Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Gynecologic procedures are among the most frequent surgical procedures done in the world. It behooves any surgeon to be familiar with the anatomy of the female reproductive tract and pelvis and with the most common gynecologic surgical diseases and procedures for these diseases. This chapter provides an overview of female reproductive tract and pelvic anatomy; discusses the most common surgical diseases of the vulva, vagina, cervix, uterus, fallopian tubes, and ovaries; and describes commonly utilized surgical procedures for these diseases.
Key components of the female reproductive tract anatomy include external reproductive anatomy (vulva) and internal reproductive anatomy (vagina, cervix, uterus, fallopian tubes, and ovaries). Other relevant pelvic anatomy components include anatomic spaces, vascular and neurologic structures, and urologic and intestinal structures. The following section provides an overview of these key components.
The main external structures of the vulva are the mons pubis, the labia majora and labia minora, the clitoral glans and clitoral hood, the urethral meatus, the vaginal introitus and hymen, and Bartholin and Skene vestibular glands ( Fig. 71.1 ). The mons pubis is the soft fatty tissue covering the pubic bone. The lower part of the mons pubis is divided by a fissure named the pudendal cleft, which separates the mons pubis into the labia majora. The labia majora and labia minora are separated by a groove called the interlabial sulci, and the labia minora fuse anteriorly to form the clitoral hood (also known as the prepuce).
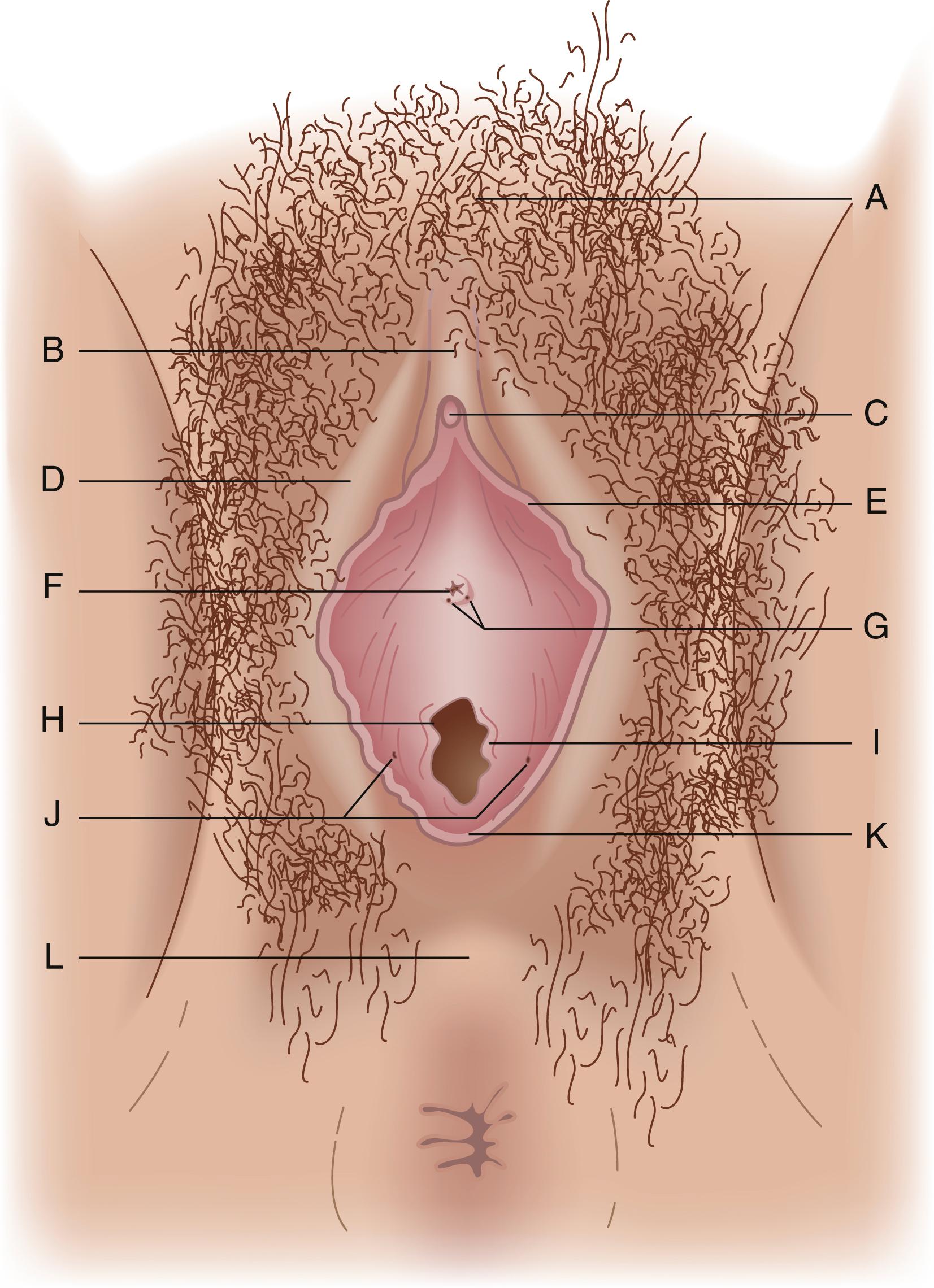
The labia cover the vestibule, the area of the vulva consisting of the vaginal introitus and urethral meatus, and is demarcated by the Hart line. The vaginal introitus is initially covered by a thin membrane called the hymen, which is usually ruptured by exercise, during insertion of tampons, or during the first episode of intercourse. Within the vestibule are Bartholin glands and Skene glands. Bartholin glands, homologues of the bulbourethral glands in males, are located posteriorly to the right and left of the vaginal introitus. These glands secrete mucous during sexual arousal to provide vaginal lubrication. Skene glands, homologues of the prostate gland in males, are located to the right and left of the urethral meatus. These glands also produce secretions that provide vaginal lubrication with sexual arousal.
These external structures are part of the urogenital triangle, which is the anterior portion of the perineum. The urogenital triangle has its apex anteriorly at the symphysis pubis and its base posteriorly formed by a line drawn between the ischial tuberosities. Beneath the aforementioned external structures lies the urogenital diaphragm, a fascial and muscular shelf extending between the pubic rami and penetrated by the urethra and vagina. The muscles of the urogenital triangle consist of the deep and superficial transverse perineal muscles, paired ischiocavernosus muscles that cover the crura of the clitoris, and bulbocavernosus muscles covering erectile vestibular bulbs that lie on either side of the vaginal introitus.
Blood supply to structures within the urogenital triangle is predominantly from a posterior direction from the internal pudendal artery, which, after arising from the internal iliac artery, passes through Alcock canal, a fascial tunnel along the obturator internus muscle below the origin of the levator ani muscle. On emerging from Alcock canal, the internal pudendal artery sends branches to the urogenital triangle anteriorly. The blood supply to the mons pubis originates anteriorly from the inferior epigastric artery, a branch of the femoral artery. Laterally, the external pudendal artery arises from the femoral artery and supplies the lateral aspect of the vulva. Venous return and lymphatic drainage from the urogenital diaphragm accompany the arterial supply and therefore drains into the internal iliac and femoral veins.
The major nerve supply to the urogenital triangle comes from the internal pudendal nerve, which originates from the S2 to S4 anterior rami of the sacral plexus and travels through Alcock canal along with the internal pudendal artery and vein. Anterior branches supply the external genitalia. The mons pubis and anterior labia are supplied by the ilioinguinal and genitofemoral nerves from the lumbar plexus that travel through the inguinal canal and exit through the superficial inguinal ring. All these paired nerves routinely cross the midline for partial innervation of the contralateral side. The visceral efferent nerves responsible for clitoral erection are derived from the pelvic splanchnic nerves and reach the external genitalia along with the urethra and vagina as they pass through the urogenital diaphragm.
It is important for the surgeon dissecting the external genitalia to be cognizant of the variability of vascular and neurologic direction from which the blood and nervous supply of the operative field is derived.
Beginning most distal and moving proximal, the internal reproductive anatomic structures include all midline structures (the vagina, cervix, and uterus) and the lateral structures (the fallopian tubes and ovaries ( Fig. 71.2 ). The borders of the vagina are the hymen inferiorly and the cervix and fornices superiorly. The lower portion of the vagina develops from the endoderm of the urogenital sinus (along with the urethra and vulvar structures). Abnormalities of development in this area can lead to transverse or horizontal septae of the vagina, which may become symptomatic during pubertal development. The upper portion of the vagina develops in tandem with the cervix, uterus, and fallopian tubes from the Müllerian ducts. Failure of fusion of these ducts as they migrate medially or failure of development completely can lead to a variety of uterine, cervix, and tube malformations.
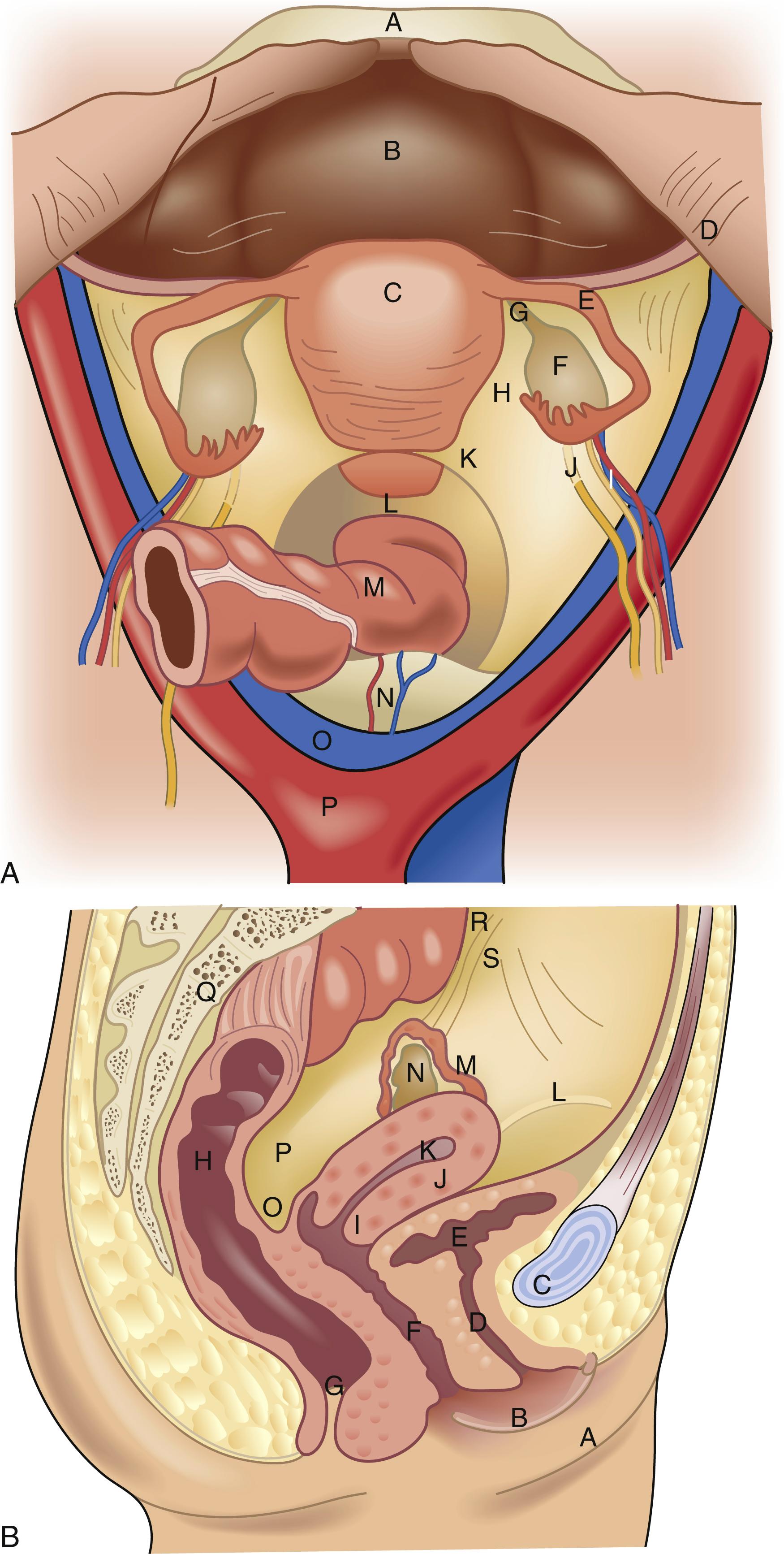
The vagina is a flexible, expandable fibromuscular tube, which, at rest, is flattened and lies in a mostly horizontal plane if the woman is in an upright posture. The layers of the vaginal walls from central to peripheral include the mucosa (which is stratified squamous epithelium), the lamina propria (also called the endopelvic fascia, which mostly consists of collagen and elastic tissue and containing the vascular and lymph supply), a muscular layer, and areolar connective tissue (which also contains a rich blood supply). The blood supply to the vagina comes mainly from the vaginal artery, a branch of the internal iliac artery. There are multiple anastomoses with other arteries, including the uterine, internal pudendal, inferior vesical, and middle hemorrhoidal arteries. The nerve supply to the vagina originates from the autonomic nervous system in the lumbosacral plexus (S2–S4), culminating in the pudendal nerve. The majority of sensory innervation lies in the distal portion of the vagina, with very little nerve density in the upper part of the vagina.
The cervix is the narrow, distal part the of uterus that can be visualized and palpated at the upper end of the vaginal canal. It is a round, often donut-like structure composed of mostly fibrous tissue and can vary in size from one female to another. The length of the cervix also varies but averages around 3 cm. It has a central canal, referred to as the os, which allows passage into or egress from the uterine cavity. The cells on the vaginal portion of the cervix (ectocervix) transition from squamous epithelium distally to columnar epithelium proximally as you travel up into the cervical canal (endocervix). This change is referred to as the transition zone and is where cervical intraepithelial neoplasia (CIN) occurs. The columnar epithelium produces mucous, which varies in texture depending on hormone influence, and facilitates sperm transport.
The blood supply of the cervix arises from a descending branch of uterine artery and lies laterally at the 3 and 9 o’clock positions on the cervix. Additionally, the azygos arteries lie in the middle portion of the cervix anteriorly and posteriorly along its axis. There are multiple anastomoses between the azygos arteries and the hemorrhoidal arteries. The cervix is innervated by the parasympathetic system, arising from lumbosacral plexus (S2–S4) with the majority of nerve endings concentrated in the endocervical region.
The uterus is an intraperitoneal muscular organ that sits posterior to the bladder and anterior to the rectum. It is held in place by several ligamentous structures. The broad ligaments extend laterally off the uterine corpus and become continuous with the pelvic peritoneum. The round ligaments originate at the uterine cornua and travel laterally through the broad ligament and exit through the inguinal ring, terminating in the labia majora. The cardinal ligaments, which attach laterally to the pelvic diaphragm and fuse medially with the vaginal endopelvic fascia, support the uterus at the level of the cervix. The uterine arteries travel within the cardinal ligament and then superiorly along the lateral aspect of the uterine body, also called the fundus. The uterosacral ligaments originate at the upper posterior cervix and attach to the third sacral vertebra, forming an arch that frames the rectum.
The nonpregnant uterus typically weighs between 40 and 80 g. It is often smaller in prepubertal and postmenopausal women compared with those in the reproductive years. The uterus has three cell layers, similar to other viscous peritoneal organs. The outer layer is called the serosa, is very thin, and is contiguous with the broad ligaments and pelvic peritoneum. The middle layer is composed of smooth muscle, which lies in three distinct layers. The uterine cavity is lined with endometrium, a mucous epithelial layer that varies in thickness depending on hormonal influences.
The blood supply to the uterus includes both the uterine arteries, which branch off the internal iliac arteries, and the ovarian arteries, which come directly off the abdominal aorta and travel adjacent to the ovary toward the uterus. The uterus has both sympathetic and parasympathetic innervation. The sympathetic innervation travels via the hypogastric and ovarian plexus. The parasympathetic innervation comes from the lumbosacral plexus (S2–S4). Afferent fibers from the uterus returning to the spinal cord travel with the sympathetic innervation within the lumbosacral plexus (T11–T12).
The fallopian tubes, also referred to as the oviducts, originate at the upper lateral aspect of the uterus (called the cornua) and extend laterally approximately 10 to 14 cm in length, coiling around the ipsilateral ovary distally. The fallopian tube has four distinct anatomic sections. The interstitial part traverses the uterine wall, is completely bordered by myometrium, and is only 1 to 2 cm in length. The isthmus portion begins as the tube leaves the uterus, measures around 4 cm in length, is very narrow, and contains the most muscular region of the tube. The ampullary region is wider, averages 4 to 6 cm in length, and has a winding course. Fertilization typically occurs in this portion of the fallopian tube. The final segment is the infundibulum, which is mostly composed of fimbriae—fingerlike projections that extend out from the tube, surround the ostia, and cause this portion of the tube to have a funnel-like shape.
Similar to the uterus, the fallopian tubes are composed of several layers. The mucosa of the tube is made up of different types of epithelial cells—columnar ciliated, secretory, and narrow peg cells. The ratio of these cell types depends on the anatomic region of the tube. Under the mucosa is the lamina propria, followed by the muscular layer, and finally the adventitial layer, which is adjacent to the peritoneal cavity.
The blood supply to the fallopian tube travels through the mesosalpinx and originates from branches of the ovarian and uterine arteries. The tubes receive innervation from both the sympathetic and parasympathetic nervous systems via the uterine and ovarian plexuses.
The ovaries are paired, oval-shaped organs lateral in location and white, usually around 2 to 3 cm in largest diameter. They are located just inferior to the pelvic brim at the infundibulum-end of the fallopian tubes. The ovaries develop from the gonadal ridge, which sits adjacent to the mesonephric duct; thus, the urinary system and reproductive system develop in close association. The ovaries are connected to the uterus via the uteroovarian ligament, which contains an anastomotic blood supply between these two structures. Additionally, the ovaries are held in place by the posterior leaf of the broad ligament and the infundibulopelvic ligament, which travels down the lateral sidewall and contains the major ovarian blood supply.
The ovary has three distinct sections: the outer cortex, the central medulla, and the hilum, which is where the mesoovarium (the structure that anastomoses the infundibulopelvic ligament and the periuterine blood supply via the broad ligament) attaches to the ovary. The ovaries contain several cell types that allow the ovaries to perform multiple functions. Within the ovaries are the oocytes, which number 1 to 2 million at birth. The ovary surface is composed of cuboidal epithelium. The oocytes mature inside small, fluid-filled cysts called follicles, which sit just under the surface epithelium. The central medulla is mostly made up of stroma and blood vessels.
As alluded to previously, the blood supply to the ovary comes from the ovarian artery, a branch off the abdominal aorta that travels along the lateral abdomen and pelvis in the infundibulopelvic ligament. Additionally, within the mesoovarium there are many anastomoses between the ovarian artery and the uterine artery. The innervation of the ovary also travels via the infundibulopelvic ligament and includes autonomic and sensory nerve fibers from the ovarian, hypogastric, and aortic plexuses.
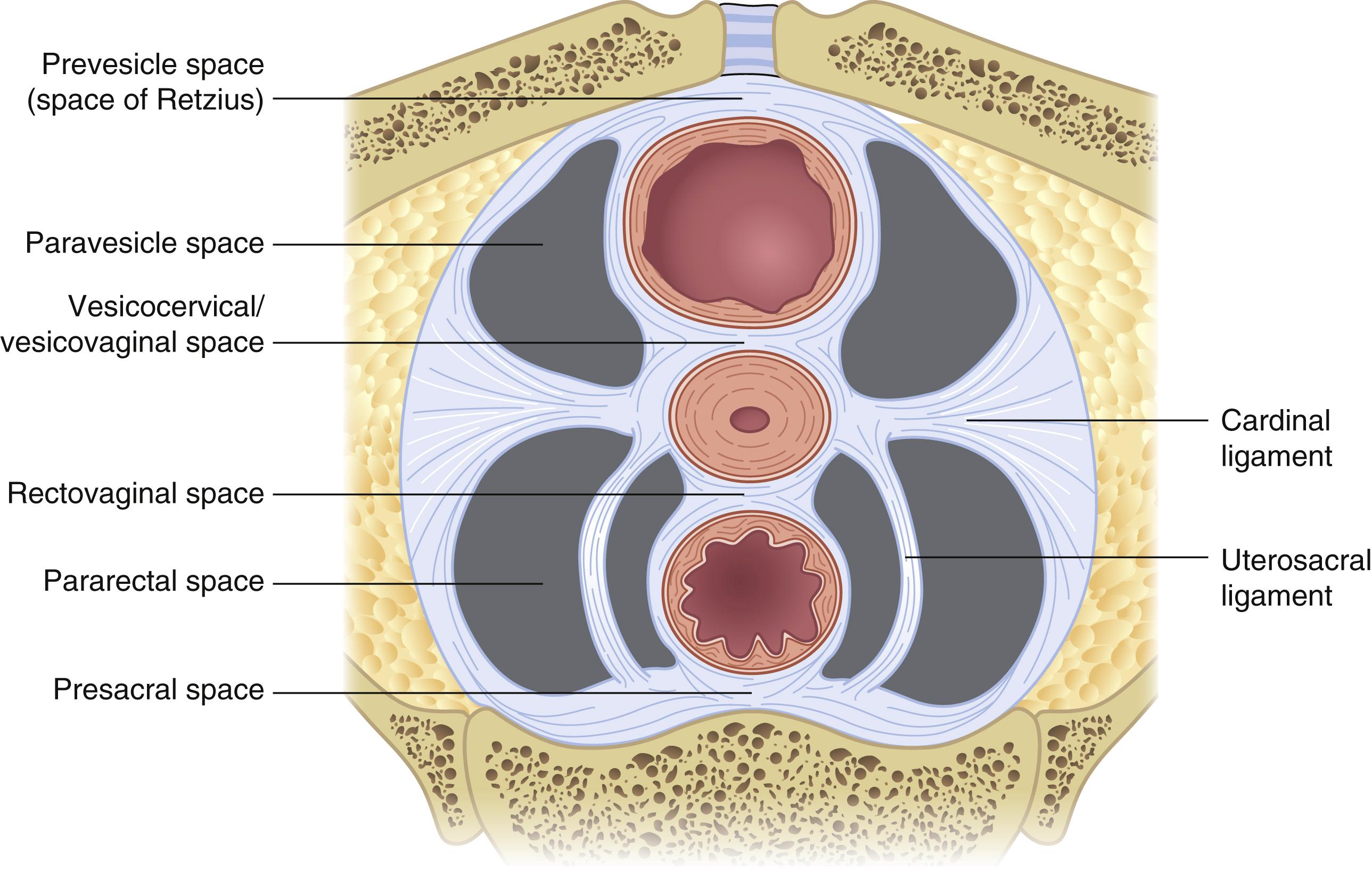
The female pelvis contains several key potential spaces, an understanding of which is required by any surgeon operating in this area ( Fig. 71.3 ). The two lateral retroperitoneal spaces include the paravesical and pararectal spaces. The paravesical space is bordered by the external iliac artery laterally, the bladder medially, the pubic symphysis anteriorly, and the cardinal ligament posteriorly. The space is entered by dissecting between the external iliac artery and superior vesical artery. The pararectal space is bordered by the internal iliac artery laterally, the ureter and rectum medially, the sacrum posteriorly, and the cardinal ligament anteriorly. The space is entered by dissecting between the vessels laterally and the ureter and rectum medially. The cardinal ligaments separate the paravesical and pararectal spaces. Key anterior to posterior spaces include the retropubic, vesicovaginal, rectovaginal, and retrorectal/presacral spaces. Developing these spaces facilitates identification of critical pelvic structures, particularly when normal anatomy is altered.
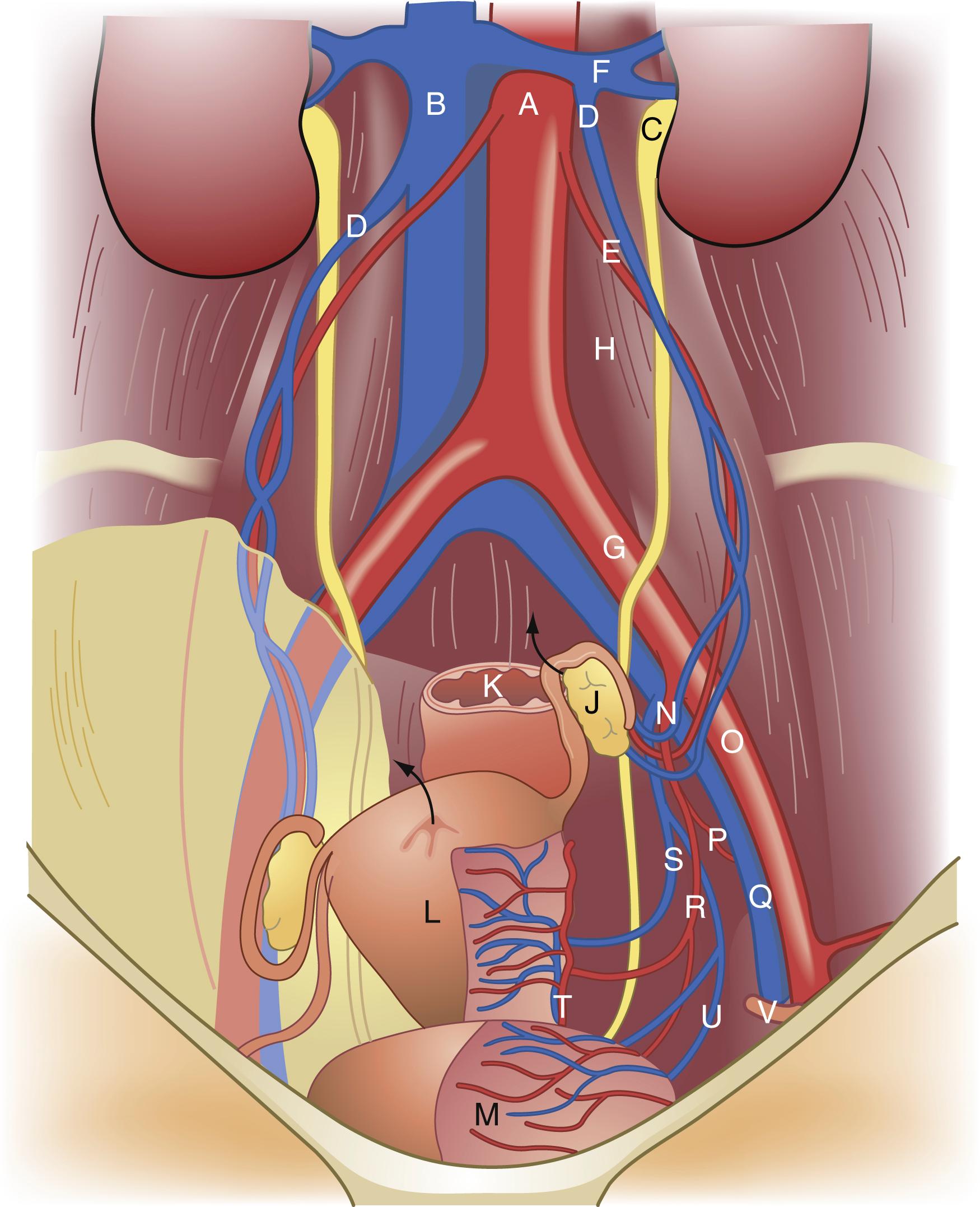
It is important for surgeons to be very familiar with the vascular anatomy of the pelvis when performing gynecologic procedures ( Fig. 71.4 ). The common iliac vessels, bifurcate at the pelvic brim into the external iliac and internal iliac vessels, which course along the pelvic sidewalls. The external iliac vessels course under the inguinal ligament to become the femoral artery and vein. The internal iliac artery bifurcates into an anterior and posterior division. The female pelvic structures derive the majority of their arterial vascular supply from vessels that branch from the anterior division. Ligation of the anterior division distal to where the posterior division branches is a technique often utilized in the setting of excessive pelvic hemorrhage. Key branches of the anterior division include the uterine and vaginal arteries the inferior, middle and superior vesical arteries and the middle rectal artery. Other key branches off the anterior division include the obturator, inferior gluteal, and pudendal arteries. The ovarian arteries, which originate from the aorta, provide another major vascular source to the pelvic structures, namely the ovaries and uterus.
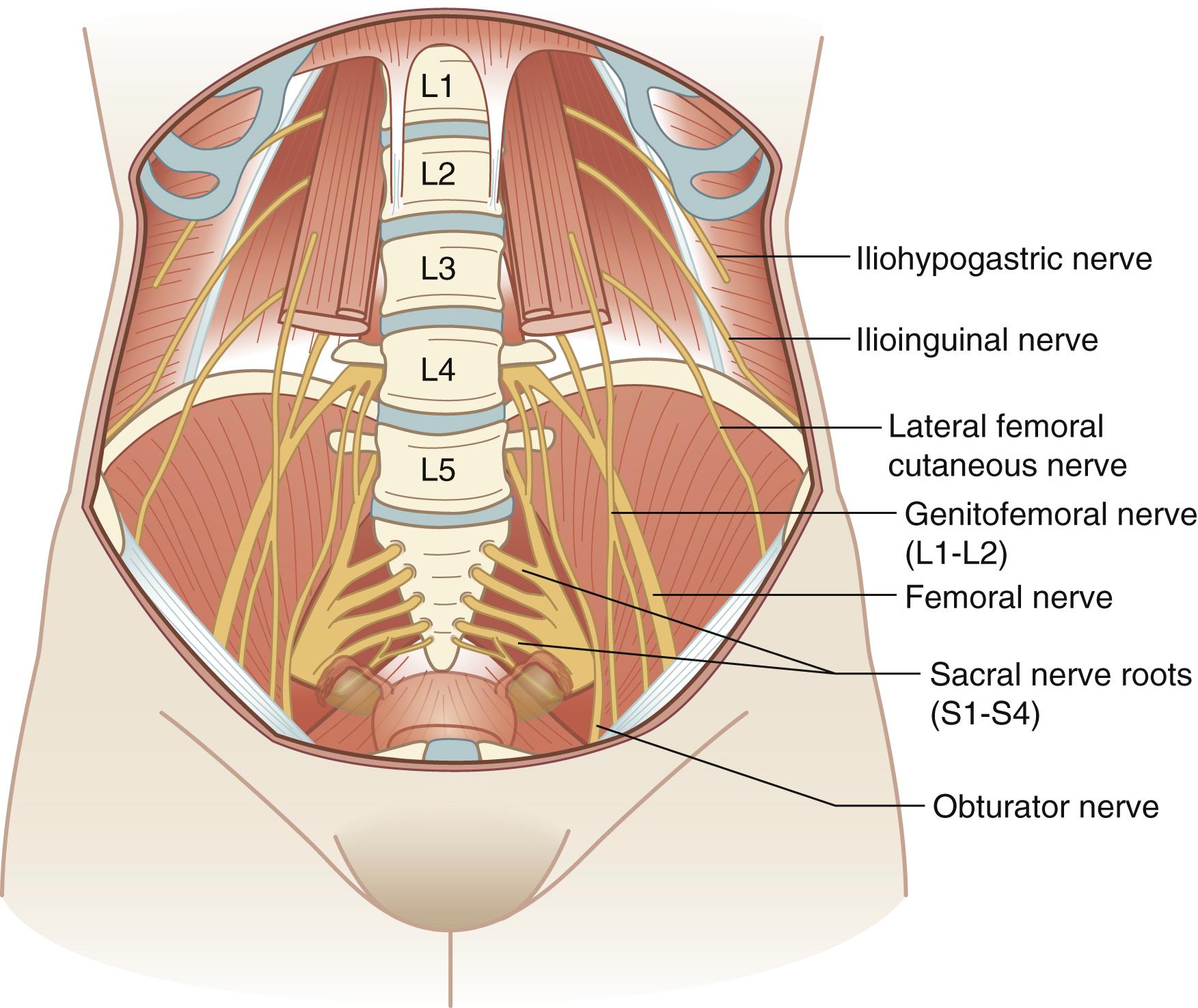
The lumbosacral nerve plexus, which originates from nerve roots from the twelfth thoracic vertebral body to the fourth sacral vertebral body (T12–S4), provides the major nerve structures in the pelvis ( Fig. 71.5 ). The primary motor nerves emanating from the lumbosacral nerve plexus include the femoral nerve, the sciatic nerve, and the obturator nerve. The primary sensory nerves include the iliohypogastric nerve, the ilioinguinal nerve, the genitofemoral nerve, the lateral femoral cutaneous nerve, the femoral nerve, the sciatic nerve, and the pudendal nerve. A description of these nerves, their origin, their motor and sensory functions, and symptoms when injured is included in Table 71.1 .
| Nerve | Origin | Motor Function | Sensory Function | Injury Symptoms |
|---|---|---|---|---|
| Ilioinguinal | T12–L1 | None | Groin, symphysis | Sharp, burning pain radiating from incision site to groin or symphysis |
| Iliohypogastric | T12–L1 | None | Mons, lateral labia, upper inner thigh | Sharp, burning pain radiating from incision site to mons, labia, or thigh |
| Genitofemoral | L1–L2 | None | Upper labia, anterior superior thigh | Pain or paresthesia labia and femoral triangle |
| Lateral femoral | L2–L3 | None | Anterior and posterior lateral thigh | Pain or paresthesia anterior and posterolateral thigh |
| Pudendal | S2–S4 | None | Perineum | Perineal pain |
| Cutaneous femoral | L2–L4 | Hip flexion, adduction Knee extension |
Anterior and medial thigh, medial calf | Unable to climb stairs |
| Obturator | L2–L4 | Thigh adduction | None | Minor ambulatory problems |
| Sciatic Common perineal Tibial |
L4–S3 | Hip extension, knee flexion Foot dorsiflexion Foot eversion Foot plantar flexion Foot inversion |
None Lateral calf Dorsum of foot Toes Plantar foot surface |
Foot drop Cavus deformity foot |
Nerve injuries occur in 1% to 2% of gynecologic cases. The most common nerves injured during gynecologic surgery include the femoral, ilioinguinal, pudendal, obturator, lateral cutaneous, iliohypogastric, and genitofemoral nerves. These nerves can be injured as a result of malpositioning the patient in the lithotomy position, incorrect placement of self-retaining retractors, nerve transection, direct nerve entrapment, or hematoma formation. Fortunately, the majority of neuropathies resolve with conservative management and physical therapy.
The ureters and bladder are key pelvic structures that require strict attention during the course of most gynecologic procedures. The ureters course retroperitoneally down the lateral pelvic walls and then cross over the common iliac arteries at the pelvic brim ( Fig. 71.2 ). At this point, the ureters are in close proximity to the ovaries and care must be taken to properly identify and avoid the ureters during ligation of the ovarian vessels. The ureters continue along the peritoneum under the uterine arteries and then traverse into the bladder laterally in an oblique fashion. The ureter is also at risk for damage during clamping, incision, and ligation of the uterine vessels, the cardinal and uterosacral ligaments, and the vaginal corners. Dissecting the ureter off its attachment to the peritoneum and isolating it with a vessel loop is a technique often useful when pathology places the ureter at risk for injury.
The bladder is intimately involved with the anterior uterus, cervix, and vagina. The bladder must be carefully dissected off these structures during a hysterectomy. This often involves incision of the vesicouterine peritoneum and identifying the vesicovaginal space between the bladder and vagina. Dissection of the bladder can be made more difficult by scarring from prior Caesarean delivery, endometriosis, or cancer.
Injuries to the ureters and bladder are overall reported to occur in less than 1% of patients undergoing major gynecologic surgery. Cystoscopy is often utilized to confirm an intact bladder and bilateral ureteral efflux after gynecologic procedures that carry a high risk of urologic injury.
The distal ileum, cecum, and appendix as well as the sigmoid colon and rectum are key gastrointestinal structures located near or within the pelvis. Gastrointestinal adhesions can occur as a result of prior surgeries, and careful attention is required to lyse adhesions in this setting in order to restore normal pelvic anatomy prior to completing any gynecologic procedure. These intestinal structures can also be involved as the result of various gynecologic pathology such as tuboovarian abscesses, endometriosis, or ovarian neoplasms. It is important to carefully inspect the intestinal organs to assure no occult injury during a gynecologic procedure, which is reported to occur in less than 1% of patients. A “bubble” test, which involves insufflating the rectum with air with compression of the sigmoid and filling the pelvic with saline or water, is often useful in detecting an occult sigmoid injury.
Common vulva and vaginal surgical diseases and procedures are listed in Table 71.2 . A description of these diseases and management options follows.
| Disease | Procedure Options |
|---|---|
| Bartholin cyst/abscess | Incision and drainage Marsupialization |
| Vulva intraepithelial neoplasia | Wide local excision Laser |
| Vulvar cancer | Radical vulvectomy with sentinel node biopsy or inguinal femoral lymphadenectomy |
| Vaginal intraepithelial neoplasia | Partial vaginectomy Laser |
| Vaginal cancer | Radical hysterectomy (rarely) |
| Prolapse | Sacral colpopexy Sacrospinous ligament suspension Anterior colporrhaphy Posterior colporrhaphy Colpocleisis |
Blockage of the greater vestibular glands can result in accumulation of mucous and may lead to obstruction and abscess formation. This most commonly presents as a vulva mass on the lower medial labia major. Bartholin cysts may be asymptomatic and found incidentally on exam; however, they may become infected and form an abscess, which presents as a painful vulva mass. Patients with a symptomatic Bartholin gland cyst or abscess often require surgical intervention. Surgical management options include incision and drainage with placement of a Word catheter or marsupialization. Marsupialization is indicated for recurrent infections or when the size of abscess precludes incision and drainage. Excision of the gland is rarely indicated.
Vulva intraepithelial neoplasia (VIN) is a premalignant condition of the vulva. VIN was previously classified using a three-tier system (VIN I, II, or III). In 2015, the International Society for the Study for Vulvovaginal Disease (ISSVD) recommended using a two-tier grading system for classical VIN, which includes low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and a third category that separates VIN differentiated type. The majority of LSIL and HSIL VIN is associated with the human papilloma virus (HPV), tends to occur in younger women, and is multifocal. The pathogenesis of VIN differentiated type is less well understood but tends to be associated with dermatosis of the vulva such as lichen sclerosis, seen more commonly in older women and is not associated HPV. Women with VIN often present with vulva itching or pain, although up to 40% of women may be asymptomatic at the time of diagnosis. Management includes colposcopy and biopsy to confirm the diagnosis. LSIL may be observed or treated with topical therapy such as imiquimod while the mainstay of treatment for HSIL is either wide local excision or laser ablation. Recurrence rates after treatment are high, and therefore, women should continue to be monitored after therapy.
Carcinoma of the vulva is a rare gynecologic cancer but still significantly contributes to overall mortality from reproductive tract cancers. Squamous cell carcinoma is the most common histology. Less common histologic subtypes include verrucous, basal cell, melanoma, sarcoma, extramammary Paget, and Bartholin gland carcinoma. Risk factors for vulva cancer include smoking, HPV infection, prior VIN, prior cervical dysplasia or cancer, lichen sclerosis, and immunodeficiency. Women most commonly present with a symptomatic nodular or ulcerative vulva lesion. Treatment is based upon clinical stage. For cancers confined to the vulva, surgical resection traditionally included radical vulvectomy with bilateral inguinal femoral lymph node dissection, which can be associated with high morbidity. Less radical techniques, in particular sentinel node dissection, have subsequently been adopted with decreased morbidity while preserving oncologic outcomes. , Treatment with chemoradiation has been the preferred strategy for larger vulvar cancers or those that involve the urethral meatus, vagina, or anus.
Vaginal intraepithelial neoplasia (VaIN) is a premalignant condition of the vagina. Like VIN, it is classified using two-tier grading system LSIL (previously VaIN 1) and HSIL (previously VaIN II/III). Patients are often asymptomatic. Risk factors are similar to that for patients with VIN. Management is also similar to that for patients with VIN, albeit laser ablation is more frequently considered over partial vaginectomy for HSIL VaIN lesions.
Vaginal cancer is also quite rare. Squamous cell carcinoma is the most common histology. Less common histologic subtypes include adenocarcinoma, sarcoma, and melanoma. Risk factors other than those mentioned above for vulva cancer include diethylstilbestrol exposure in utero and prior radiation. The most common presenting symptom is vaginal bleeding followed by vaginal discharge, change in urinary symptoms or pain. Stage is the most significant prognostic feature. The majority of patients with vaginal cancer are treated with the combination of radiation and chemotherapy. Radical hysterectomy with upper vaginectomy is an option for the rare select patient with a small stage I cancer confined to the vaginal mucosa and located in the posterior vaginal fornix.
Pelvic organ prolapse is the herniation or downward displacement of the bladder (anterior vagina), cervix or vaginal cuff (apex), or rectum (posterior vagina) beyond their normal position. Risk factors include parity, age, obesity, chronic constipation, chronic obstructive pulmonary disease, connective tissue disorders, and smoking. Presenting symptoms are often pelvic pressure or bulge and/or changes in bowel or bladder function. Treatment depends on the degree of prolapse and patient symptoms. Treatment options include nonsurgical intervention including pessary, pelvic floor exercises and physical therapy, weight loss, smoking cessation, and estrogen therapy. Surgical intervention is reserved for women who have failed or declined conservative management and remain symptomatic.
Pelvic reconstructive surgery for pelvic organ prolapse is individualized with the goal to restore normal anatomy and function of the anterior, posterior, and apical vaginal compartments. The most common procedures for repair of apical prolapse are abdominal sacrocolpopexy (or its minimally-invasive laparoscopic or robotic variants), transvaginal sacrospinous ligament suspension, and transvaginal uterosacral ligament suspension. Repair of apical prolapse abdominally with sacrocolpopexy results in a lower rate of recurrence when compared to transvaginal approaches; however, it is associated with longer surgical time and delay in return to normal activities. Long-term outcomes suggest that recurrence rates are approximately 25%. There is no significant difference in long-term outcomes for uterosacral ligament suspension when compared to sacrospinous ligament suspension with reported failure rates of 62% and 70%, respectively. Women with pelvic organ prolapse often have several compartments that are prolapsed and benefit from repair that addresses each of the compartments affected. Anterior and posterior colporrhaphy are often not needed when an abdominal sacrocolpopexy is performed as repair of apical defect often corrects the anterior and posterior defects. , However, anterior and posterior colporrhaphy are more often performed when the reconstruction is performed transvaginally in conjunction with uterosacral or sacrospinous ligament suspension.
Although most cases of pelvic organ prolapse are treated with reconstructive vaginal surgery as noted above, there are some instances in which vaginal reconstruction may not be desired or an option. For women who are not candidates for surgical reconstruction, and who are no longer sexually active, obliteration of the vaginal canal can alleviate the symptoms of pelvic organ prolapse without high surgical morbidity. Additional benefits of colpocleisis are shorter operative time, low risk of recurrence and similar patient satisfaction making this an appropriate choice for older women with multiple medical comorbidities who are not sexually active.
Incision and drainage of a Bartholin gland cyst or abscess is performed by stabilizing the cyst or abscess and making an incision over the center of the cyst or abscess on the medial aspect of the lesion just outside the hymenal ring ( Fig. 71.6 ). A common mistake is to make the incision on the lateral aspect of the vulva. It may be necessary to utilize a hemostat to break up loculations and irrigate the cyst or abscess cavity. A small Word catheter is placed into the cyst for drainage and reevaluated on a weekly basis. Bartholin abscesses are often polymicrobial. Culture of purulent fluid should be obtained to evaluate for methicillin-resistant Staphylococcus aureus. Abscesses should be treated with appropriate antibiotics.
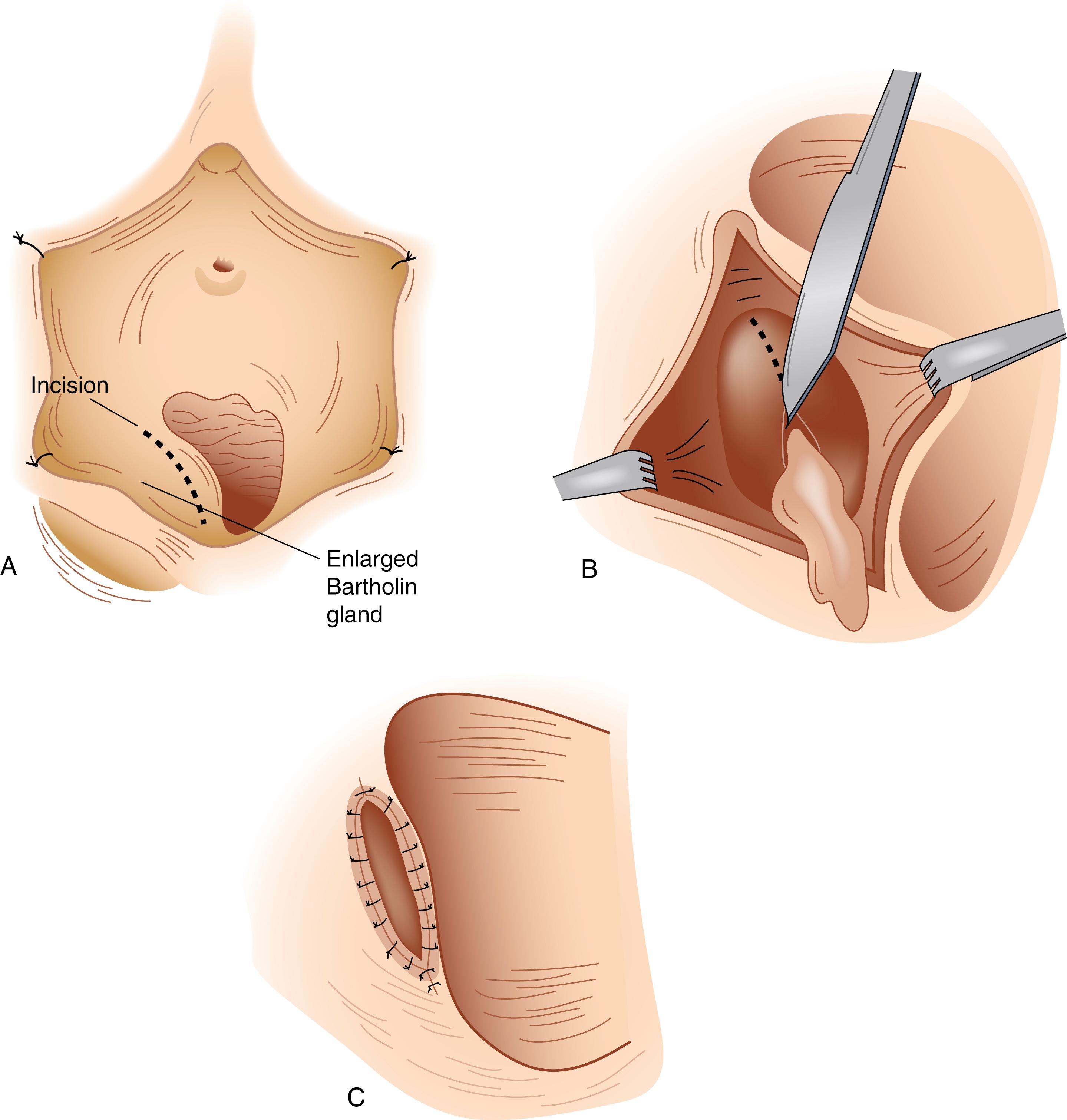
Marsupialization is performed by creating an elliptical incision in the vestibular mucosa down to the wall of the gland. The wall of the gland is incised along the entire length of the ellipse. The contents are evacuated, and the wall of the cyst is sutured to the vestibular mucosa using 3-0 synthetic absorbable sutures in an interrupted fashion or using a baseball stitch.
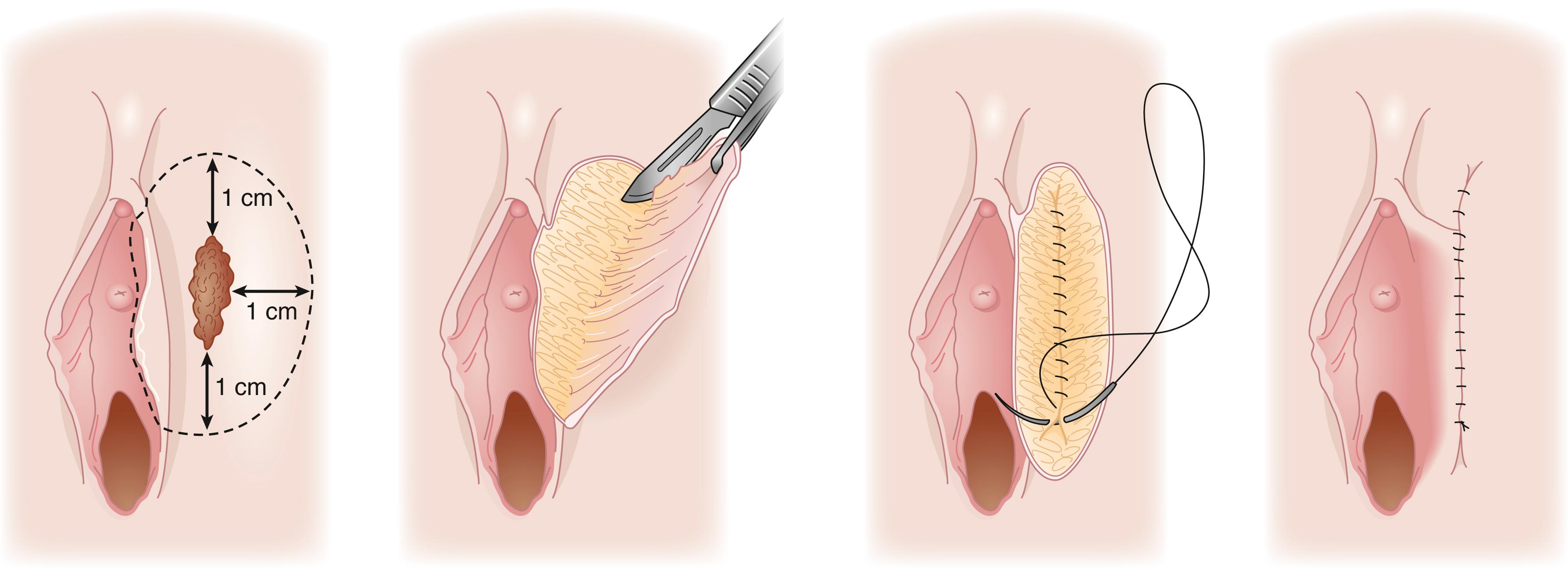
A wide local excision is a superficial excision of a vulva lesion and is most commonly utilized in the treatment of HSIL VIN ( Fig. 71.7 ). The lesion is outlined with 1 cm margins. If the lesion is in close proximity to vital structures such as anus, clitoris, or urethra smaller margins may be utilized for cosmesis or to preserve function. After making an incision along the outline, the apex of the skin is then elevated, and the epidermis is removed. The depth of incision is 2 to 3 mm. Removal of dermis is not necessary for preinvasive disease. The defect is reapproximated using running or interrupted sutures depending on surgeon preference and size of lesion.
CO 2 laser vaporization maybe utilized as alternative to surgical resection for HSIL VIN. For HSIL VIN, it is best used for patients with multifocal disease, with disease close to vital structures such as the anus, urethral or clitoris, or with extensive VIN lesions in which surgical resection would be disfiguring. CO 2 laser vaporization should be utilized only when there is a low clinical suspicion for malignancy and when vulva biopsies have confirmed no evidence of invasive cancer. A depth of 1 mm is utilized on nonhair-bearing areas of the vulva and 3 mm on hair-bearing areas of the vulva. Colposcopy is utilized to increase precision with laser therapy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here