Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The gastrointestinal (GI) tract relies on hormones and neurotransmitters to integrate signals arising in the lumen with whole-body homeostasis. For instance, satiety in the brain is, to a great extent, induced by the presence of food in the gut. This process begins with ingestion of nutrients that stimulate sensory cells in the intestinal epithelium that modulate food intake via the release of specific chemical messengers. GI hormones and neurotransmitters are intimately involved with every aspect of the digestive process including ingestion and absorption of nutrients. It is not surprising therefore that these transmitters are essential for life. In this chapter, the critical role of the regulatory transmitters in GI function is analyzed by covering the following aspects: their synthesis and secretion from sensory epithelial cells, how food or other GI luminal factors trigger their release, the most representative members, and their importance in the context of disease.
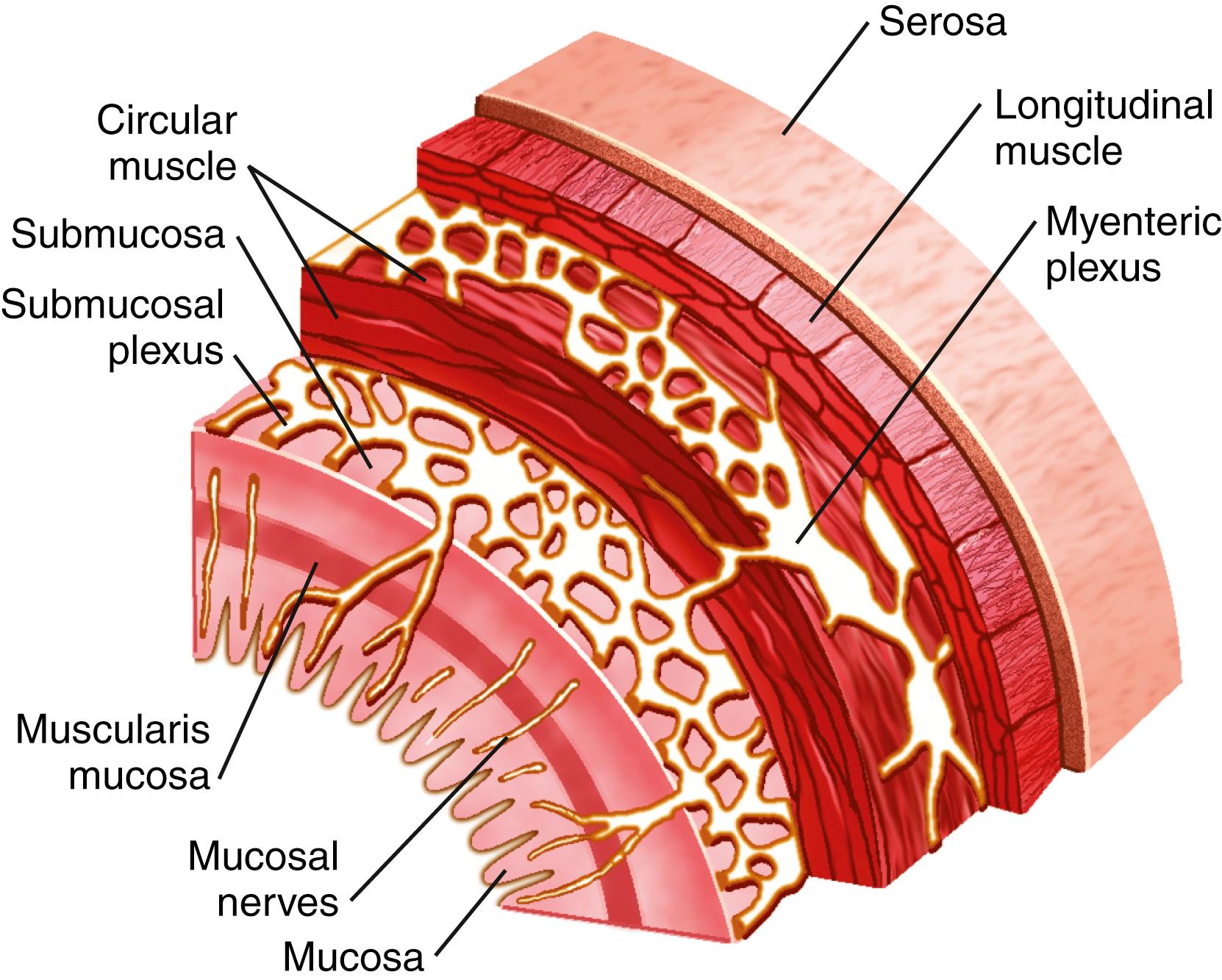
The sensory cells of the GI epithelium, enteroendocrine cells, as well as neurons of the enteric nervous system are the main producers of chemical messengers, which are released in the form of hormones or neurotransmitters. Enteroendocrine cells reside in the intestinal mucosa as single cells that are scattered among more numerous enterocytes—the absorptive cells of the gut. Most enteroendocrine cells are oriented with their apical surface open to the lumen where they are exposed to food and other contents within the gut lumen. Upon stimulation, enteroendocrine cells release from their basolateral surface hormones, which enter the paracellular space where they are taken up into the blood. In contrast to enteroendocrine cells, enteric neurons are found below the mucosal epithelium, and even though villi and crypts are richly innervated, enteric neurons are not believed to be directly exposed to food in the gut.
Unlike other endocrine organs where endocrine cells are concentrated in a single organ, the function of scattered hormone-containing cells of the GI tract has been questioned, and it becomes important to distinguish hormonal versus neuronal actions.
Criteria exist for determining if a candidate transmitter is a true hormone or a neurotransmitter. The first hormone to be discovered was secretin, when it was shown that injection of intestinal extracts into the blood stimulated pancreatic secretion. . Since then, the following criteria have been established to prove that a substance functions as a hormone. First , the stimulation of one organ must cause distant response by acting through the blood. Second , the response must be independent of neural stimulation. Third , no response should occur in the absence of the secretory organ. And fourth , the response should be reproducible by applying pure amounts of the candidate hormone onto the target tissue. There are more than 30 GI hormones that met these criteria, and their singularities are discussed in “The Transmitters” section of this chapter.
Demonstrating that a chemical is a neurotransmitter is perhaps more challenging, but the following criteria are agreed to define a neurotransmitter. First , the candidate molecule must be present within a presynaptic neuron. Second , the transmitter must be released in response to presynaptic depolarization. And, third , specific candidate-receptors must be present on the postsynaptic cell.
Hormones are commonly thought to reside exclusively in the endocrine system and neurotransmitters in the nervous system. However, these concepts were proposed when no technologies existed to visualize a single cell communicating with its surroundings. Today, it is becoming clearer that both systems are closely and synergistically related. Indeed, some cells exert both endocrine and neural actions. For example, peripheral sensory cells such as taste cells of the tongue and solitary chemosensory olfactory cells of the nose are known as paraneurons and can release both hormones in the bloodstream and neurotransmitters at synaptic connections. There is growing evidence that enteroendocrine cells have similar dual function. These observations extend the continuum between the endocrine and nervous systems.
Moreover, one transmitter can act both as a hormone or neurotransmitter depending on its location. For instance, upon the ingestion of food, cholecystokinin (CCK) is typically released from enteroendocrine cells into the bloodstream to act as a hormone. However, CCK is also abundant in nerves of the GI tract and brain, where it is released at synaptic terminals to act as a neurotransmitter. This conservation of transmitters allows the same messenger to have different physiologic actions at different locations, and is made possible by the manner in which the transmitter is delivered to its target tissues.
Enteroendocrine transmitters can be released onto their targets in the following manners: endocrine, paracrine, autocrine, or through synaptic neurotransmission ( Fig. 4.1 ).
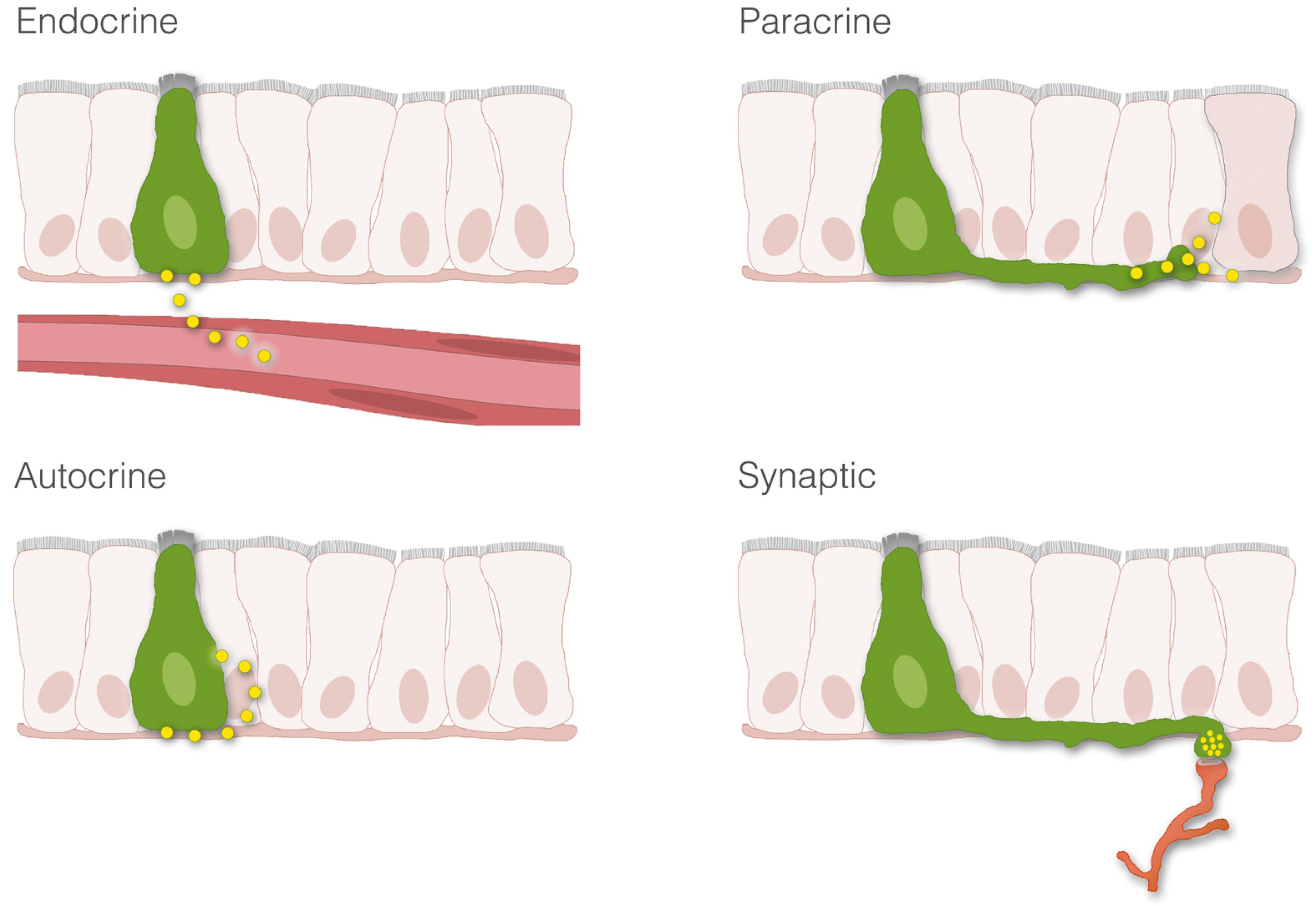
Endocrine. This type of communication occurs when transmitters are secreted into the bloodstream. The most common endocrine transmitters are peptides, lipids, and mono amines, and are collectively known as hormones . In the GI tract the most predominant type of hormone is in the peptide form (e.g., peptide YY, gastrin, secretin). Hormones bind to specific receptors on the surface of target cells at remote sites and regulate metabolic processes.
Paracrine. In contrast to endocrine mechanisms used to reach distant targets through the blood, signaling cells of the GI tract can also produce transmitters that act on neighboring cells. This process is known as paracrine signaling and is typical of enteroendocrine cells that produce somatostatin. Paracrine transmitters are secreted locally and cannot diffuse far. They bind to receptors on nearby cells to exert their biological actions. Once released, the transmitter is rapidly taken up by the target cell, catabolized by extracellular enzymes, or becomes adherent to extracellular matrix, thus limiting the transmitter’s ability to act at distant sites. Because paracrine signals act locally, their onset of action is generally rapid and can be terminated abruptly. By comparison, endocrine signaling takes much longer, and termination of signaling requires clearance of hormone from the circulation. Paracrine transmitters can be peptides (e.g., somatostatin) or monoamines (e.g., histamine).
Autocrine. Some cells possess cell surface receptors for their own messengers. In this way, when a messenger is released, it can act on the same secreting cell. This mode of transmission is known as autocrine and has been demonstrated for several growth factors. Autocrine signaling has been implicated in the growth of certain cancers, including colorectal cancer (see Chapter 1 ). .
Neurotransmission. A fourth form of signaling in the GI tract is neurotransmission. This form of signaling is primarily used by the enteric nervous system. The enteric nervous system is a complex network of nerve cells that must communicate efficiently to regulate numerous GI functions ( Fig. 4.2 ). When neurons of the GI tract are activated, signals in the form of neurotransmitters are released at nerve-to-nerve junctions known as synapses . These structures help neurons deliver neurotransmitters at specific locations on the target cell, and influence the function of other neurons, muscle cells, epithelial and secretory cells, and other specialized cells of the GI tract such as enteric glia. Neurotransmitters are critical for the processes of digestion including the coordination of gut motility and secretion. Although the GI tract secretes a variety of neurotransmitters, the most common are peptides such as vasoactive intestinal polypeptide (VIP), or small molecules, such as acetylcholine and norepinephrine. Other molecules, such as nitric oxide (NO), can simply diffuse across the synaptic cleft to exert an effect on the postsynaptic cell. Some nerves actually release peptides or neurotransmitters directly into the blood. This process is called neurocrine signaling and may be used to cause systemic effects depending on the transmitter released.
The major hormones and neurotransmitters of the GI tract are listed in Box 4.1 . Their actions depend on specific receptors located on target tissues. For instance, the specificity of neurotransmitter action is dependent on the precise location at which the nerve synapses with the target cell. Adjusting their synthesis, catabolism, or secretion regulates the transmitter concentration within the releasing cell. Once secreted, the concentration of a transmitter can be quickly modulated by catabolism or, in the case of neurotransmitters, reuptake into the secretory neuron. Many peptide transmitters have very short half-lives that are generally within the 2 to 5 minute range. This allows for rapid initiation and termination of signaling.
Gastrin
Glucose-dependent insulinotropic peptide (GIP)
Glucagon and related gene products (GLP-1, GLP-2, glicentin, oxyntomodulin)
Insulin
Motilin
Pancreatic polypeptide
Peptide tyrosine tyrosine (PYY)
Secretin
Cholecystokinin (CCK)
Corticotropin-releasing factor (CRF)
Endothelin
Neurotensin
Somatostatin
Calcitonin gene-related peptide (CGRP)
Dynorphin and related gene products
Enkephalin and related gene products
Galanin
Gastrin-releasing peptide (GRP)
Neuromedin U
Neuropeptide Y
Peptide histidine isoleucine (PHI) or peptide histidine methionine (PHM)
Pituitary adenylate cyclase–activating peptide (PACAP)
Substance P and other tachykinins (neurokinin A, neurokinin B)
Thyrotropin-releasing hormone (TRH)
Vasoactive intestinal peptide (VIP)
Peptides That Act as Growth Factors
Epidermal growth factor
Fibroblast growth factor
Insulin-like factors
Nerve growth factor
Platelet-derived growth factor
Transforming growth factor-β
Vascular endothelial growth factor
Interferons
Interleukins
Lymphokines
Monokines
Tumor necrosis factor-α
Cholecystokinin
Gastrin
Motilin
Nonpeptide Transmitters Produced in the Gut
Acetylcholine
Adenosine triphosphate (ATP)
Dopamine
γ-Aminobutyric acid (GABA)
Histamine
5-Hydroxytryptamine (5-HT, serotonin)
Nitric oxide
Norepinephrine
Prostaglandins and other eicosanoids
Newly Recognized Hormones or Neuropeptides
Amylin
Ghrelin
Guanylin and uroguanylin
Leptin
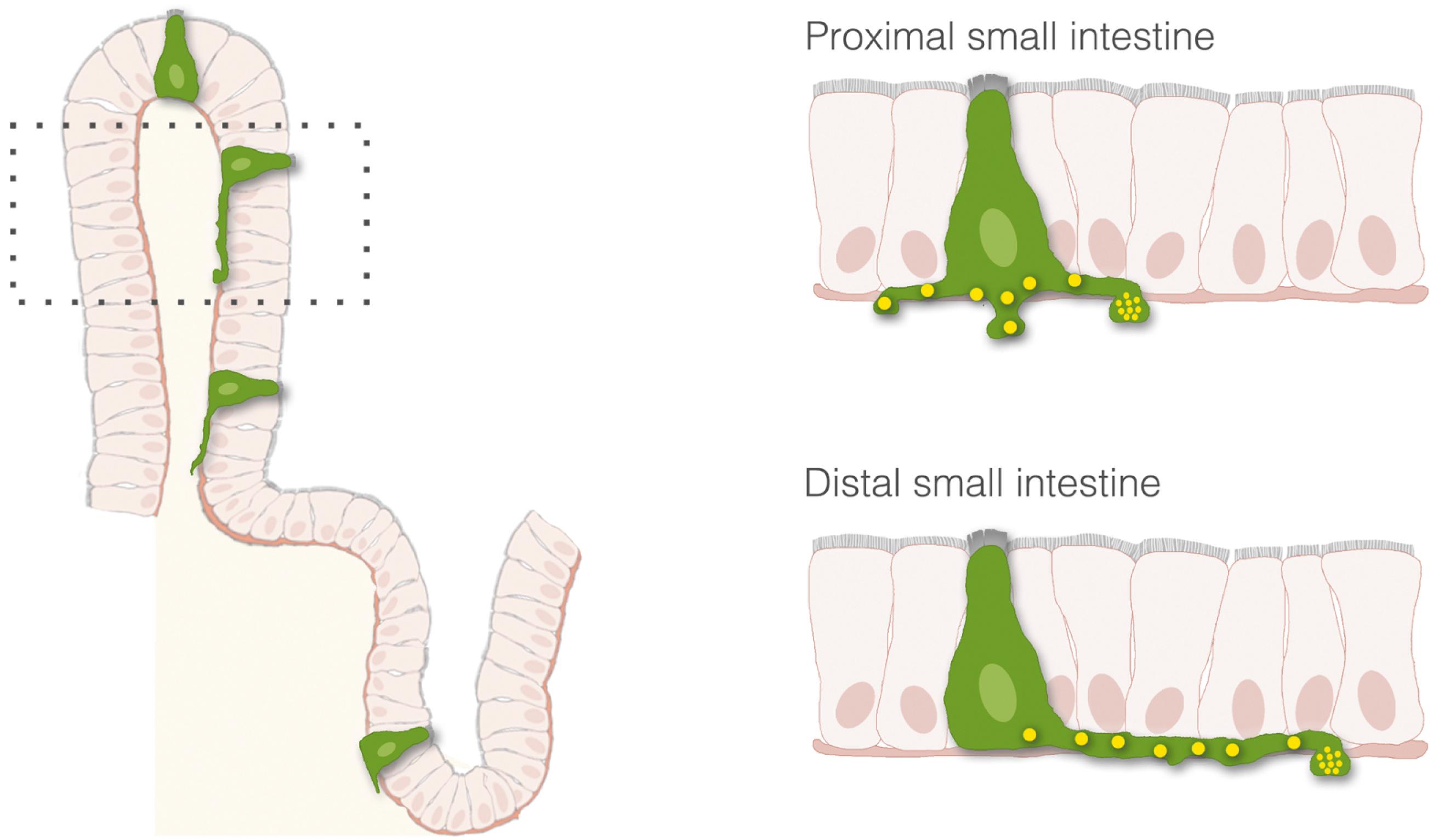
The process of nutrient sensing involves the activation of cell-surface receptors that trigger the release of transmitters. The transmitters then either enter the bloodstream or activate sensory afferent nerves. Although the cells releasing the transmitters, enteroendocrine cells, are thought to interact with nerves indirectly through paracrine or endocrine signals, a new concept is emerging, in which enteroendocrine cells and nerves actually communicate through synaptic connections. With the use of transgenic and advanced optical tools, enteroendocrine cells have been described to have several anatomical features observed in neurons, including dendritic-like spines, axon-like processes. These axon-like cytoplasmic processes vary in length from crypt to villus and from proximal to distal small intestine ( Fig. 4.3 ). Moreover, enteroendocrine cells have the molecular components, genes, and proteins of synapses, and connect to sensory neurons through synaptic-like connections ( Fig. 4.4 ). These connections may have broad applications in the biology of gastrointestinal function, including the transmission of sensory signals from nutrients and the GI microbiota. Some key components involved in the transduction of signals from the lumen of the gut to the rest of the body are described as follows.
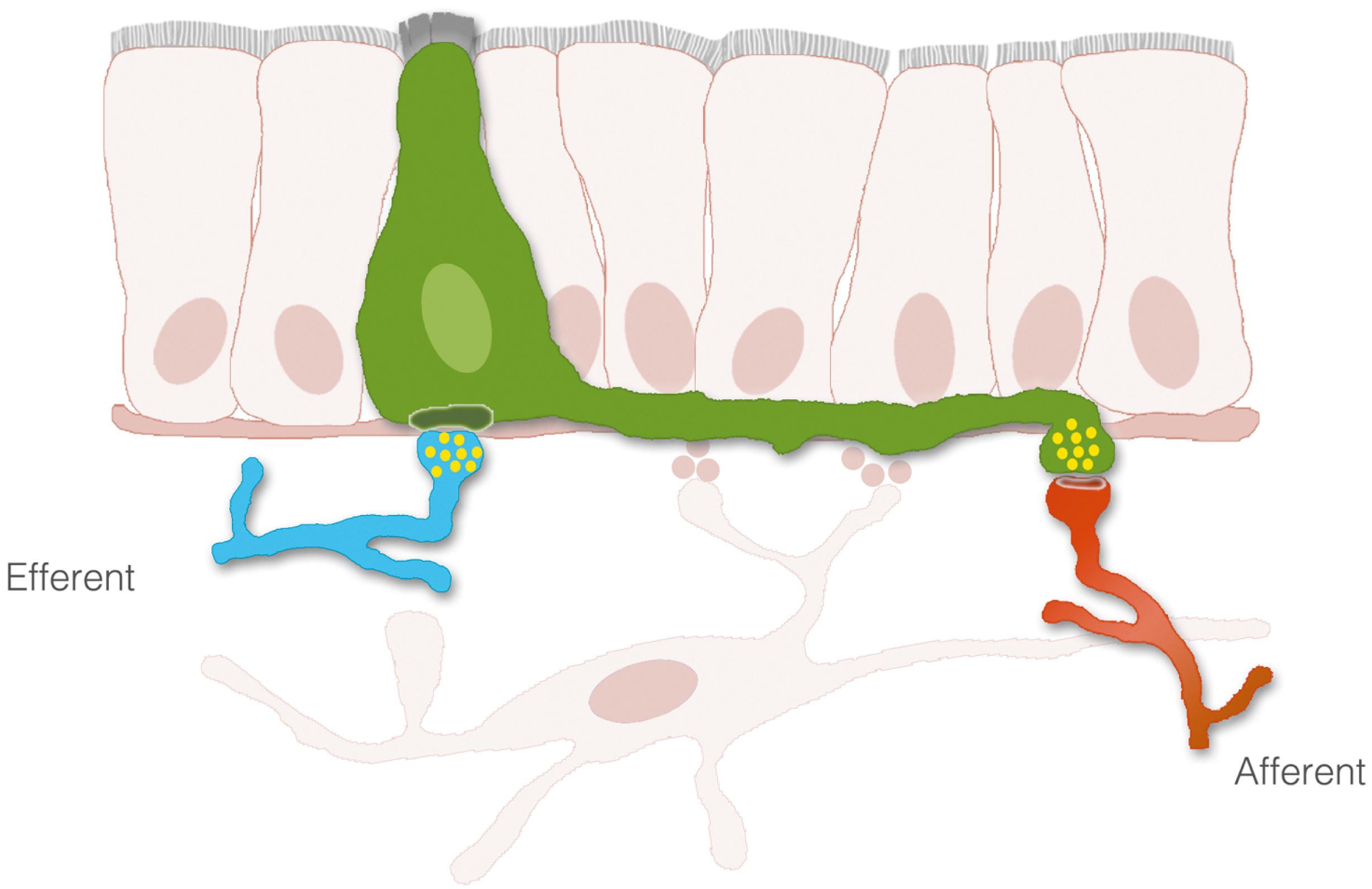
GI epithelial cells recognize molecules in the lumen using membrane bound receptors. When activated, receptors transduce signals from the outside of the cell into the cytoplasm. Although the process is rather complex, there are key checkpoints at which the signaling cascade can be regulated. Some of these checkpoints occur at the moment of receptor activation, desensitization, internalization, and/or resensitization. Because of their regulatory potential, these are attractive targets for therapeutic intervention.
Receptors are grouped into major families depending on their structures and signaling mechanisms. The major families of cell surface receptors include G protein-coupled receptors (GPCRs), enzyme-coupled receptors, and ion channels. The following are some of the main aspects of each receptor family.
GPCRs are typified by their seven transmembrane domains. They are the most common family of protein receptors and have broad physiological applications, ranging from sensing light in the retina to allow vision to sensing nutrients in the gastrointestinal tract to regulate food intake. When stimulated by a specific ligand, GPCRs undergo conformational changes leading to their association with a G protein —hence their name. These G proteins are bound to the intracellular surface of the cell membrane and are composed of three distinct subunits—α, β, and γ. It is the Gα subunit that confers the name of the G protein ( Table 4.1 ). For instance, G proteins that stimulate an effector (e.g., adenylate cyclase) are classified as Gs (for stimulatory), whereas those that inhibit an effector are called Gi (for inhibitory). When the G protein acts on the effector , this causes a rapid increase in the intracellular concentrations of a second messenger (e.g., cyclic AMP or calcium). The second messenger then changes the activity of one or more protein kinases to catalyze the phosphorylation of an existing protein and ultimately modify the physiological activity.
| Class | Signaling |
|---|---|
| Gαs | Adenylate cyclase, calcium channels |
| Gαi and Gαo | Adenylate cyclase, cyclic guanosine monophosphate, phosphodiesterase, c-Src, STAT 3 |
| Gαq | Phospholipase C-β |
| Gα12 and Gα13 | Sodium-hydrogen exchange |
In general, the GCPR signaling mechanism involves the following events. When the ligand or first messenger binds to the receptor, the receptor changes its conformation and binds to the G protein complex. In the resting state, the G protein complex does not interact with the receptor. However, once bound, there is a molecular substitution in the Gα subunit —a guanosine diphosphate (GDP) is replaced by a guanosine triphosphate (GTP). This replacement causes the activation of the Gα subunit. The active Gα subunit then separates from the β and γ subunits, and moves laterally in the membrane to activate an effector . Working through different Gα subunits, the activity of an effector can be up- or downregulated. When the interaction is completed, the GTP bound to the Gα subunit is hydrolyzed back to GDP and dissociated from Gα. In this way, Gα moves back to reunite with the other two subunits. The effector then induces an increase in the intracellular concentration of a second messenger . The two most common second messengers are cyclic adenosine monophosphate (cAMP) and calcium. The mechanisms involving each second messenger are briefly outlined as follows.
Signaling through cyclic adenosine monophosphate (cAMP). This second messenger is a classic downstream effector of β adrenergic receptors, a family of GPCRs that have been well characterized. These receptors are coupled to Gαs and activate adenylyl cyclase, which catalyzes the conversion of ATP to cAMP. High concentrations of cAMP then modify the activity of protein kinase A (PKA) that ultimately modulates rate-limiting enzymes involved in important physiological functions. For instance, modulation of glycogen phosphorylase increases the conversion of glycogen to glucose-1 phosphate, leading to a rise in blood glucose levels.
Signaling through calcium (Ca 2+ ). GPCRs associated with Gαq subunits use Ca 2+ as a second messenger. An increase in intracellular concentrations of Ca 2+ can result from the activation of voltage-gated Ca 2+ channels, ligand gated Ca 2+ channels, or the release of cytosolic Ca 2+ activated by membrane phospholipids. The latter is triggered by activation of GPCRs associated with Gαq. When active, Gαq moves along the cell membrane to activate the enzyme phospholipase Cβ. Phospholipase Cβ then cleaves the membrane phospholipid phosphatidyl inositol bisphosphate into diacylglycerol and inositol 1,4,5-trisphosphate (IP 3 ) , generating two potential signaling molecules. Diacylglycerol in the presence of Ca 2+ activates protein kinase C. In addition, a rise in Ca 2+ levels from internal stores can also activate Ca 2+ —calmodulin kinase. In this way, two different kinases are activated: Ca-calmodulin kinase by increasing cytosolic Ca 2+ and protein kinase C by the action of diacylglycerol and Ca 2+ . These kinases then catalyze the phosphorylation of target proteins within the cell. Following receptor activation, IP 3 moves from the plasma membrane into the cytoplasm to bind IP 3 receptors located on the endoplasmic reticulum and mitochondria. IP 3 receptor binding causes release of Ca 2+ from intracellular organelles to further increase cytoplasmic Ca 2+ concentrations. Ultimately, Ca 2+ cytoplasmic concentrations are restored to normal by active transport out of the cell or by reuptake into intracellular Ca +2 stores.
If the cell is overstimulated, a process of adaptation occurs to prevent the cell from overresponding. Attenuation of signaling occurs through either ligand-induced receptor desensitization or receptor internalization . The receptor is desensitized by means of phosphorylation. Phosphorylation can also further label the receptor for internalization, which is accomplished by activation of specific receptor kinases and the recruitment of arrestin-like molecules that uncouple the receptor from the G protein. Uncoupling and subsequent receptor internalization ends signaling and eventually restores cell responsiveness.
The most representative of the enzyme-coupled receptors are the tyrosine kinase receptor family. These receptors are primarily targets of growth factors, such as epidermal growth factor. These receptors are unique in that they are both a receptor and a tyrosine kinase. When activated, the receptors catalyze the transfer of phosphate from ATP to the target proteins. Enzyme-coupled receptors are composed of three domains: a ligand-binding extracellular domain, a transmembrane domain, and a cytoplasmic domain. The cytoplasmic domain contains a protein tyrosine kinase region and substrate region for agonist-activated receptor phosphorylation. In this way, phosphorylation from other kinases or autophosphorylation can occur to modulate the activity of the tyrosine kinase receptor. In general, receptor tyrosine kinases exist in the cell membrane as monomers. However, with ligand binding, these receptors dimerize, autophosphorylate, and initiate other intracellular signal transduction pathways that ultimately modulate physiological function. Receptor tyrosine kinases are further discussed in Chapter 1 in relation to cellular growth and neoplasia.
There are several other types of enzyme-coupled receptors, including receptor guanylate cyclases, nonreceptor tyrosine kinases, receptor tyrosine phosphatases, and receptor serine/threonine kinases. Although these receptors act through different enzymes, the signaling principles remain similar to those of tyrosine kinase receptors.
Ion channel–coupled receptors are involved in rapid signaling between cells. This type of receptors is important in tissues where electrical impulses drive signaling, like nerve cells and muscle. For instance, in nerve cells, ion channels open or close in response to a relatively small number of neurotransmitters and allow the flow of particular ions across the plasma membrane. The kinetics of the flow depend on the concentration inside and outside the cell. This flow of ions regulates the excitability of the target cell to ultimately trigger processes such as neurotransmission, muscle contraction, electrolyte and fluid secretion, or hormone release.
An example of this type of receptor is the transient receptor potential cation channel M5, or better known as TRPM5. This ion-channel receptor is activated by elevated intracellular Ca 2+ concentrations and is a key component in the transduction of the taste signals bitter, sweet, and umami. Moreover, it has been recently shown to mediate the release of opioids and hormones like CCK from enteroendocrine cells. Thus ion channel-coupled receptors can be attractive targets to modulate the function of sensory cells in the epithelium of the GI tract.
Lipids in the intestinal lumen are potent inducers of satiety and modulators of whole-body metabolism. Although the mechanisms are not completely understood, it has been recently demonstrated that specific lipids are recognized by cell surface receptors, which activate the release of several hormones, including CCK, peptide YY, and glucagon-like peptide 1.
The lipids can be in the form of triglycerides or free fatty acids of various chain lengths. Different lipids are recognized by different receptors. For instance, the Gq coupled GPCRs 40 (i.e., FFAR1) and 120 respond to medium- and long-chain fatty acids; whereas the Gα i coupled GPR41 (i.e., FFAR3) and GPR43 (i.e., FFAR2) bind to short-chain fatty acids of 2 to 5 carbons. It is possible that some GPCRs respond to lipids in the lumen of the gut. Other non-GPCRs are also involved in lipid sensing, such as the immunoglobulin-like domain containing receptor (ILDR). ILDR is expressed in CCK cells and is activated by the combination of fatty acids and lipoproteins suggesting that fatty acids must be absorbed to stimulate CCK secretion. Although the specific location of most nutrient receptors has yet to be determined, it may be that at least some lipids need to be digested and absorbed prior to activating hormone release. This hypothesis is supported by studies, in which the infusion of lipid in the intestine triggers hormone secretion but only if chylomicrons, lipoprotein particles formed from absorbed lipids, are allowed to form.
Some lipid-generated sensory signals appear to travel through afferent fibers of the vagus nerve. For instance, infusion lipids into the duodenum increases brown fat temperature, and this effect is abolished if lipids are infused along with tetracaine— an potent local anesthetic used to block vagal afferents activation. Signals traveling through afferent nerves or the bloodstream ultimately induce homeostatic changes (e.g., satiety, body temperature, GI motility) in response to the presence of nutrients in the GI lumen.
Proteins can also be potent stimulants of GI hormone secretion. Most proteins stimulate hormone secretion only when digested to peptones and amino acids (AAs). Recently, enteroendocrine cells have been found to express several classes of amino acid receptors that mediate hormone secretion. For instance, the calcium sensing receptor (CaSR), which was originally identified for its ability to detect and respond to extracellular Ca 2+ and regulation of calcium homeostasis in the kidney and parathyroid gland, also recognizes L-amino acids and di- and tri-peptides. A clear role for CaSR has been established in the regulation of L-amino acid-stimulated gastrin and gastric acid secretion. The aromatic AAs phenylalanine and tryptophan are the most potent AAs for stimulating CaSR and are also the most potent for stimulating CCK secretion. The discovery of CaSR in CCK cells and its link to secretion support its physiological importance as a nutrient sensor in the GI tract. Besides CCK, CaSR appears to also mediate the secretion of GIP, GLP-1, and PYY .
Another amino acid sensing receptor closely related to CaSR is the G protein-coupled receptor, GPRC6A. GPRC6A responds to basic AAs and is expressed in taste cells and enteroendocrine cells of the distal small intestine where mediates the secretion of GLP-1. Genetic deletion of GPRC6A leads to diet-induced obesity, implying that this receptor is important for metabolic regulation. Finally, the taste receptors, T1R1/T1R3, also recognize acidic AAs and do not appear to be restricted to taste cells of the tongue but instead are distributed in chemosensory cells throughout the body. Together, CaSR, GPFC6A, and T1R1/T1R3 respond to all of the 20 L-AAs and represent a comprehensive mechanism to sense amino acid nutrient stimuli.
Partially digested protein in the form of peptones can also stimulate hormone secretion. The G protein–coupled receptor GPR93 is not only a lysophosphatidic acid receptor but is also activated by peptone. GPR93 is expressed in enterocytes and enteroendocrine cells, where its activation has been coupled to CCK secretion. Thus GPR93 may be the mechanism by which peptone stimulates CCK release following a meal.
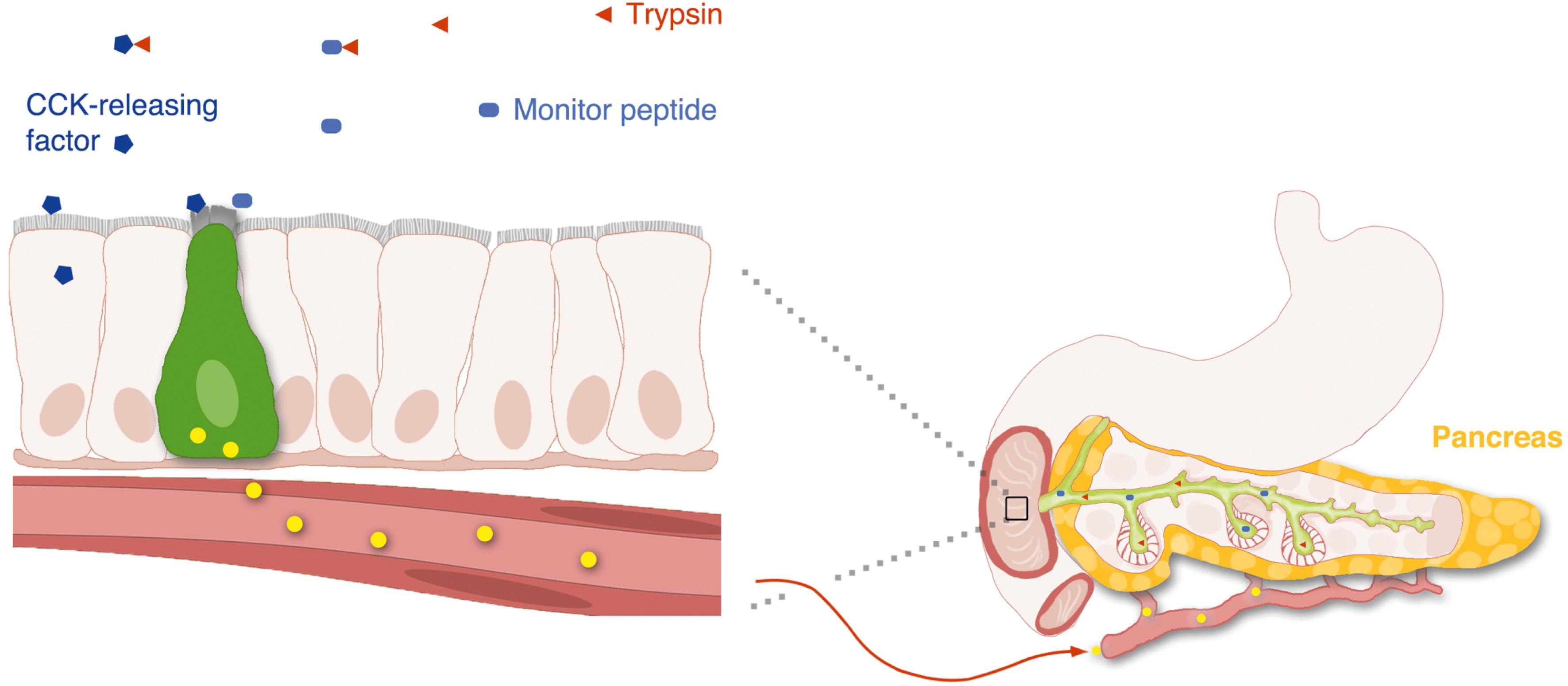
Some intact proteins stimulate hormone secretion indirectly through a class of endogenous luminally active hormone releasing factors, including luminal cholecystokinin releasing factor (LCRF) and diazepam binding inhibitor (DBI). . The most potent proteins are those that compete for trypsin binding and allow the endogenous releasing factor to escape proteolytic digestion within the gut lumen.
Sensing tastants in various foods is important to regulate pleasure, reward, food intake, and other important metabolic functions. The GI tract detects chemicals and toxins through specific receptors expressed by specialized chemosensory cells. These cells are best characterized in the tongue, where they are concentrated in taste buds. Taste receptor cells can detect chemicals that give rise to the five different flavors: sweet, salty, sour, bitter, and umami—the savory taste of soy sauce. Although this is an active area of research, only the sensing mechanisms for sweet, bitter, and umami flavors are well understood. These three flavors are mediated by the activation of two families of GPCRs: taste-1 receptors (T1Rs) and taste-2 receptors (T2Rs). In humans, there are 30 T2R proteins and three T1Rs, named T1R1, T1R2, and T1R3.
Sweet and umami flavors are recognized by T1Rs. In the tongue, T1R1 and T1R2 are expressed in separate taste receptor cells, but always along with T1R3. In this way, the receptors form heterodimers that allow the detection of sweet ligands in the case of T1R2 + T1R3, and umami in the case of T1R1 + T1R3. T1Rs are also expressed in enteroendocrine cells. Here, the binding of glucose to T1R2 + T1R3 receptors in enteroendocrine cells in the gut lumen leads to secretion of incretin hormones, like glucagon-like peptide-1 (GLP-1). GLP-1 ultimately modulates a wide variety of functions, including insulin secretion, nutrient absorption, and gut motility. Consequently, gut-expressed taste signaling has become an active area of research to develop therapies for diet-related disorders like type II diabetes.
Bitter perception functions as a warning signal against the ingestion of toxic substances through direct taste aversion, induction of the pharyngeal gag reflex, and nausea. The wide array of T2Rs present in the tongue as well as in the gut are set to recognize bitter compounds, such as toxic alkaloids in plants. It is believed that bitter compounds that bypass T2Rs in the tongue are recognized by T2Rs in the gut, serving as a backup mechanism for inducing a protective response such as vomiting. Activation of T2Rs and their associated Gα-gustducin protein result in a rapid increase in cytosolic Ca 2+ , which stimulates membrane depolarization and hormone release. In the gut, bitter chemicals can stimulate the release of CCK from enteroendocrine cells to slow gastric emptying and decrease appetite, thus reducing the likelihood of toxin absorption.
Enteroendocrine cells typically have a small narrow opening to the luminal surface. Although it has been long been assumed that nutrients stimulate enteroendocrine cells at their apical portion, there are some reports that absorbed and not luminal nutrients stimulate gut hormone release. Thus it is possible that the apical portion of enteroendocrine cells open to the gut lumen may serve to sense bacterial inputs. Evidence supporting this hypothesis comes from the fact that some bacterial toll-like receptors (e.g., TLRs 4, 5, and 9) are exclusively expressed in enteroendocrine cells. When these specific TLRs are stimulated with bacterial ligands (e.g., LPS or flagellin), CCK and several chemokines are secreted. Remarkably, cytokines and defensins are secreted from an enteroendocrine cell line (i.e., STC-1) only in response to bacterial ligands and not to fatty acids. Moreover, silencing MyD88, a central mediator of TLR signaling, reduces CCK secretion stimulated by bacterial ligands but not by fatty acids. This evidence suggests that there may be two different sensing pathways in enteroendocrine cells—one for bacteria and another for nutrients.
It has long been assumed that chemosensory receptors on enteroendocrine cells reside on the apical surface, which is open to the gut lumen. However, this has not yet been demonstrated, and recent evidence suggests that some nutrients stimulate enteroendocrine cells when exposed to the basal lateral surface. Because gut microbiota reside in the lumen of the GI tract, it is likely that toll-like receptors are located on microvilli. In the future, elucidating the location of receptors on enteroendocrine cells may facilitate the design of drugs to target specific receptors and modulate the secretion of hormones involved in appetite regulation and insulin secretion.
There is evidence that GI hormones can be released by certain non-nutrient factors present in the lumen of the gut ( Fig. 4.5 ). CCK was the first hormone shown to be regulated by an intraluminal releasing factor. Luminal CCK-releasing factor was purified from intestinal washings and shown to stimulate CCK release when instilled into the lumen of animals. Other luminal factors causing the release of CCK are the diazepam-binding inhibitor and the pancreatic monitor peptide. It has been proposed that a secretin-releasing factor regulates secretin secretion in an acid-sensitive way. The pancreatic secretory trypsin inhibitor, better known as monitor peptide , is an endogenous trypsin inhibitor produced by pancreatic acinar cells. When secreted into the duodenum, monitor peptide directly stimulates CCK secretion from I cells. These proteins act directly on enteroendocrine cells, most likely through cell surface receptors. The existence of these releasing factors highlights the existence of underappreciated bioactive molecules within the lumen of the gut.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here