Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ability of the left ventricle to function as one of the body’s two main pumping chambers depends on how efficiently it ejects and subsequently fills with oxygenated blood. This implicit duality indicates that heart failure may occur either when the left ventricle is unable to eject blood at a rate sufficient to meet tissue metabolic requirements or when it cannot fill completely without elevated pressures. Simply, heart failure is defined as a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood. Tissue hypoperfusion results in clinical symptoms such as fatigue, dyspnea, weakness, edema, and weight gain. Structural abnormalities of the pericardium, myocardium, endocardium, heart valves, or great vessels can result in heart failure. Functional impairment of the myocardium, during either systole or diastole, is more commonly associated with this clinical syndrome. Heart failure with reduced ejection fraction (HFrEF) is classified as an ejection fraction of 40% or less, whereas heart failure with preserved ejection fraction (HFpEF; previously termed diastolic heart failure) is diagnosed in patients with an ejection fraction of 50% or more. A patient with clinical symptoms with an ejection fraction between 40% and 50% is labeled as having borderline HFpEF. The distinction between these two types of heart failure based solely on ejection fraction is clearly somewhat artificial, as HFrEF and HFpEF most likely represent different phenotypes rather than unique clinical entities. Diastolic dysfunction is present in both HFrEF and HFpEF, and myocardial contractility is abnormal in HFpEF when quantified using more sensitive indices (e.g., left ventricular [LV] end-systolic pressure volume relations, tissue Doppler imaging) despite relatively normal ejection fraction. Indeed, the patterns of LV dilation and remodeling (e.g., concentric vs eccentric hypertrophy), and not ejection fraction per se, are major distinguishing features between HFrEF and HFpEF, along with their differential responses to medical treatment. Nevertheless, the classification system remains useful because ejection fraction is easily measured using echocardiography and because the risk factors, responsible mechanisms, treatment, and outcomes of HFrEF and HFpEF have been established based on this definition. Patients suffering from HFpEF or HFrEF present for surgery on a regular basis and anesthesiologists must be familiar with clinical characteristics and treatment of these disorders to provide successful perioperative care for these often-complex patients.
Heart failure is an emerging worldwide epidemic. Current estimates suggest that more than 8 million patients in the United States alone will be treated for the condition by 2030 at an annual cost of $53 billion. Approximately half of the patients who present with heart failure have an LV ejection fraction that is relatively normal (>50%). The proportion of patients with HFpEF is steadily increasing compared with those in whom reduced ejection fraction (<40%) is present (HFrEF) in large part because the prevalence of conditions with which the former is more closely associated, including poorly controlled essential hypertension, atrial fibrillation, diabetes mellitus, obesity, metabolic syndrome, chronic obstructive pulmonary disease (COPD), renal insufficiency, anemia, and generalized deconditioning, continues to rise as the population ages ( Table 10.1 ). In contrast, patients with HFrEF are more likely to have modifiable risk factors (e.g., tobacco abuse, hyperlipidemia) and have a higher prevalence of myocardial ischemia and myocardial infarction (MI), previous percutaneous coronary intervention, coronary artery bypass graft surgery, and peripheral vascular disease compared with those with HFpEF. Nevertheless, as many as 70% of patients with HFpEF also have evidence of coronary artery disease.
| HFrEF | HFpEF | |
|---|---|---|
| Clinical Features | ||
| Relative age | Younger | Older |
| Sex | Men > women | Women > men |
| Medical comorbidities | CAD CABG/PCI Tobacco abuse HLD PVD |
HTN Atrial fibrillation DM Obesity/metabolic syndrome COPD CRF Anemia Deconditioning |
| Physical findings | S 3 gallop | PND Peripheral edema JVD Elevated PCOP |
| Pathophysiology | ||
| Myocardial contractility | Markedly depressed | Depressed |
| LV ESPVR | Reduced E es | Elevated E es (systolic stiffening) |
| LV end-diastolic pressure | Increased | Increased |
| LV relaxation | Delayed | Delayed |
| Passive myocardial stiffness | Normal to increased | Markedly increased |
| LV size | Enlarged | Normal |
| LV geometry | Eccentric hypertrophy | Concentric hypertrophy |
| LV mass | Increased | Increased |
| Cardiac myocyte dimension | Increased length | Increased diameter |
| Extracellular matrix | Less interstitial fibrosis | Greater interstitial fibrosis |
| BNP concentration | Markedly elevated | Modestly increased |
| LV afterload | Increased secondary to neurohormonal activation | Prominent arterial stiffening |
| LV-arterial coupling | Variable E a | Elevated E a |
| LA systolic and diastolic dysfunction | Less common | More common |
| PAH/RV dysfunction | Common | Common |
| Chronotropic incompetence | Less common | More common |
| Echocardiography | ||
| LV ejection fraction | <40% | >50% |
| Tissue Doppler velocity | Reduced | Reduced |
| LV diastolic dysfunction | Secondary to LV systolic dysfunction | Pathognomonic |
| LV dimensions | Enlarged | Normal |
| LV wall thickness | Variable | Markedly increased |
| Positive Response to Treatment | ||
| Diuretic | Yes | Yes |
| β blocker | Yes | No |
| RAAS inhibitor | Yes | No |
| Digoxin | Yes | No |
| PDE V inhibitor | No | No |
| Nitrate | Yes | No |
| Statin | Yes | Questionable |
| Exercise training | Yes | Yes |
| Weight loss | Yes | Yes |
| Control of medical comorbidities | Yes | Yes |
A recent survey indicated that 52% of heart failure cases were classified as HFpEF, whereas 33% were HFrEF and 16% had modestly depressed LV systolic function (ejection fraction between 40% and 49%; “borderline”). Women are more likely to be affected by HFpEF than men (as opposed to the male predominance of HFrEF), and this gender disparity widens with advancing age. For example, the incidence of HFpEF in women older than 80 years of age was reported to be 8% to 10% compared with 4% to 6% in men. The signs, symptoms, degree of functional impairment, morbidity, and mortality of both forms of heart failure are very similar, although death from a primary cardiovascular cause is more common in patients with HFrEF, whereas hospital admission and readmission for noncardiac causes occur more often in those with HFpEF.
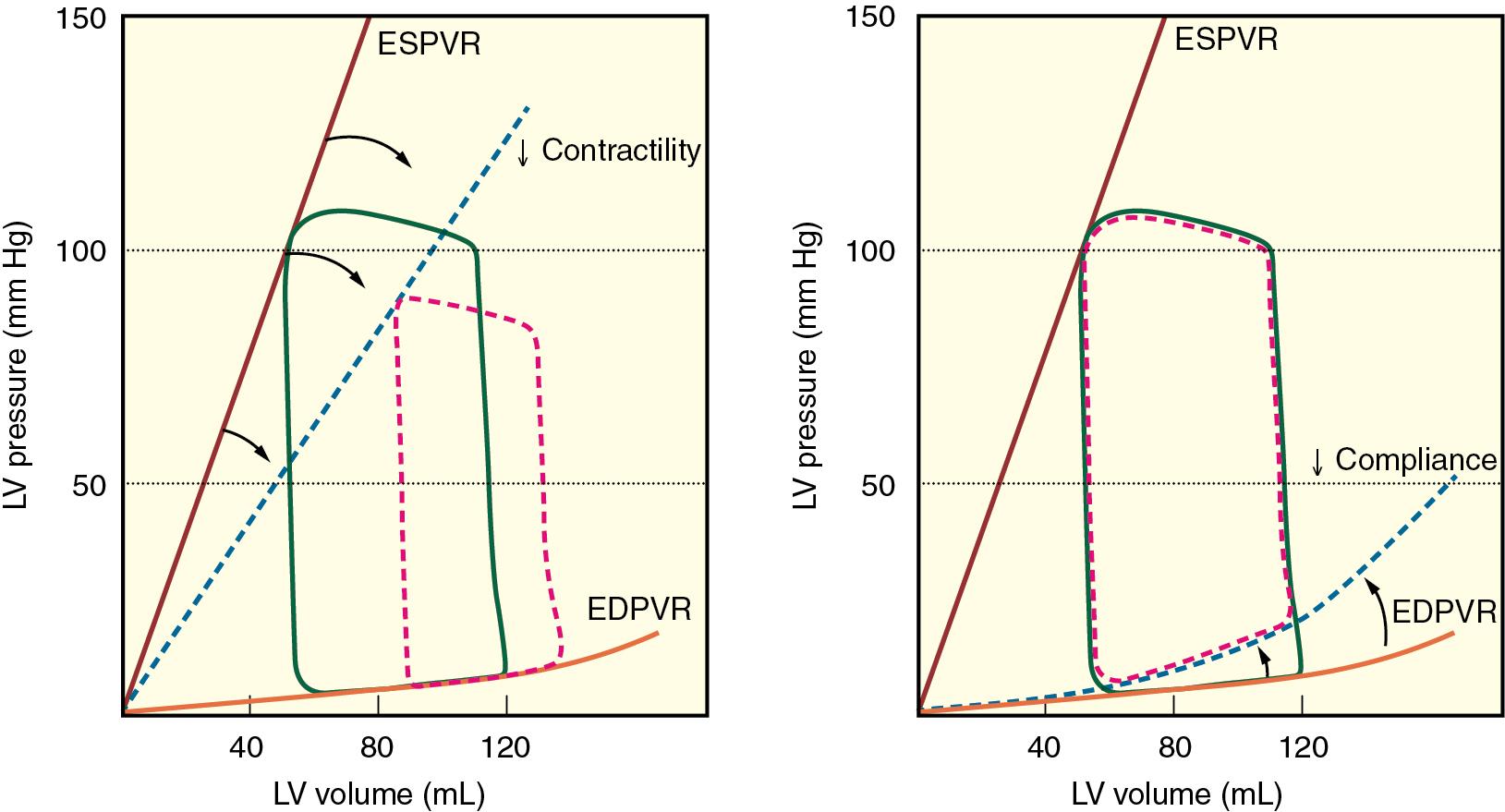
Whereas fundamental abnormalities of contractile function characterize HFrEF, left ventricular diastolic dysfunction (LVDD) is a primary determinant of HFpEF. LV diastole encompasses a complicated sequence of temporally interrelated heterogeneous events ( Fig. 10.1 ; Tables 10.2 and 10.3 ). Pulmonary venous blood flow, left atrial (LA) function, mitral valve dynamics, pericardial restraint, and the active (relaxation) and passive elastic (compliance) properties of the left ventricle during diastole determine its ability to properly fill. LV diastolic function is considered normal when these factors combine to provide an LV end-diastolic volume (i.e., preload) that provides sufficient cardiac output to satisfy cellular metabolism without elevations in pulmonary venous and mean LA pressures (normal values of ∼10 mm Hg for each). It is essential to recognize that no single index of LV diastolic function completely characterizes this period of the cardiac cycle or selectively identifies patients at highest risk of developing heart failure resulting from abnormal relaxation, filling, and compliance. The vast majority of indices of LVDD are also highly dependent on heart rate, loading conditions, and myocardial contractility, and as a result abnormalities in diastolic function require thoughtful interpretation. Despite these inherent limitations, invasively derived LV end-diastolic pressure-volume relations uniformly demonstrate that higher LV filling pressures are required to achieve normal end-diastole volume in patients with HFpEF ( Fig. 10.2 ). The steeper rise of the end-diastole pressure-volume curve is indicative of delayed LV relaxation (such as is commonly observed in pressure-overload hypertrophy resulting from long-standing essential hypertension) and an increase in passive myocardial stiffness resulting in a reduction in LV compliance that restricts LV filling and precipitates LA hypertension, LA systolic and diastolic dysfunction, pulmonary venous congestion, and exercise intolerance.
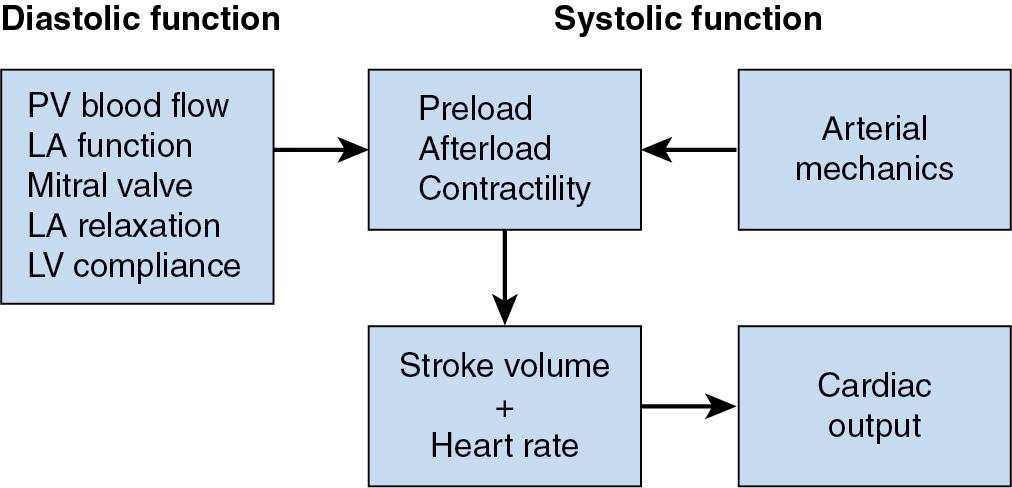
| Heart rate and rhythm LV systolic function Wall thickness Chamber geometry Duration, rate, and extent of LV relaxation LV elastic recoil and untwisting Magnitude of diastolic suction LA-LV pressure gradient Passive elastic properties of LV myocardium Viscoelastic effects (rapid LV filling and LA systole) LA structure and function Mitral valve structure and function Pulmonary venous blood flow Pericardial restraint RV loading conditions and function Ventricular interdependence Coronary blood flow and vascular engorgement Extrinsic mediastinal compression |
| Age >60 years Acute myocardial ischemia (supply or demand) Myocardial stunning, hibernation, or infarction Ventricular remodeling after infarction Pressure-overload hypertrophy (e.g., aortic valve stenosis, essential hypertension) Volume-overload hypertrophy (e.g., aortic or mitral valve regurgitation) Hypertrophic obstructive cardiomyopathy Dilated cardiomyopathy (e.g., viral, postpartum, idiopathic) Restrictive cardiomyopathy (e.g., amyloidosis, hemochromatosis) Pericardial diseases (e.g., tamponade, constrictive pericarditis) |
Relaxation is an active, energy-dependent process requiring extrusion of calcium (Ca 2+ ) from the myoplasm and its resequestration into the sarcoplasmic reticulum, thereby facilitating prompt dissociation of the contractile proteins and rapid recoil of elastic elements compressed during contraction. Delays in relaxation are a form of “active stiffening” because failure of actin-myosin cross bridges to dissociate occurs when energy supply is inadequate (e.g., ischemia) or intracellular Ca 2+ homeostasis is dysfunctional (e.g., heart failure resulting from a variety of causes). LV relaxation is highly dependent on afterload, and this afterload sensitivity is markedly enhanced in the failing heart. The phenomenon is particularly notable in HFpEF because hypertension is almost invariably present, thereby producing further delays in LV relaxation, compromising early LV filling by attenuating the LA-LV pressure gradient, blunting torsional recoil, inhibiting the diastolic suction mechanism (which facilitates LV filling during hypovolemia and exercise), and raising LV diastolic pressures. Failure of LV relaxation to proceed more rapidly in response to tachycardia during exercise also exacerbates this process and is an important mechanism responsible for the often-profound exercise intolerance that patients with HFpEF experience despite only modestly depressed LV systolic function. In addition, delays in LV relaxation prolong compression of intramyocardial coronary arterioles and restrict early diastolic coronary blood flow, actions that contribute to the development of subendocardial ischemia and further compromise exercise tolerance in HFpEF.
Hooke’s law governs the passive mechanical behavior of myocardium, an elastic material that develops a resisting force (stress) as its length (strain) increases. It can be easily shown that an exponential relationship between stress and strain accurately models the passive stiffness of myocardium. An increase in this myocardial stiffness occurs in HFpEF that contributes to the steeper slope of the LV end-diastolic pressure-volume curve. The determinants of myocardial stiffness are multifactorial, as components of the cardiac myocyte, extracellular matrix, interstitium, and coronary microvasculature have all been implicated in the presence of a chronic proinflammatory state characterized by elevated nitrosative-oxidative stress that contributes to interstitial fibrosis and cardiac myocyte stiffening. Cardiac myocyte diameter and myofibrillar density were greater, and excess collagen volume was present in endomyocardial biopsies obtained from patients with HFpEF. These factors increase myocardial stiffness. Excessive collagen deposition resulting from an imbalance between synthesis and degradation contributes to increased extracellular matrix stiffness. Coronary microvascular density is reduced in HFpEF and is correlated to the magnitude of myocardial fibrosis, factors that contribute to impaired coronary blood flow reserve. Microvascular endothelial dysfunction also leads to a relative paucity of nitric oxide bioavailability, a decrease in intracellular cyclic guanosine monophosphate concentration, and a decline in protein kinase G activity, actions that adversely modulate the phosphorylation state of specific isoforms of titin. A large, highly elastic protein, titin acts as a bidirectional length sensor to exert restoring forces as a cardiac myocyte approaches its maximum and minimum length, thereby preventing excessive stretch and compression, respectively. Sufficient diastolic recoil of titin and other elastic elements (e.g., elastin, collagen, actin, myosin) within the myocardium is an essential determinant of both LV compliance and diastolic suction. The changes in titin isoform phosphorylation state and expression occur in HFpEF that have also been closely linked to increases in myocardial stiffness.
Cardiac catheterization is required to quantify the LV compliance curve using end-diastolic pressure-volume relations in patients with HFrEF or HFpEF. This approach is clearly impractical for the vast majority of patients with heart failure who present for diagnostic evaluation. Instead echocardiography is used to estimate LV compliance based on two-dimensional imaging and Doppler measurement of blood flow and mitral annular velocities. Evidence of impaired LV filling, attenuated systolic pulmonary venous blood flow, reduced tissue Doppler velocities, enlarged LA size, and increased LV wall thickness with normal chamber dimensions are common echocardiographic findings in many patients with HFpEF. However, one-third of patients present with subclinical HFpEF, and LVDD may only be detected using echocardiography with provocative testing. It is noteworthy that the presence of LVDD alone does not establish the diagnosis of HFpEF without clinical symptomatology, as approximately 70% of patients over the age of 75 years have some echocardiographic evidence of LVDD. Nevertheless, the presence of moderate or severe LVDD in patients without heart failure is a predictor for the subsequent development of HFpEF. LVDD also invariably accompanies HFrEF, as LVDD most often occurs as a consequence of LV systolic dysfunction under these circumstances. Regardless of the cause, the severity of LVDD and its response to medical therapy are important determinants of exercise tolerance and mortality in patients with HFrEF or HFpEF. The anesthesiologist should recognize that LVDD has significant implications on LV response to acute alterations in loading conditions that occur during and after surgery independent of LV systolic dysfunction, as higher LV filling pressures may be required to achieve adequate stroke volume, and patients may be more susceptible to hypotension when anesthetics or other vasodilators are administered.
The defining characteristic of HFrEF is a reduction in myocardial contractility, most commonly resulting from ischemia, infarction, or cardiomyopathy (e.g., viral, postpartum, idiopathic). Pressure-volume analysis provides a useful illustration of the pathophysiology of myocardial contractility as an underlying cause for HFrEF. A shallow slope of the LV end-systolic pressure-volume relation (E es ) indicates that contractility is reduced compared with the normal heart (see Fig. 10.2 ) and is usually accompanied by compensatory LV dilation (movement of the pressure-volume diagram to the right to higher volumes) along the LV end-diastolic pressure-volume curve. The increase in LV end-diastolic volume may preserve stroke volume and cardiac output but occurs at the cost of higher LV filling pressures and pulmonary venous congestion. Chronic neurohormonal activation (e.g., sympathetic predominance of autonomic nervous system tone, stimulation of the renin-angiotensin-aldosterone axis) also serves to mitigate decreases in cardiac output and mean arterial pressure (MAP) observed in HFrEF, but this response also inadvertently increases heart rate, LV afterload, and myocardial oxygen consumption that further undermine LV systolic function.
In contrast to the marked depression of E es and LV dilatation observed in HFrEF, a steep slope of the LV end-systolic pressure-volume (consistent with systolic stiffening) ( Fig. 10.3 ) and lower LV volumes are characteristic features of HFpEF. Despite the increase in baseline E es , myocardial contractility is abnormal in patients with HFpEF as indicated by tissue Doppler echocardiography, strain imaging, and estimates of LV pressure-volume relations. Depression of myocardial contractility is substantially less severe under resting conditions in patients with HFpEF versus HFrEF, but the ability to augment contractility and raise cardiac output during physiologic stress is markedly attenuated. The limitation of systolic reserve is an important cause of exercise intolerance in patients with HFpEF. The systolic stiffening of HFpEF also has important implications for vasodilator therapy. Afterload reduction substantially improves stroke volume with only modest declines in arterial pressure in HFrEF because of the shallow slope of the LV end-systolic pressure-volume relation, but this beneficial effect is not observed in the presence of systolic stiffening associated with HFpEF, as vasodilation causes substantial hypotension with only a modest increase in stroke volume (see Fig. 10.3 ). As a result, vasodilator therapy is a mainstay in the treatment of HFrEF but not HFpEF.
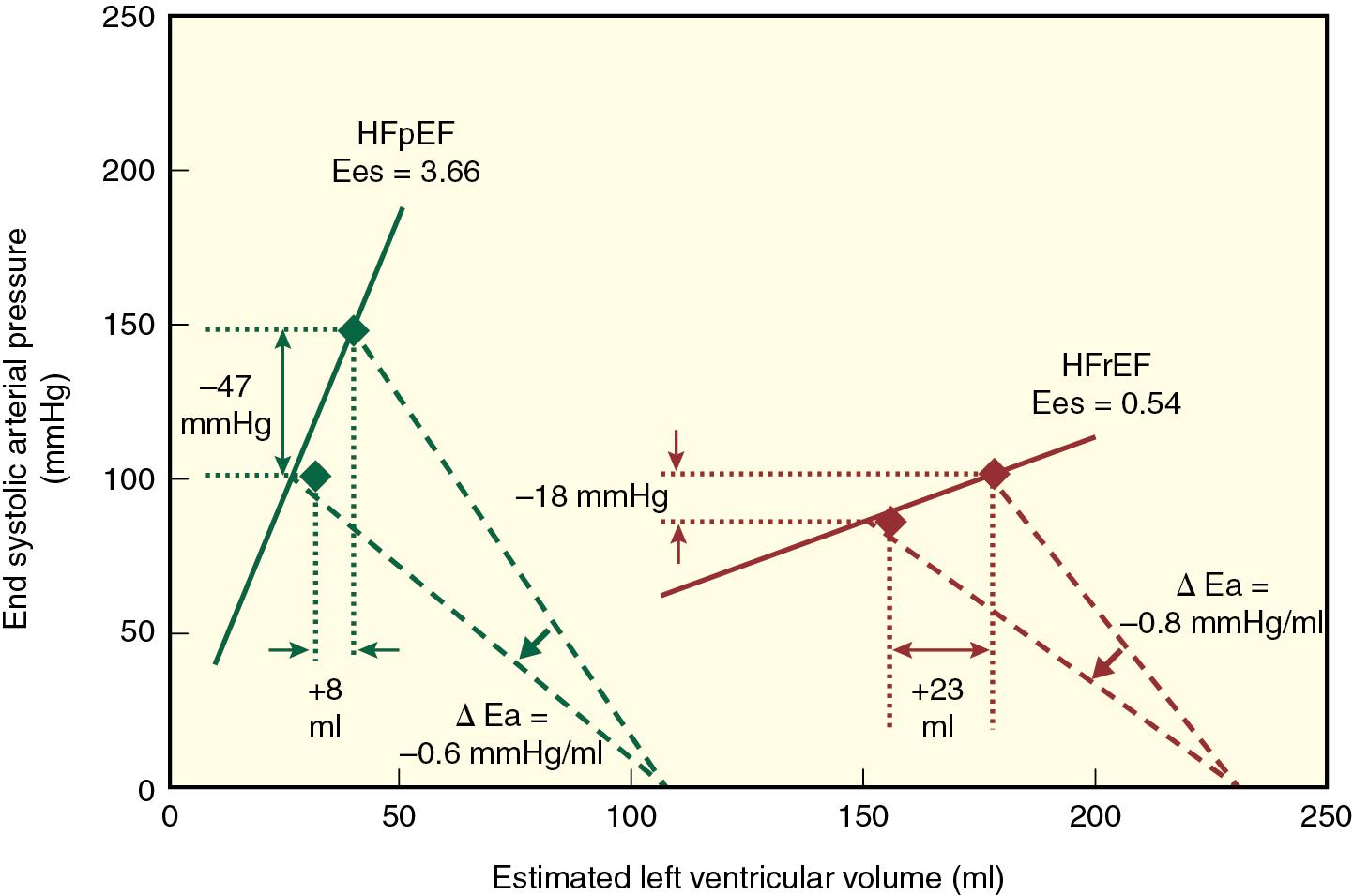
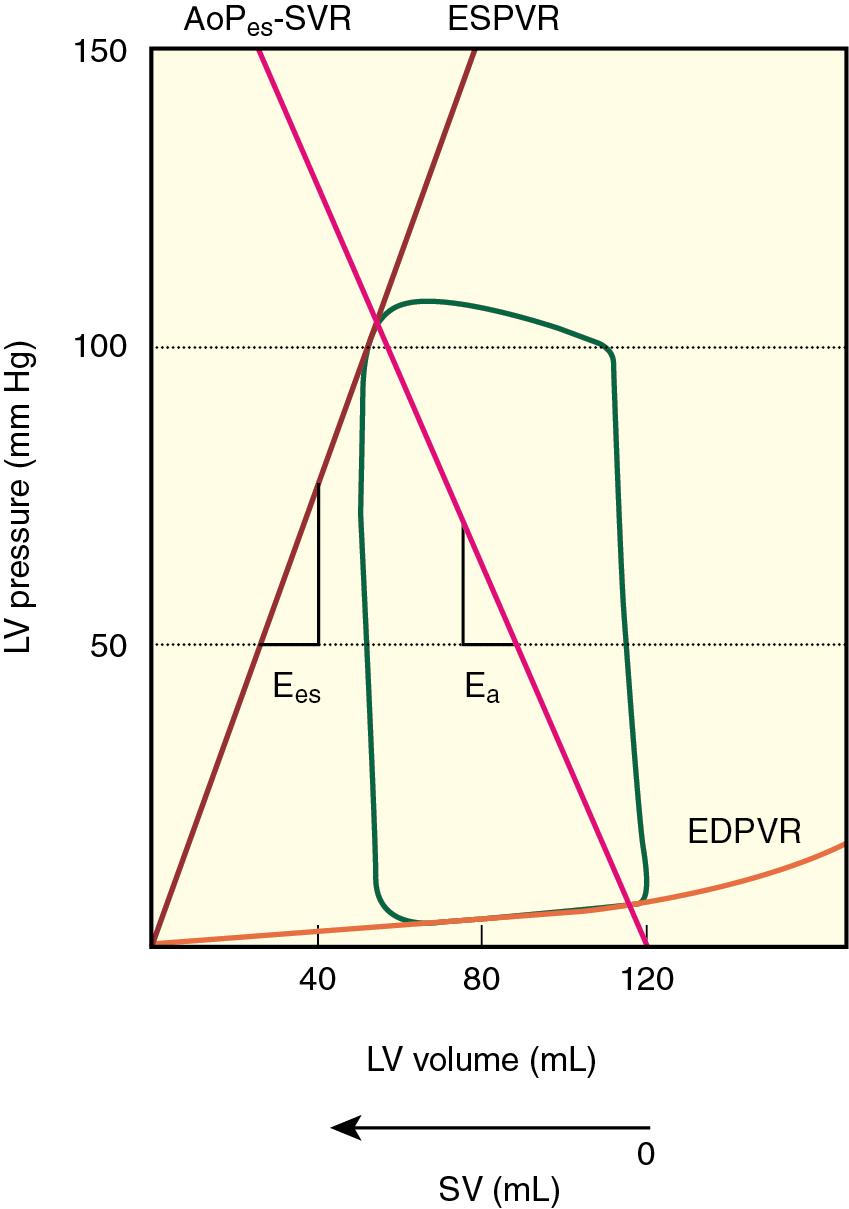
A reduction in compliance of the aorta and proximal great vessels is a cardinal feature of HFpEF that substantially increases arterial pulse pressure, LV afterload, and myocardial oxygen demand. The magnitude of arterial stiffening observed in HFpEF has been shown to exceed that predicted on solely the basis of the presence of chronic hypertension or advanced age alone. When combined with LV systolic and diastolic stiffening, this increase in arterial stiffness is an important contributing factor to marked lability in arterial pressure that commonly occurs in patients with HFpEF. A series elastic chamber model of the cardiovascular system facilitates understanding of this interaction between arterial and LV systolic stiffening in HFpEF. Effective arterial elastance (E a , the ratio of LV end-systolic pressure and stroke volume) is an estimate LV afterload that combines its compliance and resistance components ( Fig. 10.4 ). Both E a and E es are increased in patients with HFpEF (reflecting increased arterial and LV systolic stiffening, respectively), assuring optimal mechanical energy transfer (i.e., stroke volume) from the left ventricle to the great vessels and proximal arterial circulation under resting conditions (see Fig. 10.3 ). However, the steep slopes of E a and E es imply that even modest reductions in preload or afterload produced by diuretics or arterial vasodilators may precipitate large declines in arterial pressure. In contrast, LV-arterial coupling is abnormal at rest in HFrEF, primarily because myocardial contractility is depressed (reduced E es ) while arterial stiffness (E a ) remains relatively unaffected. In this situation, a decrease in LV afterload (E a ; produced by a vasodilator, for example) is beneficial because matching between the LV and arterial circulation improves and greater forward flow occurs as a result. The elevated LV-arterial stiffening observed in HFpEF also adversely affects the hemodynamic responses of these patients during exercise. Vasodilation is poorly tolerated, and limited LV systolic reserve attenuates increases in myocardial contractility resulting from activation of the sympathetic nervous system. This phenomenon can be especially pronounced in women because arterial stiffness is often greater in women than men, an observation that may contribute to the greater prevalence of HFpEF in women.
The left atrium’s systolic contribution to LV filling directly increases through the Frank-Starling mechanism when LV compliance is reduced or LV diastolic pressures are elevated. However, this compensatory augmentation of LA contractility declines as heart failure progresses because LA afterload continues to increase and the left atrium dilates, stretching its myofilaments beyond their ideal operational length, thereby attenuating the chamber’s pumping ability and rendering it less capable of actively contributing to LV preload. LA contractile failure eventually occurs as LV compliance continues to decline and end-diastolic pressure increases. LA dilation resulting from chronic increases in LA pressure also reduces the chamber’s compliance and its ability to collect pulmonary venous blood. Indeed, remodeling and reduced compliance of the left atrium occur in response to LV diastolic dysfunction, effects that further restrict pulmonary venous blood flow into the left atrium during its reservoir and conduit (diastolic) phases. This LA systolic and diastolic dysfunction occurs in both forms of heart failure but is especially apparent in patients with HFpEF. LA dysfunction in HFpEF has been identified as another factor that limits exercise tolerance in these patients and is associated with pulmonary arterial hypertension (PAH), right ventricular (RV) dysfunction, and mortality. LA dilation is a primary cause of atrial fibrillation, which is present in as many as two-thirds of patients with HFpEF. When LVDD is present in HFpEF or HFrEF, the onset of new atrial fibrillation often acutely precipitates signs and symptoms of acute decompensation because the loss of LA contraction and elevated heart rate (reduced diastolic filling time) lead to inadequate LV filling and cardiac output. More profound exercise limitations, lower quality of life, increased rate of rehospitalization, and higher mortality are observed in patients with atrial fibrillation versus sinus rhythm in HFpEF.
Whereas chronic heart failure is classified as those with long-standing disease, acute heart failure is generally described as rapid onset of clinical symptoms of heart failure that requires immediate treatment as these patients often present in life-threatening conditions. Patients may require hospitalization with treatment aimed at decreasing volume status and stabilizing hemodynamics. The term acute heart failure applies to both patients who present with worsening symptoms of their preexisting condition (acute decompensated heart failure [ADHF]) and those who present for the first time with signs and symptoms of heart failure (de novo acute heart failure [de novo AHF]). ADHF symptoms include signs of fluid retention leading to congestion (weight gain, dyspnea) as the result of decompensation due to inadequacy or failure of compensatory mechanisms. As opposed to decompensation of a chronic state, de novo AHF is characterized by a sudden increase in intracardiac filling pressures and/or acute myocardial dysfunction. This rapid change in myocardial dynamics leads to an overall decrease in peripheral perfusion and pulmonary edema. Cardiac ischemia due to coronary artery occlusion is the leading cause of de novo HF, therefore management is focused on not only stabilizing hemodynamics but restoring myocardial perfusion to improve myocardial contractility. Less common nonischemic etiologies of de novo HF include viral, drug-induced (toxic), and peripartum cardiomyopathy. Patients who present with de novo HF may experience long-term cardiac dysfunction (chronic heart failure); however, management of underlying insults (e.g., thyroid storm) may lead to complete restoration of myocardial function.
The diagnosis of heart failure is based on a well-known constellation of clinical signs and symptoms, among which fatigue, tachypnea, dyspnea at rest or on exertion, paroxysmal nocturnal dyspnea, orthopnea, a S 3 gallop, jugular venous distention, peripheral edema, exercise intolerance, and evidence of reduced tissue perfusion are the most prominent. Whereas paroxysmal nocturnal dyspnea, pulmonary edema, and dependent edema are more common in patients with HFpEF, S 3 gallop is heard more frequently in those with HFrEF. Notably, jugular venous distention, among other clinical signs, was shown to be the strongest predictor of elevated LV end-diastolic pressures (LVEDPs) in patients with HFpEF. When ejection fraction is reduced, the presence of heart failure symptoms usually establishes the diagnosis of HFrEF following standard guidelines in the absence of other causes (e.g., pericardial or valvular disease). In contrast to HFrEF, the initial diagnosis of HFpEF is often more difficult to make, especially when the patient is asymptomatic or only mildly symptomatic at rest. Many of heart failure symptoms are also relatively generic in nature and can suggest a large differential of other possible cardiac and noncardiac etiologies that require careful assessment to exclude. Cardiac catheterization to define elevated LV systolic and diastolic stiffness using pressure-volume analysis or provocative testing (e.g., exercise, rapid intravascular volume expansion) to fully manifest overt clinical symptoms or characteristic echocardiographic findings may be required for HFpEF to become readily apparent. Direct measurement of RV filling pressures under resting conditions or during exercise also offers useful information about the severity of HFpEF. Mean pulmonary capillary occlusion pressure greater than 15 mm Hg at rest or 25 mm Hg during exercise provides strong evidence of HFpEF and is a predictor of mortality.
Chest radiography (posteroanterior and lateral views) may detect the presence of pulmonary disease, cardiomegaly, pulmonary venous congestion, and interstitial or alveolar pulmonary edema. An early radiographic sign of LV failure and associated pulmonary venous hypertension is distention of the pulmonary veins in the upper lobes of the lungs. Perivascular edema appears as hilar or perihilar haze. The hilum appears large with ill-defined margins. Kerley lines, which reflect edematous interlobular septae in the upper lung fields (Kerley A lines), lower lung fields (Kerley B lines), or basilar regions of the lungs (Kerley C lines) and produce a honeycomb pattern, may also be present. Alveolar edema produces homogeneous densities in the lung fields, typically in a butterfly pattern. Pleural effusion and pericardial effusion may be observed. Radiographic evidence of pulmonary edema may lag behind the clinical evidence of pulmonary edema by up to 12 hours. Likewise, radiographic patterns of pulmonary congestion may persist for several days after normalization of cardiac filling pressures and resolution of symptoms.
The American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) published guidelines for the diagnosis of HFpEF ( Table 10.4 ). The ACC/AHA recommendations depend on three factors: heart failure symptoms, ejection fraction greater than 50%, and evidence of LVDD. This approach is useful for patients with clear symptomatology but may be too simplistic when subclinical HFpEF is present. The ESC criteria are more specific and incorporate several echocardiographic indices based on two-dimensional measurements (LV size, wall thickness, and mass; LA size), transmitral blood flow velocity (early LV filling peak “E” velocity), and tissue Doppler imaging (mitral annulus e’ wave velocity, E/e’ ratio). As should be clear from this definition, the ESC guidelines rely entirely on resting echocardiographic assessment and, like the ACC/AHA guidelines, are limited because they do not incorporate provocative testing.
American College of Cardiology Foundation/American Heart Association
|
European Society of Cardiology
|
Abnormalities on a 12-lead electrocardiogram (ECG) are common in the majority of heart failure patients; however, they are typically the result of the underlying pathology. For instance, there may be evidence of LV hypertrophy, previous MI, various arrhythmias (e.g., atrial fibrillation), and conduction abnormalities that include but are not limited to left or right bundle branch block and a widened QRS complex. Due to the common presence of these abnormalities, the ECG has a low predictive value for the diagnosis or risk prediction of heart failure.
Efforts continue toward the discovery of a circulating biomarker that will facilitate early diagnosis, to effectively monitor treatment regimens, and to allow for risk stratification within the population. Measurement of brain natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) concentrations is an important part of the ESC criteria, as these assessments have been shown to be very useful prognostic indicators in patients with HFpEF. Natriuretic peptide concentrations are related to LV end-diastolic wall stress, which is higher in HFrEF because LV dilation resulting from eccentric remodeling is common. In contrast, concentric hypertrophy and relatively normal LV chamber size are characteristic features of HFpEF. This LV geometry is associated with lower LV end-diastolic wall stress, which is reflected in lower BNP or NT-proBNP levels. Thus changes in BNP and NT-proBNP levels are reflective of hemodynamic alterations within the heart.
While BNP and NT-proBNP remain the most important heart failure biomarkers for diagnosis and risk prediction, other potential biomarkers have emerged that are worth mentioning. For instance, levels of highly sensitive troponins (e.g., troponin T and I; high-sensitivity troponin T/I [hs-TnT/I]) released systemically due to myocardial damage serve as a measure of risk prediction. Both C-reactive protein (CRP) and growth differentiation factor-15 (GDF15) increase within the circulation and represent the inflammatory component of heart failure in noninfected patients. Although not routinely used clinically, next-generation biomarkers, including soluble suppression of tumorigenicity 2 (sST2), galectin-3 (Gal-3), and a few candidate microRNAs (miRNAs) that are primarily released secondary to myocardial fibrosis and hypertrophy, may prove useful to diagnose heart failure and improve risk prediction.
Regardless of the criteria used to identify HFrEF or HFpEF, these diagnoses are essential for anesthesiologists to recognize because they predict major adverse cardiovascular events, including mortality in patients undergoing cardiac or major noncardiac surgery independent of ejection fraction.
Both the New York Heart Association (NYHA) and the ACC/AHA have created complementary classification systems for heart failure patients. The NYHA system focuses primarily on the degree of limitation during physical activity, whereas the ACC/AHA provides information regarding both the presence and severity of the disease. Since progression of heart failure is linked to reduced 5-year survival, it is important to note that these stages are progressive and do not allow for regression to a lower classification. Often patients are classified using a combination of both scoring systems. For example, a patient diagnosed with severe aortic stenosis using echocardiography, who experiences shortness of breath with ordinary activity, would be classified as NYHA II, ACC/AHA stage D (2D). Categories of both the NYHA functional classification and the ACC/AHA stages of heart failure can be found in Table 10.5 .
| NYHA Functional Classification | |
| Class I: | No limitation of physical activity. Usual physical activity does not result in clinical symptoms. a |
| Class II: | Slight limitation of physical activity. The patient is comfortable at rest; however, activity may cause clinical symptoms. |
| Class III: | Marked limitation of physical activity. Patient is comfortable at rest; however, low physical activity causes clinical symptoms. |
| Class IV: | Patient is unable to perform any physical activity without clinical symptoms and experiences these symptoms at rest. |
| ACC/AHA Stages of Heart Failure | |
| Class A: | No structural evidence of cardiovascular disease. No functional limitation in ordinary physical activity. |
| Class B: | Evidence of minimal structural cardiovascular disease. Comfortable at rest but slight limitation during ordinary activity. |
| Class C: | Evidence of moderately severe structural cardiovascular disease. Comfortable only at rest and with limitation in activity due to clinical symptoms. |
| Class D: | Objective evidence of severe structural cardiovascular disease. Experiences clinical symptoms at rest. |
a Clinical symptoms include fatigue and/or shortness of breath.
Survival of patients with HFrEF has steadily improved during the past three decades, but mortality in those with HFpEF remains essentially unchanged. These epidemiologic data emphasize the relative futility of standard pharmacologic therapy (which is clearly beneficial in patients with HFrEF) when applied to those with HFpEF. Indeed, mitigation of heart failure symptoms, aggressive treatment of associated conditions (e.g., hypertension, diabetes), weight loss, and exercise training are the primary objectives for patients with HFpEF ( Fig. 10.5 ) because β blockers and inhibitors of the renin-angiotensin-aldosterone system (RAAS), medications that form the foundation upon which chronic HFrEF management rests, have been consistently shown to lack verifiable efficacy in HFpEF.
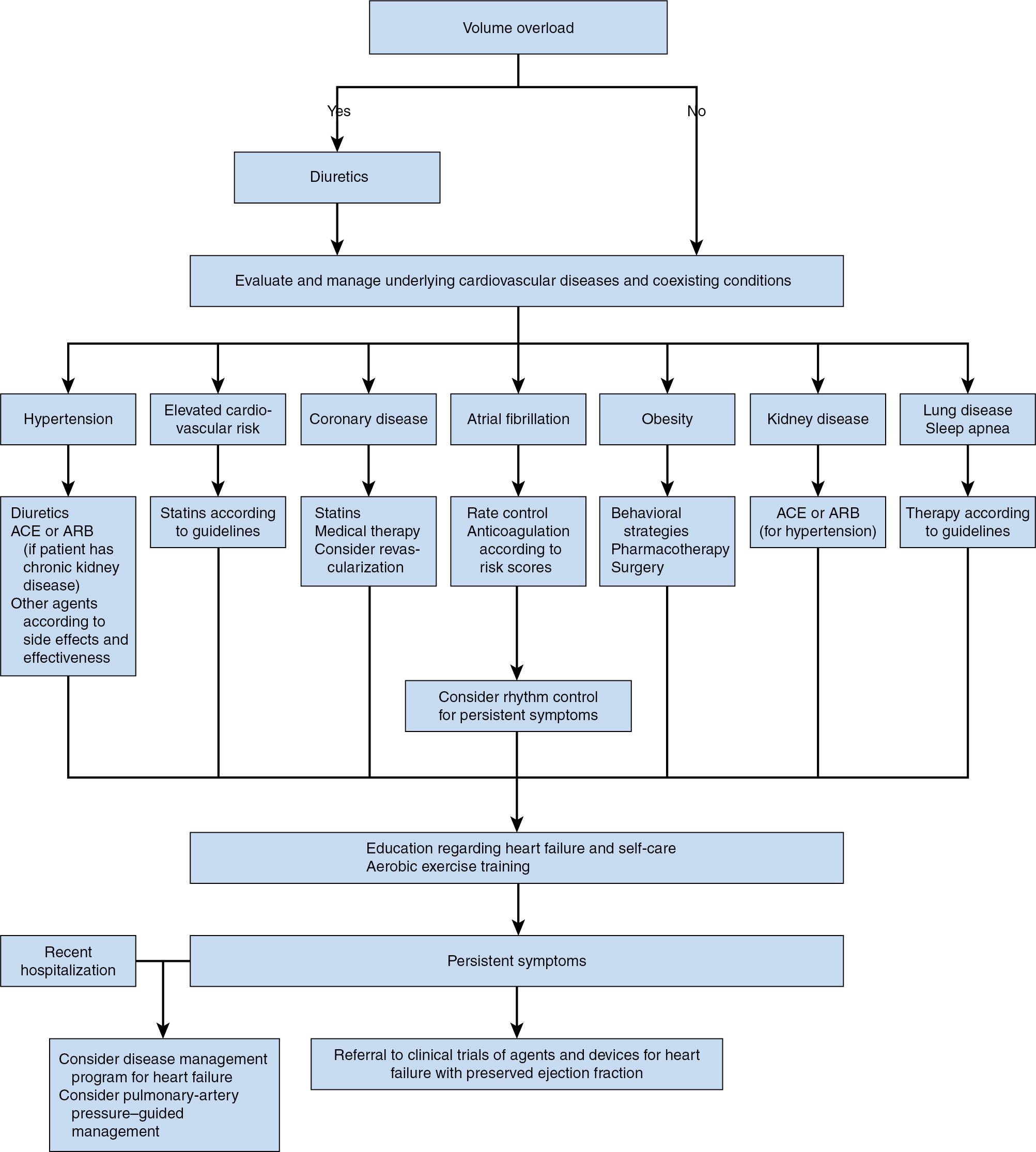
Furosemide and other loop diuretics reduce LV filling pressures, decrease pulmonary venous congestion, and improve clinical symptoms in patients with acute or chronic HFrEF and HFpEF. These medications are strongly recommended by the ACC/AHA and ESC guidelines. Thiazide diuretics may also be useful in patients with poorly controlled hypertension to reduce the incidence of new HFpEF, an observation that stresses the importance of control of comorbid conditions when managing this condition. An implanted wireless pulmonary artery pressure (PAP) monitor was shown to provide unique information to guide diuretic treatment, decrease LV filling pressures, and reduce heart failure hospitalizations in NYHA class III patients with either HFrEF or HFpEF. These interesting results not only suggest that novel hemodynamic monitoring devices may eventually prove to have utility for heart failure treatment but also emphasize the central role of diuretic therapy in its management.
Several major randomized controlled clinical trials demonstrated that β blockers are beneficial for the treatment of HFrEF. These medications are strongly recommended for this clinical indication (class I evidence) in the most recent ACC/AHA and ESC guidelines. In contrast, whether β blockers have utility in patients with HFpEF could not be definitively established in the multitude of observational studies and clinical trials that addressed this question to date. The β blocker nebivolol reduced mortality and cardiovascular-related hospital admission in elderly patients with heart failure, but no differences were observed in those with HFrEF versus HFpEF. Notably, the definition of HFpEF used in this study included patients with ejection fraction greater than 35% (as opposed to the currently accepted value of 50%), suggesting that any salutary effect of nebivolol observed in the HFpEF group may have resulted from inclusion of a substantial number of patients with HFrEF. Another randomized controlled trial showed that long-term treatment with nebivolol did not reduce clinical symptoms or improve exercise capacity (as quantified using a 6-minute walk distance test) in patients with HFpEF. Cardiovascular mortality and hospitalization related to heart failure were similar in patients with HFpEF who received carvedilol compared with placebo, although this study’s findings were later criticized because the dose of carvedilol may have been insufficient to positively influence outcome. A propensity-matched cohort study examined the impact of β blockers on mortality in 8244 patients with HFpEF. The results indicated that use of β blockers was associated with modest, but statistically significant, declines in 1- and 5-year mortality compared with conventional treatment. Conversely, a large retrospective study of 4123 Medicare patients with HFpEF indicated that β blockers did not affect mortality or rehospitalization rates. Two meta-analyses also suggested that all-cause mortality may be marginally lower in HFpEF patients treated with β blockers, but no differences were observed in hospitalization rates for heart failure. To date, no large-scale randomized clinical trial of β blockers has been performed to further define the role of this class of medications in HFpEF. Thus use of β blockers in patients with HFpEF should be restricted to other clinical indications (e.g., treatment of hypertension or MI; rate control in atrial fibrillation).
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are mainstays of medical therapy for HFrEF. Five major randomized clinical trials conducted to date studied the effects of ACE inhibitors and ARBs on mortality and hospitalization rates in patients with HFpEF, but their collective findings have been remarkably disappointing. The ARB candesartan did not change cardiovascular mortality during a 3-year follow-up in 1514 patients with HFpEF compared with placebo. This trial did show that hospitalization rates were lower in those treated with candesartan, but these patients also had a higher rate of adverse side effects. Similar to the findings of this trial, the ACE inhibitor perindopril did not improve 3-year survival compared with placebo in elderly HFpEF patients (≥70 years). Nevertheless, a modest increase in exercise tolerance and a decrease in NYHA classification were observed in those treated with perindopril, most likely because hypertension was more effectively managed with the ACE inhibitor. Another RAAS inhibitor trial compared the effects of the ARB irbesartan against placebo in patients with HFpEF. After more than 4 years of follow-up, no differences in mortality or hospitalization rate for heart failure were observed between groups. Not surprisingly, a meta-analysis incorporating these three trials also concluded that ACE inhibitors or ARBs did not reduce mortality or hospitalization in those with HFpEF.
The effects of the aldosterone antagonist spironolactone were subsequently compared with placebo in another randomized double-blind trial of 3445 patients with HFpEF. No differences in the primary composite end points, including cardiovascular mortality, survival after cardiac arrest, and total hospital admissions for heart failure, were observed between groups. Nevertheless, the rate of heart failure hospital admission was lower in those receiving spironolactone, suggesting that the medication may have some modest utility in these patients. Most recently, an eagerly awaited randomized clinical trial demonstrated that the angiotensin receptor neprilysin inhibitor valsartan-sacubril did not reduce cardiovascular mortality or total hospitalizations for heart failure in patients with HFpEF compared with placebo. These latter findings were particularly disheartening not only because valsartan-sacubril had been previously shown to increase survival and decrease hospitalization rate in patients with HFrEF compared with the ACE inhibitor enalapril, but also because the drug combination favorably reduced NT-proBNP concentration, LA size, and NYHA functional class compared with the valsartan alone in those with HFpEF. Despite these negative results, ACE inhibitors and ARBs continue to be useful for control of arterial pressure in patients with hypertension and HFpEF.
The results of clinical trials in which other medications were studied have also failed to yield encouraging results in patients with HFpEF. The effects of digoxin on cardiovascular mortality and heart failure hospitalizations were compared with placebo in patients with HFpEF. No differences between groups were observed after 37 months of follow-up. Another trial examined the effects of chronic treatment with phosphodiesterase (PDE) fraction V inhibitor sildenafil on functional capacity in HFpEF. Sildenafil did not improve peak oxygen consumption, 6-minute walk distance, quality-of-life scores, or indices of LV diastolic function after 24 weeks of treatment compared with placebo. Similar results were reported in a clinical trial of isosorbide mononitrate. More recently, exercise capacity, daily activity, NYHA functional class, E/e’ ratio (an echocardiographic estimate of LA pressure), and NT-proBNP concentrations were similar in patients with HFpEF treated with a 4-week course of an inhaled inorganic nitrite compared with those receiving placebo. Some data suggest that statins may have beneficial effects on survival and hospitalization rates in patients with HFpEF, but the evidence supporting this contention is inconsistent. A small observational study indicated that statins, but not β blockers, RAAS inhibitors, or Ca 2+ channel antagonists, reduced mortality and cardiovascular-related hospitalization in HFpEF. These findings were supported by the results of two meta-analyses and a retrospective propensity-matched study from the Swedish Heart Failure Registry. Nevertheless, rosuvastatin failed to provide these salutary effects in HFpEF when a subanalysis of a large sample of heart failure patients was conducted. A multicenter randomized controlled trial is needed to clearly define whether statins play an important role in HFpEF.
Exercise training augments aerobic fitness, reduces clinical symptoms, and increases self-reported quality of life in patients with HFrEF and HFpEF. These salutary effects result primarily from enhanced peripheral oxygen utilization, but some direct cardiac benefits may be accrued as well. When combined with exercise training, weight loss achieved through caloric restriction provided additive benefits on aerobic capacity. The type, intensity, and duration of exercise and the magnitude of weight loss required to maintain such favorable effects are yet to be clearly defined, but such lifestyle modifications also significantly mitigate the impact of major risk factors for heart failure, including hypertension and diabetes. Simple diet modifications may also be helpful to partially reverse some of the adverse hemodynamic consequences of heart failure. For example, a 3-week course of a salt-restricted Dietary Approaches to Stop Hypertension (DASH) diet improved indices of LV diastolic function, decreased arterial stiffness, and facilitated more favorable LV-arterial coupling in patients with HFpEF.
Hypertension is a major risk factor for HFrEF and HFpEF, so it is not surprising that control of arterial pressure (using diuretics, β blockers, or RAAS inhibitors) is of paramount importance to reduce the incidence and severity of heart failure, especially in the elderly. A recent large randomized clinical trial showed that the incidence of heart failure was reduced by 37% when systolic arterial pressure was maintained less than 120 mm Hg compared with 140 mm Hg in nondiabetic, hypertensive patients with increased cardiovascular risk. This aggressive control of hypertension was especially beneficial in elderly patients. The results of these and other trials examining the impact of hypertension control on cardiovascular outcome emphasize one of the most notable consequences of such an intervention is the reduction in heart failure risk. Unfortunately, this strategy does not appear to extend to patients with type 2 diabetes mellitus, as intensive blood pressure control was not associated with a lower risk of heart failure in this patient population. In contrast, tighter glycemic control using sodium-glucose cotransport protein 2 (SGLT2) inhibitors has been shown to substantially reduce heart failure hospitalizations and mortality in diabetics. Coronary artery disease is very common in patients with both phenotypes of heart failure, and the current ACC/AHA guidelines recommend coronary artery surgery if myocardial ischemia is determined to be a contributing factor. Atrial fibrillation is also frequently observed in patients with HFrEF or HFpEF, a complication that is independently associated with RV dysfunction, tricuspid regurgitation, exercise intolerance, and mortality. Reestablishing and maintaining sinus rhythm may be an important theoretical goal in patients with heart failure and atrial fibrillation, but this objective is sometimes not feasible despite antiarrhythmic medications or multiple catheter-based or surgical radiofrequency or cryoablation interventions because of fundamental time-dependent alterations in LA structure and function. Nevertheless, rate control in chronic atrial fibrillation appears to be an acceptable alternative to restoration of sinus rhythm in patients with HFrEF. Indeed, symptom reduction is the primary goal of atrial fibrillation management following established guidelines. A highly anticipated new clinical trial will undoubtedly provide additional insight into the impact of coexisting disease management on outcome in elderly patients with HFpEF.
As patients may present either in ADHF or de novo HF, the anesthesiologist may be faced with stabilizing these patients for emergent or urgent surgery. Likewise, decompensation may occur in heart failure patients during routine elective cases. The hemodynamic profile includes a low cardiac output state, high ventricular filling pressures, and hypertension or hypotension.
Despite limited clinical trial data showing efficacy, diuretics remain a first-line therapy for patients presenting in acute heart failure. Per the 2013 ACC/AHA heart failure guidelines, diuretics should be immediately administered in patients with significant fluid overload. Rapid administration of a loop diuretic not only controls the symptoms related to volume overload (dyspnea) but may also improve in-hospital mortality as shown in a large multicenter, prospective observational study. However swift administration of diuretics may not be ideal in patients with severe hypotension or cardiogenic shock, in which case the patient may first require hemodynamic or mechanical circulatory support prior to diuretic therapy. Furosemide, bumetanide, and torsemide, used as continuous infusions or as bolus administrations, are commonly used to encourage diuresis in this patient population. The reduction in intravascular volume leads to decreased central venous and pulmonary capillary wedge pressures (PCWP), thus reducing overall pulmonary congestion. There is further evidence that furosemide, and potentially other loop diuretics, cause the release of prostaglandins and decrease acute pulmonary edema irrespective of the effect on intravascular volume.
The use of vasodilators has also proven to be efficacious in correcting elevated filling pressures and reducing afterload. Similar to diuretics, evidence pertaining to efficacy in the acute heart failure setting is lacking. Careful consideration of the vasodilator is critical and is based on the underlying hemodynamics. Use of nitroprusside is an effective approach to rapidly decrease left ventricular afterload in patient with severe hypertension, whereas nitroglycerin, which primarily decreases venous tone, is commonly used as an adjunct to diuretic therapy. As with many heart failure treatments, the routine use of vasodilators in acute situations does not improve outcomes, including mortality or rehospitalization rates.
Vasopressin receptor antagonists such as tolvaptan have emerged as potential adjunct therapy with the goal of reducing arterial constriction, hyponatremia, and volume overload associated with acute heart failure. The ACC/AHA guidelines recommend reasonable, short-term use of vasopressin antagonists in hospitalized patients with persistent severe hyponatremia due to volume overload, placing them at risk for cognitive dysfunction.
The mainstay treatment for patients presenting with acute reduced contractility, or cardiogenic shock, are positive inotropic agents. Several pharmacologic agents are available to be administered intravenously with the goal to increase cyclic adenosine monophosphate (cAMP) levels. The rise in cAMP promotes an increase in intracellular calcium and efficient excitation-contraction coupling. Each inotropic agent increases cAMP via different mechanisms, giving each a unique set of side effects. Catecholamines (epinephrine, norepinephrine, dopamine, dobutamine) directly interact with β receptors on the myocardium to activate adenylyl cyclase to increase cAMP, whereas PDE inhibitors (milrinone) indirectly increase cAMP by inhibiting its degradation. Mechanism of action as well as hemodynamic effects of commonly used inotropic agents are shown in Table 10.6 .
| Medication | Mechanism | CO | MAP | HR |
|---|---|---|---|---|
| Epinephrine Norepinephrine |
β1 = β2 > α α > β1 > β2 |
↑↑ ↑ |
↑↑ ↑ |
↑↑ ↔ or ↓ |
| Dobutamine Dopamine Milrinone Levosimendan |
β1 > β2 > α D > β (α with HD) PDE inhibition Calcium sensitization |
↔ or ↓ ↑ ↑ ↑↑ |
↑↑ ↑ ↔ or ↓ ↔ or ↓ |
↑↑ ↑↑↑ ↔ or ↑ ↑↑ |
A paradigm shift with regard to inotrope development has occurred within the last decade. As opposed to traditional inotropes that increase intracellular levels of calcium, which increase myocardial oxygen consumption, heart rate, and cause dysrhythmias, myofilament calcium sensitizers are a novel approach to increasing contractility. To date, only three such drugs have been developed: levosimendan, pimobendane, and omecamtiv mecarbil. Of the three, levosimendan has been utilized the most in a clinical setting. Interestingly, levosimendan is also capable of eliciting vasodilation, and at higher doses it can inhibit PDE. These pleiotropic effects extend beyond the heart and possibly protect renal, hepatic, and neural cells from reperfusion injury. Omecamtiv mecarbil is also emerging as a potential therapy with recent a clinical trial (GALACTIC-HF) showing a lower incidence of heart failure events or death compared to placebo.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here