has also been found that immunoglobulin G (IgG)-sensitized red blood cells are rapidly removed from the circulation by the spleen and that unusually rapid clearance persists well into the convalescent phase. Undoubtedly, the activity and number of macrophages are increased during human malarial infection, which may therefore contribute to the increased removal of uninfected cells.
In rodent malaria, a model of chronic anemia has been developed in which Plasmodium berghei –infected semiimmune mice show a fivefold to tenfold increase in the clearance of uninfected erythrocytes, although the extent of parasitemia remains below 1%. In this study, clearance of uninfected red blood cells was delayed in mice depleted of macrophages, thus supporting the view that removal of uninfected erythrocytes is an important factor in persons with anemia.
All the available evidence therefore points to increased reticuloendothelial clearance in P. falciparum malaria that persists long after recovery. These changes are presumably a host defense mechanism to maximize the clearance of parasitized erythrocytes. Clinical studies have also uncovered a curious phenomenon whereby some erythrocytes appear to be returned to the circulation after having had their parasites removed, but killing parasites usually means destroying erythrocytes, and it appears that the survival of uninfected red blood cells is reduced in the process.
These observations in patients are consistent with findings at the cellular level. Active erythrophagocytosis is a conspicuous feature within the bone marrow of persons with P. vivax and P. falciparum malaria, and it is highly probable that it also occurs within the spleen. Children with acute P. falciparum malaria have high circulating levels of interferon-γ and TNF, a synergistic combination of cytokines that activate macrophages. Transgenic mice that constitutively overexpress human TNF are anemic and show enhanced clearance of autologous erythrocytes, which is presumed (though not proved) to be due to erythrophagocytosis. When infected with Plasmodium yoelii or P. berghei , transgenic mice suppress parasite density much more effectively than their littermates do. This finding supports the notion that with relatively nonspecific effector mechanisms such as erythrophagocytosis within the spleen, anemia is part of the price that the host has to pay for protection against overwhelming parasitemia.
A peculiar example of malaria-associated hemolysis is blackwater fever. The classic form of this syndrome, often reported in colonial times among Europeans living in Africa, was characterized by fever and massive intravascular hemolysis associated with low or no parasitemia; it led to renal failure and was associated with high mortality. It was suspected that intermittent quinine ingestion might have led to a drug-induced immune hemolysis, although this mechanism was never proved and recent reports have implicated newer antimalarial drugs such as halofantrine and mefloquine/artemisinin combinations. Classic blackwater fever is now much less common, but it is important to remember that massive intravascular hemolysis with hemoglobinuria remains an important complication of P. falciparum malaria in Southeast Asia in both children and adults. Blackwater fever is sometimes associated with high parasitemia or destruction of G6PD-deficient red blood cells by oxidant antimalarial drugs. A survey of cases in Vietnam found considerable overlap of quinine ingestion, G6PD deficiency, and concurrent malaria, suggesting that these different factors may interact, but they certainly do not all have to be present for blackwater fever to occur. It is conceivable that hemolysis is caused by the rupture of a large number of sequestered parasites, but the possibility of another hemolytic process has yet to be ruled out.
The increased clearance of uninfected erythrocytes is due not only to the activation of splenic macrophages but also to extrinsic and intrinsic factors that enhance their recognition and phagocytosis. Uninfected erythrocytes have reduced deformability, which leads to enhanced clearance in the spleen. The mechanism responsible for the loss of deformability is not completely understood, however. Increased oxidation of membranes in uninfected erythrocytes has been demonstrated in children with severe P. falciparum malaria, and the ongoing inflammatory insults associated with acute malaria (proinflammatory cytokines) or the direct effects of parasite products have been shown to cause loss of red blood cell deformability. Intriguingly, a severe reduction in red blood cell deformability measured on admission is also a strong predictor not just for anemia but also for mortality, both in adults and children with severe malaria.
It is also possible that at least part of the hemolysis in P. falciparum malaria might have an immune basis. A positive direct Coombs antiglobulin test (DAT) is seen in some patients with malaria. As with all studies of malaria, considerable differences in the incidence of positive DAT results in malarial infection seem to exist in different populations and at different ages. For example, in Thai children or adults during their first attack or with subsequent attacks in persons who live in areas where a degree of immunity has not developed, DAT results are invariably negative. Furthermore, studies using labeled immunoglobulin have shown that the number of antibodies bound to the red blood cells of patients with malaria of this type is no different from that in uninfected control subjects of the same age and in the same location.
The position is different in African children who have experienced repeated attacks of malaria. Positive DAT results have been found in up to 50% of these children, and the incidence is significantly higher in children with active malarial infection than in those who are not infected. The phenomenon is age related and is more common in young children. DAT results may remain positive for several weeks after an acute infection. Use of specific antisera has revealed that the most common type of red blood cell sensitization occurs with C3; other specificities include IgG alone, IgG plus C3, IgG plus C3 and C4, and various other combinations of IgG with complement components. The IgG that can be eluted from these cells has specific activity against P. falciparum schizont antigen, which suggests that the erythrocyte coating results from passive attachment of circulating complement-fixing malarial antigen-antibody complexes. It is therefore likely that the development of positive DAT results is part of the immune response to P. falciparum .
Other studies have suggested that the P. falciparum ring surface protein 2 (RSP-2) may be one component of the immunoglobulin-antigen complexes deposited on uninfected erythrocytes. This protein is expressed on infected erythrocytes shortly after merozoite invasion and may mediate some adhesion of infected erythrocytes to endothelial cells. RSP-2 is also deposited on uninfected erythrocytes and forms immune complexes that contribute to the phagocytosis of uninfected erythrocytes. Limited clinical studies have shown that high levels of anti–RSP-2 antibodies are found in the sera of immune adults and children with severe anemia. This antigen is also present on the surface of erythroblasts in the bone marrow of patients infected with P. falciparum , thus indicating that clearance or damage to developing erythroid cells by RSP-2 and anti–RSP-2 could contribute to the development of anemia.
However, it is far from clear whether the presence of positive DAT results indicates that immune destruction of red blood cells occurs in African children with malaria. Several studies have shown that no correlation exists between the degree of anemia and positive DAT results, and other measured parameters of hemolysis have not correlated with coating of red blood cells. Nonetheless, immune destruction may occasionally be of importance. A small number of patients with severe anemia and positive DAT results have been described who had active C3 components on their red blood cells. In these patients, striking monocyte erythrophagocytosis of nonparasitized erythrocytes was seen.
In some patients with P. falciparum infection, IgM antibodies have been reported to develop against triosephosphate isomerase, and disappearance of the antibodies coincides with disappearance of the hemolysis. Because these antibodies can induce erythrocyte lysis and complement activation in vitro, it has been postulated that they may contribute to a hemolytic process in vivo.
In short, little evidence exists for immune destruction of red blood cells in P. falciparum malarial infections in nonimmune adults. Although a varying proportion of children with chronic malaria in Africa have positive DAT results, only occasionally is evidence of genuine immune destruction of red blood cells seen. Of course, it is possible that a sensitized red blood cell population is very rapidly destroyed and that subsequent serologic studies have missed this event. However, the persistence of shortened red blood cell survival of nonparasitized cells in the absence of any consistent serologic abnormalities suggests that another factor must be involved in the shortened red blood cell survival in persons with malaria. This factor appears to be nonspecific “overactivity” of the monocyte/macrophage populations of the spleen and liver, as previously discussed.
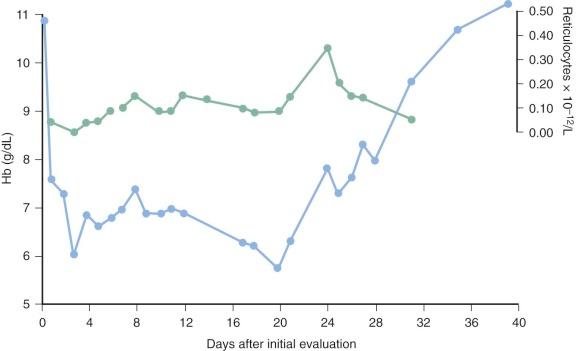
In addition to the hemolytic components of the anemia of P. falciparum malaria, an inappropriate marrow response undoubtedly occurs. For many years it has been noted that the reticulocytosis in response to the decrease in hematocrit is often inappropriately low and delayed ( Fig. 38-6 ). It remains unclear whether defective erythropoietin production is a significant cause, with some studies suggesting an appropriate rise or even enhanced response and other investigators observing a blunted response, but there is no question that significant dysfunction of marrow takes place and appears to have a complex multifactorial basis.
It is now clear that disturbances in iron metabolism are mediated by hepcidin. Hepcidin is regulated by proinflammatory mediators such as TNF and IL-6, which are elevated in both murine infections and in patients presenting with severe falciparum malaria, although other parasite-derived and host factors may be involved in stimulating hepcidin production. Hepcidin levels decrease in the most severely ill children because hypoxia inhibits hepcidin production. High hepcidin levels stimulated by blood stage malaria infection are associated with inhibition of liver stage malaria infection in mice and reduced incorporation of iron into red blood cells in humans. Evidence from murine malaria suggests that a low level of serum iron caused by an elevated level of hepcidin reduces parasite growth and progression to severe disease. These experimental and clinical findings are consistent with a role for iron as a growth factor for malaria parasites, as well as a role for an increased level of hepcidin and a reduction in available iron as a protective innate response to malaria infection.
Some of the features of iron metabolism and bone marrow structure and function in persons with acute malaria resemble those of other acute infections; thus the serum iron level rapidly decreases and iron appears to be sequestrated into the storage compartments of the marrow. Serum ferritin levels are extremely high during the acute phase, although studies of isoferritins suggest a complex source. In many instances it appears that much of the ferritin is released as a consequence of liver damage.
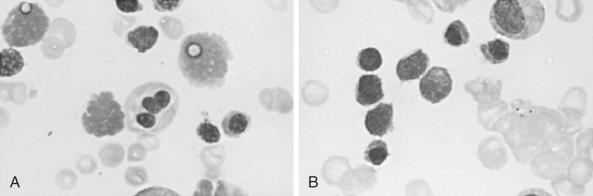
During acute malarial infections, marked dyserythropoietic changes in the bone marrow may occur. Although changes are most marked in African children with chronic relapsing malaria, they have also been observed in nonimmune Thai people and in travelers returning to the United Kingdom. These morphologic abnormalities, which have been studied by both light and electron microscopy, consist of erythroblast multinuclearity, karyorrhexis, incomplete and unequal amitotic nuclear divisions, and cytoplasmic bridging ( Fig. 38-7 ). Upon electron microscopic examination, binucleate or multinucleate erythroblasts, bizarre myelination with loss of parts of the nuclear membrane, and widening of the space between the two layers of the nuclear membrane have been observed. Some degree of iron loading of the mitochondria and a reduction in electron density of the cytoplasmic matrix appear to occur, together with a paucity of ribosomes. In addition, widespread sequestration of erythrocytes in the sinuses of the bone marrow occurs in persons with an acute infection.
With use of [ H]thymidine autoradiography and Feulgen microspectrophotometry, it is clear that a significant abnormality of red blood cell proliferation in the bone marrow occurs in persons with acute malaria. Changes include an increased proportion of red blood cell precursors in the G 2 phase and arrest in progress of cells in the S phase. These findings are nonspecific and have been observed in other conditions associated with ineffective erythropoiesis.
In addition to these dyserythropoietic changes, erythrophagocytosis is particularly common in the bone marrow in persons with P. falciparum malaria. This phenomenon is not restricted to the marrow and may be seen in the spleen and other organs and, as mentioned earlier, may play a role in the hemolytic component of the disease.
Although the mechanism of these dyserythropoietic changes is unknown, it is possible that they are an exaggerated example of the bone marrow suppression that occurs in other situations of chronic infection. An important factor may be the high levels of TNF production that occur during malarial infection because this cytokine has been strongly implicated in the anemia of chronic infection and severe malarial anemia has been associated with certain TNF promoter polymorphisms. TNF suppresses the proliferation of erythroid progenitor cells in human marrow culture, although the effect declines as the cells differentiate. On the other hand, TNF stimulates fibroblasts to secrete growth factors for colony-forming unit–granulocyte-erythrocyte-monocyte-macrophage and burst-forming unit–erythrocyte. In nude mice implanted with transfected Chinese hamster ovary cells that can constitutively express the human TNF gene, erythropoiesis is preferentially suppressed, with a marked reduction of colony-forming unit–erythrocyte and burst-forming unit–erythrocyte in the marrow and spleen. Chronic malaria has specific features that might augment these effects because huge numbers of pigment particles are ingested by the resident macrophages of the spleen and marrow, thereby providing a sustained stimulus for TNF production at the site of erythropoiesis. Furthermore, experimental findings in mice are consistent with a role for TNF in the dyserythropoietic changes of malaria.
Other cytokines have also been implicated. IL-10 is an antiinflammatory cytokine that inhibits TNF, and two clinical studies in African children with severe malarial anemia have found a low ratio of IL-10 to TNF in plasma, thus leading the investigators to propose that defective IL-10 production may pave the way to marrow suppression by TNF. IL-12 protects against severe anemia in experimental murine malaria, which might reflect both its antiparasitic actions and effects on erythropoiesis.
Several studies have suggested that a parasite by-product of hemoglobin digestion, hemozoin, may have an indirect or direct role in impaired erythroid development. Hemozoin stimulates the secretion of biologically active endoperoxides from monocytes, such as 15( S )-hydroxyeicosatetraenoic acid and hydroxynonenal, via oxidation of membrane lipids, which may affect erythroid growth.
Hemozoin and TNF-α also have additive effects on erythropoiesis in vitro, and in a clinical study, hemozoin-containing macrophages and plasma hemozoin were associated with anemia and reticulocyte suppression. Moreover, bone marrow sections from children who died with severe malaria show a significant association between the quantity of hemozoin (located in erythroid precursors and macrophages) and the proportion of erythroid cells that were abnormal. These findings are consistent with a direct inhibitory effect of hemozoin on erythropoiesis.
Modest thrombocytopenia is not uncommon during acute P. falciparum attacks, and, occasionally, the platelet count decreases to as low as 10,000 to 20,000/mL. The mechanisms have not been determined but probably involve platelet activation and adhesion of infected erythrocytes to platelets, and experimental studies in mice suggest that platelets sequester in venules during malarial infection. Adherence of platelets to epithelium appears to be mediated through a specific receptor-ligand interaction involving lymphocyte function antigen–1. Evidence indicating reduced thrombopoiesis during acute infection also exists.
In some patients with severe P. falciparum malaria, a severe bleeding diathesis may develop during the acute phase of the illness. Although it has been suggested that the diathesis results from disseminated intravascular coagulation, studies in Thailand have not substantiated this supposition. Rather, the bleeding appears to reflect gross thrombocytopenia together with liver damage. However, it is becoming apparent that approximately 10% of African children with severe malaria also have bacteremia, and thus when disseminated intravascular coagulation occurs, the cause may be complex.
In endemic regions, it is extremely common for women to have malaria while they are pregnant. The placenta provides a favorable environment for parasite replication, and specific pathways of adhesion of infected erythrocytes to the surface of syncytiotrophoblasts have now been unraveled. A specific variant antigen, PfEMP1, which is expressed on the surface of infected erythrocytes (varCSA-2), mediates adhesion of infected erythrocytes to chondroitin sulfate. Primigravidae are particularly susceptible to malarial infection and may become systemically unwell, but immunity to this variant antigen modulates the duration and severity of infection in subsequent pregnancies. These observations suggest that varCSA-2 may be an excellent candidate antigen for a pregnancy-specific malaria vaccine. Apart from the effects on the mother, malaria is an important cause of low birth weight and increased perinatal mortality. Studies of cord hemoglobin levels indicate that it is also a significant cause of fetal anemia. Because the severity of the fetal anemia is out of proportion to the degree of maternal anemia, it has been suggested that intrauterine hemolysis may be involved. Malaria during pregnancy is a preventable illness, although it has become more difficult to control with the rise of resistance to antimalarial drugs. Studies in different parts of Africa have found placental malaria to be an important risk factor for anemia in the first 6 months of life.
Given the high prevalence of malaria in pregnancy and the strong association of placental malaria with infantile anemia, it is surprising how few infants have overt symptoms of congenital malaria in the tropics. On the rare occasions when it does occur, it can result in a perplexing hematologic disorder. The manifestations of malaria may not be seen until several months after birth, presumably because of passive immunization from the mother and possibly the fact that P. falciparum tends to grow less effectively in cells that contain relatively large amounts of fetal hemoglobin. The disease is characterized by a febrile illness associated with anemia that may often be profound. As in conventionally acquired malaria, the pathophysiologic course of the anemia is a complex combination of hemolysis and marrow suppression. Thus it is important to obtain a careful travel history from the parents of any infant with unexplained hemolysis. This condition is commonly misdiagnosed because it is not considered as a possibility.
Although it is generally believed that malaria may be associated with particularly severe infections in persons who have had a splenectomy, no clear evidence was found in a review of this important question. Until this question is resolved, it is wise to advise visitors to the tropics who have had their spleens removed and splenectomized patients who live in regions where malaria is endemic to be particularly careful about continuing to maintain malaria prophylaxis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here