Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Imagine that your hearing sensitivity for pure tones is exquisite—not affected by frequent exposure to loud music or other noises—but that you have great problems in understanding speech even in a quiet environment. This occurs if you have a temporal processing disorder. Although hearing loss (HL) is in the ear, hearing problems originate in the brain. We often take hearing for granted; not realizing what good hearing allows us to do. Without hearing, communication with our fellow humans largely disappears. Substitutes for loss of hearing are sign language, which replaces hearing with vision, and cochlear implants, which restore hearing to a large extent. For hard of hearing persons, amplification with hearing aids restores the sense of sound but does not generally result in normal perception, except if the HL is of the conductive type. Here we will identify some effects of noise exposure on the central nervous system (CNS). In addition, we will present some annoying side effects of HL: a primary one being tinnitus, but an even more disturbing one is an increased probability of dementia.
The commonly accepted level duration of exposure trade-off is shown in Fig. 4.1 together with equivalent exposure levels for permanent threshold shift (PTS), temporary threshold shift (TTS), and “assumed safe” conditions. It should be noted that “safe” means a very low likelihood of cochlear damage, and that all these conditions are based on 8 h exposure per day. The region indicated by “?” does not fulfill that criterion, and our own results have shown that there are specifically long-lasting effects on the auditory thalamus and cortex ( Section 4.5 ). Nevertheless, there were no HLs, not even temporary, and no outer hair cell (OHC) abnormalities. So according to these criteria the time–intensity trading “red line” in Fig. 4.1 , demarcating “safe” from “threatening,” continues in the “?” region.
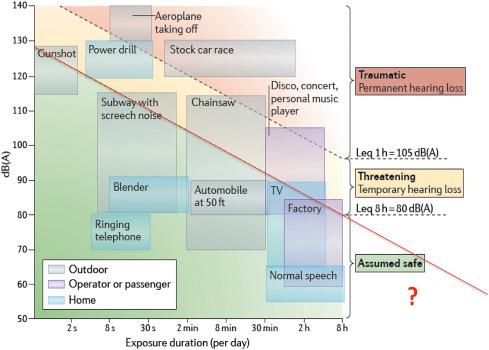
The vertical axis in Fig. 4.1 is in dB(A), where 0 dB(A) is the minimal audible sound intensity at each frequency in people with normal hearing. dB(A) and dB SPL are very close (±5 dB) between 0.5 and 6 kHz ( Fig. 4.2 ). Regulations on admissible sound levels do not generally apply to recreational areas such as bars, sports, and concert venues (with some countries, such as the United Kingdom, being an exception), and if they do apply then typically it is only to what is audible outside the venue. While ear protection inside these establishments is advisable, it is generally not complied with. For instance, identified possible hazardous noise sources in two hockey arenas, namely, enhanced reverberant conditions from enclosed environment, loud public address systems, and design and building materials. They applied personal dosimeters worn by both workers and fans and area noise monitors to evaluate exposure levels in both venues. Noise monitor measurements for both venues recorded peak levels of 105–124 dB(A) with equivalent continuous SPLs 81–96 dB(A), and 110–117 dB(A) with equivalent continuous SPLs 85–97 dB(A). Taking 80 dB(A) and 8 h (Leq 8 h) exposure as the industrial norm, and using an exchange of 3 dB (the National Institute of Occupational Safety & Health, NIOSH norm) for time doubling or halving, would then result in an allowable exposure time at 100 dB of about 5 min/day ( Fig. 4.1 ). Leq is a measure of the total sound energy averaged over the duration of the observation period (e.g., 8 h). Formally, this is 20log 10 of the ratio of a root mean square dB(A)-weighted sound pressure during the stated time interval to the reference sound pressure, divided by the exposure duration ( Fig. 4.2 ). This gives a single value of sound level for any desired duration based on the amount of sound energy contained in the time-varying sound. Note that the use of Leq is often discouraged for very long durations (from months to years) a region indicated in Fig. 4.1 with the red question mark.
Depending on the exposure level and duration, several consequences result for the auditory system. They all share CNS changes, which may or may not be structural. These consequences are shown in Table 4.1 and further explained in the text.
| PTS noise | TTS noise | “Safe” noise–long duration | |
|---|---|---|---|
| Hearing loss | Yes | No | No |
| ABR threshold shift | Yes | No | No |
| ABR wave I amplitude | Decreased | Decreased | Normal |
| Otoacoustic emission loss | Yes | No | No |
| Central gain change | Yes | Yes | Yes |
| SFR | Increased | Increased | Decreased/increased |
| Xcorr | Increased | ? | Increased |
| Tonotopic map | Reorganized | ? | Reorganized |
| Speech-in-noise deficit | Yes | Yes | Yes |
| OHC | Damage | Normal | Normal |
| IHC | Damage likely | Modiolar ribbon synapses | Normal |
| ANFs | Loss in HL range | Loss of high-threshold ANFs | Normal |
| Central damage | Yes | Yes | Yes |
The effect of loud sound on the cochlea depends on the type used. Impulse and impact noises, such as gunfire, are characterized by high intensity and short duration and may produce immediate mechanical alterations to the cochlea. Continuous exposure at moderate to severe noise levels typically produces more subtle changes. Narrow-band noise exposure at approximately 110 dB SPL for 2 h resulted after more than 4 months postexposure in about 40 dB threshold elevation in cat ears and was correlated with loss or damage to hair cells. The orderliness of the stereocilia, on both IHC and OHC, showed the closest correlation with the thresholds of firing of ANFs ( ).
Scanning electron microscope examination of guinea pig cochleas immediately after a 1 h exposure to a pure tone ranging from 96 to 129 dB SPL showed little hair cell loss but widespread damage to the stereocilia ( ). The stereocilia are the locus of the mechanically sensitive ion channels that mediate transduction ( ; see chapter: Hearing Basics ). In general, the OHCs are more vulnerable to noise trauma than the IHCs, regardless of the type of noise. This susceptibility may be an inherent property of the OHC biochemistry ( ) since there is a relation between the pathology seen with noise exposures and the vulnerability of OHCs to ototoxic drugs ( ). As stereocilia damage progresses from disarray to fusion or loss, interfering with the patency of the transduction channels, the ANF firing thresholds increase. Subsequent cellular impairment involves protein-, lipid-, and glucose synthesis needed for cell repair and survival, and this results in permanent cell injury or cell death, leading to a permanent HL ( ). Noise exposure also induces neurotoxicity caused by excessive release of glutamate by the IHCs, resulting in the influx of large and toxic quantities of Ca 2+ ions in the postsynaptic area, and disruption of the synapse. This synaptic damage may extend well beyond regions with hair cell damage and likely can be attributed to excessive activation of those physically nondamaged hair cells. Glutamate neurotoxicity can recover in about 1 week after the trauma ( Fig. 4.3 ; ; ).
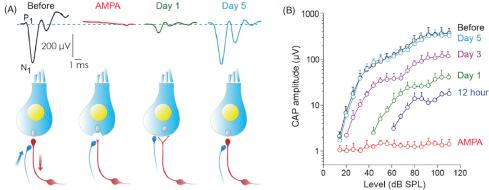
In a mouse model, acoustic trauma causes retrograde degeneration of ANFs. It could be secondary when it follows IHC loss or primary as a result of damage of specific ribbon synapses in the IHCs ( ). The first affected synapses are those with high-threshold, low-SFR, nerve fibers. The ANF degeneration starts when the peripheral dendrites to the IHCs are irreversibly damaged ( ). The integrity of its peripheral dendrite, and likely also the ribbon synapse, seems to be essential for the neuron to survive ( ). Without the peripheral dendrite synapsing on the IHCs there is no action potential activity in the ANFs and that may be required for their viability. ANFs with elevated thresholds at the characteristic frequency as a result of noise exposure in general have frequency-tuning curves with an abnormal shape ( ). A strong correlation was found between cochlear regions with fused IHC stereocilia and ANF regions with V-shaped tuning curves and a reduction in SFR. Selective damage to the OHCs did not reduce the SFR, indicating that SFR in ANFs only depends on spontaneous transmitter release by the IHCs.
The noise level and exposure durations that produce a PTS and structural changes are more extensively discussed in Chapter 6 , Causes of Acquired Hearing Loss. Besides inducing a loss of sensitivity for sound, as reflected in the audiogram, there are also changes in the CNS. I reiterate here ( ) that most studies on PTS in the central auditory system have been based on recordings from the brainstem (medulla, olive, pons) and midbrain. Adult chinchillas exposed to an octave-band noise centered at 4 kHz for 105 min at a level of 108 dB SPL ( ) showed besides hair cell damage also partial deafferentation of the ipsilateral cochlear nucleus (CN). Most of this loss resulted subsequently to the degeneration of the ANFs. New growth of axons and axonal endings was observed in the CN following the deafferentation. Sixteen days following this exposure, the chinchillas showed transient axonal degeneration in the dorsal CN that was no longer visible at longer survival times. Meanwhile, ANF degeneration continued to extend basally in the cochlea, and 2 weeks to 2 months later was followed by spread of axonal degeneration into the corresponding high-frequency region of the ventral cochlear nucleus (VCN). Following a 3 h exposure to the same sound, there was degeneration of hair cells and ANFs ( ). Secondary synaptic degeneration in the VCN was visible 1–16 weeks after the trauma. After several months, however, all these changes reversed and eventually the ANF endings again showed a normal appearance ( ). For periods of 6 and 8 months after this single exposure they observed a chronic, continuing process of neurodegeneration involving excitatory and inhibitory synaptic endings. This was accompanied by newly formed synaptic endings, which repopulated some of the sites vacated previously by axosomatic endings on globular bushy cells in the anterior part of the VCN. Noise-induced hearing loss (NIHL) thus resembles in part a neurodegenerative disease with the capacity for synaptic reorganization within the CN ( ). After noise exposure and recovery for up to 32 weeks, neuronal cell bodies lost both excitatory and inhibitory endings at first and later recovered a full complement of excitatory but not inhibitory terminals ( ). In addition to changes in the CN, there was transsynaptic terminal axonal degeneration in nuclei of the superior olivary complex, lateral lemniscus, and inferior colliculus (IC) ( ). A consequence of these subcortical structural changes may be cortical tonotopic map reorganization. have shown that a high-frequency HL in humans causes atrophy in prefrontal cortex. Typically, high-frequency HL is also accompanied by increased SFR from the CN to auditory cortex and includes increased neural synchrony, and tonotopic map reorganization in primary auditory cortex ( ).
Lower level exposures often lead to TTSs ( Fig. 4.1 ). Using confocal imaging of the inner ear in the mouse, showed that acoustic overexposure for 2 h with an 8–16 kHz band of noise at 100 dB SPL caused a moderate, but completely reversible, threshold elevation as measured by ABR. They observed no permanent changes in the otoacoustic emissions, which indicated that the exposure left OHCs, and therefore likely the less susceptible IHCs as well, intact. Despite the normal appearance of the cochlea and normal ABR thresholds there was an acute loss of the ribbon synapses located on the medial side of the IHC that are connected to high-threshold, low-SFR ANFs. This corresponded to the loss of suprathreshold ABR wave-1 amplitude for frequencies that showed a TTS. After some delay, this was followed by a progressive diffuse degeneration of the auditory nerve for these TTS frequencies. Likely consequences following TTS in humans, where clinical audiograms are normal, are difficulties understanding speech particularly in background noise, because of the loss of the high-threshold, low-SFR fiber activity, which is difficult to saturate by noise.
Recently, showed that repeated TTS noise exposures (also 8–16 kHz noise presented at 100 dB SPL for 2 h) in initially 4-week-old CBA/CaJ mice do affect permanent hearing thresholds (HTs). Although ABR thresholds recovered fully in once- and twice-exposed animals, the growth function of ABR wave-1 amplitude (CAP activity) was significantly reduced at high SPL. However, a third dose of the same noise exposure resulted in a PTS. The pattern of PTS resembled that of age-related HL, i.e., high-frequency hearing impairment. found that threshold elevation at the frequency locus matched with synaptic ribbon loss in the IHCs. They suggested that accumulation of afferent synapse damage over time with recurrent noise exposures could be a major contributor to age-related hearing impairment. Recently, showed that stimulation at 94 dB SPL (2 h with an 8–16 kHz band of noise) did not result in significant synaptic ribbon loss. However, it cannot be excluded that repeated exposure at this level could cause ribbon loss, or even PTS.
reported that at least 4 months of passive exposure of adult cats to a 4–20 kHz tone pip ensemble presented at 80 dB SPL, termed an enhanced acoustic environment (EAE), decreased the responsiveness of primary auditory cortex to sound frequencies in the exposure band, and increased the responsiveness to frequencies at the outer edges of the band. The reduced responsiveness to the EAE frequencies may be partially the result of habituation (see chapter: Brain Plasticity and Perceptual Learning ). The increased responsiveness to frequencies above and below the EAE frequency band may result from reduced lateral inhibition provided by the neurons responding to the EAE frequencies. No HL as measured by ABR was found. Recordings of the SFR in control and EAE cats were split into three groups according to the location of the recording electrodes along the posteroanterior axis: posterior (<10% of the posteroanterior sulci distance), middle (10–70% of the posteroanterior sulci distance), and anterior (>70% of the posteroanterior sulci distance). These locations correspond to regions with CFs of less than 4, 4–20, and more than 20 kHz, respectively. found that the “SFR was not significantly different between control and EAE cats for recordings with CFs normally corresponding to the EAE spectrum (middle area). On the other hand, the SFR was significantly increased for recordings normally corresponding to CFs of less than 5 kHz (posterior area) and more than 20 kHz (anterior area).” During 15 min periods of silence, also recorded the spontaneous multi-unit activity (MUA) from pairs of electrodes and then calculated the neural synchrony (peak cross-correlation coefficient; ) between them. found that the synchrony strength was lower in control cats ( Fig. 4.4A ) than in EAE cats ( Fig. 4.4B ) in posterior, middle, and anterior areas. Even the local neural synchrony (i.e., for electrode distances of ≤1 mm) was significantly increased in EAE cats as compared with control cats throughout the entire mapped area. In addition, in EAE cats, the neural synchrony was significantly enhanced in the posterior and anterior areas of A1 as compared with the middle area.
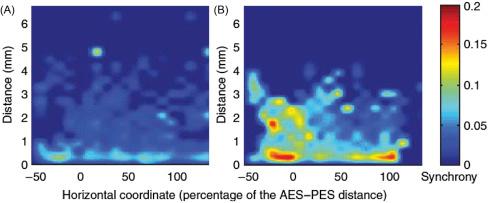
subsequently explored the effects of “safe” noise exposures, i.e., considerably below the 80 dB(A), 8 h equivalent, but with longer durations (in the “?” region of Fig. 4.1 ). They demonstrated qualitatively similar plastic changes for a 6-week EAE exposure at a level of 68 dB SPL. Again, no peripheral HL was induced by the exposure, and the resulting reorganization of the A1 tonotopic map occurring mostly during the 8- to 12-week recovery period (and much less during the exposure) resembled that following a restricted lesion of the sensory epithelium. They concluded that exposure-induced effects were present in the thalamus, as deduced from changes in short-latency, sound-evoked local field potentials (LFPs), but were further modified in A1. In contrast to the exposure at 80 dB SPl for at least 4 months ( ), found that the SFR was not significantly changed for the EAE frequency range in exposed cats, but was significantly enhanced in the non-EAE frequency ranges as compared with controls in the same frequency range. The SFR in the EAE range was also significantly lower than in the non-EAE range in exposed cats ( Fig. 4.5A ).

also computed correlations in spontaneous spike firing between MUA pairs recorded on separate electrodes in AI. Averaged peak correlation coefficients are plotted in Fig. 4.5B as a function of the interelectrode distance, pooled in half-octave distance bins; data from control cats (2982 pairs) are shown as solid lines, data from cats exposed at 68 dB SPL and allowed no recovery (401 pairs) are shown as dashed lines, and data from cats exposed at 68 dB SPL and allowed 8–12 weeks of recovery (1250 pairs) are shown as dotted lines. As is usually observed (e.g., ), there was a decrease in the synchrony of spontaneous firing with increasing interelectrode distance in all three groups. In the shortest distance bin (0.25–0.35 mm), corresponding to the distance between nearest neighbors on the electrode arrays, the synchrony measured in both the no recovery and the 8- to 12-week recovery groups was approximately double that measured in controls ( p <10 −8 ). However, increased synchrony was observed only in the outside-EAE regions up to distances of approximately 2 mm in both exposed groups ( Fig. 4.5C ; boxes and asterisks indicate significant differences from control data, at least at p <0.05, for the no recovery and 8- to 12-week groups, respectively). Beyond 2 mm, the data were noisier (fewer pairs) and not statistically different. Importantly, the increase in synchrony showed no sign of reverting to normal 12 weeks after the cessation of exposure in either the EAE or outside-EAE regions. If one considers increased SFR and synchrony as substrates of tinnitus, one cannot escape the notion that long-term exposure to nontraumatic noise can cause tinnitus.
The effect was similar but significantly weaker following an intermittent exposure (12 h-on/12 h-off) of the same type, duration, and intensity ( ). ’s “intent with the intermittent exposure (12 h-on/12 h-off) was to simulate the alteration of noisy-work/quiet-rest environments, albeit at substantially lower intensity levels (~68 dB SPL) than are presently considered harmful to human hearing (~85 dB(A) for 8 h/day; OSHA, Standard 1926.52). The current standards for occupational noise exposure are apparently intended to just prevent permanent absolute threshold shifts ( ), though it has long been suspected that problems such as poor speech intelligibility in noise, as well as tinnitus, could arise after long-term exposure at lower intensities ( ). It may be that cortical response suppression and eventual tonotopic map reorganization, as observed in our studies, represent the neurophysiological underpinnings of such problems.”
Do these surprising findings have a bearing on potential changes in the brain occurring from daily exposure to occupational noise? found qualitatively similar effects of passive exposure occurred when the EAE presentation was limited to 12 h/day. Compared to continuous exposure at the same SPL and over a similar duration (6–12 weeks), this intermittent exposure produced a smaller decrease in AI-neuron firing rate and LFP activity in response to sound frequencies in the exposure range, and an increase in LFP amplitude only for frequencies above the exposure range. As expected at these moderate exposure levels, cortical changes occurred in the absence of concomitant HL (i.e., absolute ABR-threshold shifts). Since there was some overlap in the amount of change in neural activity between the present intermittently exposed group and the continuously exposed group, it is expected that recovery from the effects of the intermittent exposure would also take a long time.
Animal data on sound localization with normal hearing have been presented in Chapter 1 , Hearing Basics. Here we first present electrophysiological and psychoacoustical data from normal hearing humans and then proceed to the effects of HL and aging.
Using magneto-encephalography (MEG) in humans to record long-latency cortical potentials, found that sounds originating from locations within the same hemifield resulted in the same N 1m , (the magnetic equivalent of the N 1 ; cf. Fig. 8.1 ) activity distribution regardless of the spatial separation between the sound sources. When sounds were presented from locations in opposite hemifields it resulted in different N 1m distributions, suggesting activation of separate groups of neurons. These results are consistent with a rate code of spatial location formed by two opponent populations, one tuned to locations in the left and the other to those in the right.
In an EEG study, also aimed at distinguishing these two dominant models of sound localization, found that the N 1 and P 2 amplitudes were highly consistent with the predictions of the opponent-channel model. They concluded that the majority of interaural time difference (ITD)-sensitive neurons—underlying the N 1 and P 2 —in each hemisphere are tuned to ITDs from the contralateral hemifield. then measured the P 1 –N 1 –P 2 complex in response to changes in sound lateralization elicited by perceptually matched changes in ITD and/or interaural level difference (ILD). The P 1 –N 1 –P 2 responses to the ITD and ILD changes showed different morphology, however, they originated from overlapping areas of the cortex and showed clear evidence for functional coupling. suggested that in auditory cortex sound laterality is coded in an integrative way, but that independent information about ITD and ILD cues is preserved.
Evidence for a third, frontal, channel had been found in psychoacoustic ITD studies with low-frequency tones ( ) and ILD studies with both high- and low-frequency tones ( ). then used a selective adaptation paradigm in combination with transposed tones to further probe for the existence of three (left, right, and midline) perceptual channels for sound source azimuth based on ITDs at high frequencies. They reported little evidence for a midline ITD channel at high frequencies.
Normal hearing listeners can localize well in both the horizontal and vertical planes based on the binaural difference cues, ILDs and ITDs. The smallest error for broadband sounds in a study by was 2° in the front and 9° at 60° azimuth in a two-dimensional head pointing task. However, controversy exists regarding how well hearing-impaired listeners can localize sound. Vertical localization and front-back discrimination rely on high-frequency spectral cues (>5 kHz) that are created by reflection and diffraction of sound by the external ear and, in particular, the pinna. These cues are often referred to as “monaural spectral cues” since the analysis depends only on a signal being present in one ear, although the spectral cues would be available to both ears. Listeners with high-frequency HL have more difficulty than normal hearing listeners when tested in the sagittal plane and with elevation ( ).
Binaural performance can vary markedly across subjects. Subjects with unilateral or asymmetric losses tend to show larger than normal thresholds for detecting ITDs and ILDs. Subjects with symmetrical losses sometimes show normal or near-normal localization for broadband noise stimuli. However, they often show impaired performance for narrowband stimuli ( ). He mentioned that carried out speech-in-noise experiments using 17 subjects with mild to moderate symmetrical HLs and 17 subjects with mild to moderate asymmetrical losses. For speech and noise presented at 0° azimuth, the speech reception thresholds (SRTs) were approximately 2.5 dB higher than found for normally hearing subjects. However, when the noise azimuth was changed to 90° and the speech was still presented at 0°, the hearing-impaired subjects showed SRTs that were 5.1–7.6 dB higher than normal. In summary, hearing-impaired subjects tested under conditions where speech and noise are spatially separated, perform more poorly, relative to normal, than when the speech and noise come from the same position in space. The disadvantage appears to arise mainly from the inaudibility of high frequencies in the ear at which the speech-to-noise ratio is highest ( ).
described potential origins of these localization deficits: (1) The relatively low SL of the stimuli because ITD discrimination in normally hearing subjects worsens markedly below about 20 dB SL; (2) differences in travel time or phase of spike initiation between the two ears; (3) abnormalities in phase locking. Abnormalities in ITD discrimination may occur as well, due to: (1) relatively low SL of the stimuli, (2) abnormal intensity coding, and (3) differences in intensity coding at the two ears. Some people with cochlear damage have essentially no ability to use spectral cues provided by pinna transformations to assess sound elevation.
Compared with young human adults, older adults typically localize sound sources less accurately. reported that: “Findings underscore the distinct neural processing of the auditory spatial cues in sound localization and their selective deterioration with advancing age.” In contrast, “found similar localization abilities in azimuth for all listeners, including the older adults with high-frequency hearing loss.” According to a review by : “This contradiction could reflect stimulus or task differences between the two studies, or it could reflect differences in the participant groups.” With respect to age, in the study the older adults were 70–81 years of age, whereas in the study they were 63–80 years of age. High-frequency HLs also began on average at a lower frequency in the population than in the group. Thus, it could be HL as well as age that underlie the localization problems.
Animals often use acoustic signals to communicate in groups or social aggregations in which multiple individuals signal within a receiver’s hearing range. Consequently, receivers face challenges related to acoustic interference and auditory masking that are not unlike the human cocktail party problem, which refers to the problem of perceiving speech in noisy social settings. ( )
One well-known example of difficulties that humans face in auditory scene analysis ( ) is described as the “cocktail party problem” ( ). This refers to the difficulty that even normal hearing persons sometimes have to understand speech in noisy social settings. studied how one can distinguish one speaker among a multitude of others in a crowded noisy scene such as a cocktail party. He asked: “On what logical principles could one design a machine whose reaction, in response to speech stimuli, would be analogous to that of a human being? How could it separate one of two simultaneous spoken messages?” described two distinct challenges for a listener in a “cocktail party” situation. The first is the problem of sound segregation. The auditory system must derive the properties of individual sounds from the mixture entering the ears, i.e., through a binaural filter to assess spatial proximity. The second challenge is that of directing attention to the sound source of interest while ignoring the others, i.e., an attention filter, and of switching attention between sources, as when intermittently following two conversations. Most of our cognitive processes can operate only on one thing at a time, so we typically select a particular sound source on which to focus. This process affects sound segregation, which is biased by what we attend to.
In a recent study, used 21 and 29 Hz amplitude-modulated sounds and recorded neural responses directly from the auditory cortex of epileptic patients. They found that different mechanisms were involved in the segregation or grouping of overlapping components as a function of the acoustic context. In their study, sound onset asynchrony was manipulated to induce the segregation or grouping of two concurrent components. This was done by starting the 21 Hz component 800 ms before the 29 Hz component (pitch continuity of the 21 Hz component), resulting in the perception of two streams. When the 21 and 29 Hz components were started synchronously this led to the percept of one sound. According to the authors, synchronization of transient responses could account for grouping of overlapping auditory components.
Considering the effects of HL on spatial filtering, recognized that the process of understanding speech in simultaneous background noise involves separating the acoustic information into discrete streams. However, they found no significant relationship between spatial-processing ability and aging. In addition, no significant relationship was found between cognitive ability and spatial processing.
Recently, I summarized the cocktail party problem as: “It is amazing that in a noisy, multiple-people-talking environment, listeners with normal hearing can still recognize and understand the attended speech and simultaneously ignore background noise and irrelevant speech stimuli. … Temporal structure has a key role in stream segregation. Timing synchrony of frequency partials allow fusion into a more complex sound, and if the frequency partials are harmonic the fusion is more likely. In contrast, timing asynchrony is a major element to distinguish one stream from two streams. Animal experiments have highlighted the role of temporal aspects by comparing behavioral data and recordings from the forebrain in the same species. Feature dependent forward suppression in auditory cortex may underlie streaming. Modeling studies suggest that stream formation depends primarily on temporal coherence between responses that encode various features of a sound source. Furthermore, it is postulated that only when attention is directed towards a particular feature (e.g. pitch) do all other temporally coherent features of that source (e.g. timbre and location) become bound together as a stream that is segregated from the incoherent features of other sources” ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here