Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The immune system can be viewed as a process evolving over 1000 million years ( Fig. 26.1 ). The classic immune system can be divided into an innate immune system that evolved early and an adaptive immune system that has evolved over the past ~ 500 million years. Immunocompetence was observed in its basic form in the prokaryotes as they had the ability to detect danger and neutralize it using restriction enzymes and clustered regularly interspaced palindromic repeats (CRISPRs). Single-cell organisms have retained a complex innate immune system. The acquisition of mobility provided a means to move toward nutrients and away from danger and the development of additional discriminating or recognizing sensors was useful in sexual reproduction. Phagocytosis required the development of receptors allowing recognition of nonself entities. Hundreds of millions of years were marked by whole-genome replications with mast-, macrophage-, and eosinophil-like cells preceding the presence of adaptive immune cells. The insertion of recombinases (RAG1 and RAG2) into the genome predated the divergence of jawless (e.g., lampreys) and jawed vertebrates (e.g., sharks, primates) about 450–550 million years ago. This evolution was marked by the development of two distinct, but similar, adaptive immune systems that used different recombinatorial systems. The RAGs and the RAG-dependent rearrangement of variable (V), diversity (D), and joining (J) gene segments in the jawed vertebrates heralded the appearance of adaptive immunity in mammals, providing a foundation for antigen-specific immunoglobulins and T cell antigen receptors.
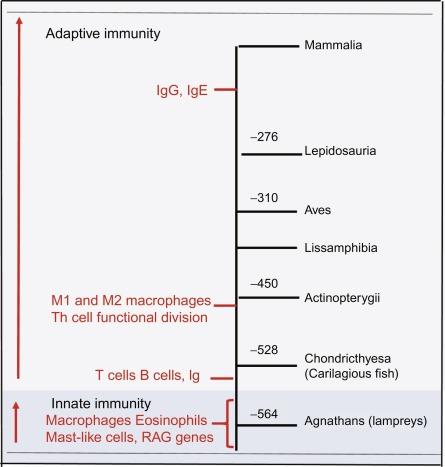
Host defense against pathogens is a biological imperative in more complex organisms. While the innate system relies on the hosťs ability to generate receptors that recognize conserved features of pathogens, the adaptive system has an exquisite mechanism of cell surface receptors that recognize a theoretically unlimited number of structurally diverse antigens. Both T and B cells in jawed vertebrates have sublineages with T cells expressing either the αβ or the γδ T cell receptor (TCR), with the latter composing only 1%–10% of the T cell repertoire. Of interest is that jawless vertebrates also have distinct T and B cell lineages suggesting vertebrates have a common ancestry in humoral and cellular immunity. The range of TCR or T cell repertoire achieves its diversity through gene recombination, deletion and insertion, and shuffling. Reduction in TCR diversity occurs with age, immunosuppressive drugs, and is considered a risk factor in autoimmune diseases. Experimental estimates put the TCR diversity at 2 × 10 6 for mice and 2 × 10 7 for humans. TCR diversity is a product of structural diversity, defined as the combination of variable, joining, and constant regions, as well as functional diversity, defined as the ability of T cells to have specialized effector functions. The mechanism by which B-cell antigen receptors encode immunoglobulins is similar to that used to generate antigen receptors by T cells. Evolutionary immunologists have focused their investigations on the identification of TCR, B cell receptor (BCR), RAG1/RAG2, and major histocompatibility complex (MHC) class I and class II genes as the central components of the adaptive immune system. An important function of T cell diversity is to keep up with emerging pathogens, which provided the major driving force for the development of adaptive immunity. There are factors, however, that limit diversity such as positive and negative intrathymic selection and the reality that the number of T cells cannot exceed the total number of cells in the body. While the random rearrangement of adaptive antigen receptors provides a high level of diversity of antigen receptor specificity, there is a risk of generating autoreactive T and B lymphocytes.
The major difference between B and T cells is that all the effector functions of B cells rely on secreted antibodies, with discrete heavy chain region isotypes, while the effector functions of T cells depend on cell-cell interactions and the TCR serves only to recognize antigens. Immunoglobulins exits as soluble antibodies or are part of membrane-bound receptors on B cells. With an alteration in RNA splicing, a membrane-bound receptor becomes a soluble product, a process that is correlated with the differentiation from receptor-expressing B cells into immunoglobulin-secreting plasma cells. The antigen-binding variable lymphocyte receptors (VLRs) expressed in extant jawless vertebrates are thought to represent the precursors of modern immunoglobulins. Genes encoding Ig heavy and light chains appeared before the division into cartilaginous and bony fishes, resulting in expression of Ig molecules that are characteristic of higher vertebrates. Generation of immunoglobulins requires the development of germinal centers, which are specialized areas in lymphoid structures that foster interaction among T and B cells. The first evidence of a dedicated mucosal Ig isotype came with the emergence of IgT in an ancestor of modern bony fishes. In mammals, as part of the appropriate and optimal immune response to enteric stimuli, antigen-stimulated B cells undergo somatic hypermutation and class switch recombination, resulting in production of different isotype classes such as IgM, IgE, and IgA. IgM exists in all jawed vertebrates and may be the earliest Ig. Mammalian IgE evolved from amphibian IgY, while IgG emerged from amphibian IgX.
The gut adaptive immune system evolved in the presence of pathogens with the goal of increasing survival. It is not surprising, therefore, that a prime directive of the immune system is to recognize self versus nonself in order to distinguish harmless versus harmful signals. Thus, host defense is divided into control of, or tolerance to, the invading pathogen. Host resistance is accomplished by avoiding initial infection, more rapid recovery after infection, or remaining immune for longer periods of time after infection. The ability to attenuate the deleterious effects of infection on survivorship is tolerance. Importantly, tolerance does not always prevent the growth of the pathogen.
The classic definition of innate immunity is a nonspecific, hard-wired, and rapidly mobilized defense mechanisms against pathogens and is restricted to the ability of the host to produce antigen-specific effectors. Innate mechanisms are often sufficient for protecting the host, and genome sequencing indicates that innate immune receptors are highly immutable and impervious to change except on an evolutionary scale. There is much more diversity in the adaptive immune system although 99% of multicellular organisms lack an adaptive response. The exclusivity of the adaptive immune response to vertebrates parallels longer-lived multiorgan species that require both long-term highly specific recognition of foreign (nonself) antigens. The primary adaptive immune response is slower than the innate, but an important feature of the adaptive response is the ability of effector lymphocytes (T cells) to develop immunological memory to eliminate pathogens more quickly upon subsequent exposure. This also shortens the subsequent duration of associated functional changes such as reduced mucosal barrier function. In addition, there are a number of nonhematopoietic cells, including epithelial cells, glial cells, and neurons, which perform immune functions.
The strict division of immunity into innate and adaptive responses may be an artificial concept with the recognition that there are a number of cells that straddle the boundary of innate and adaptive immunity, including macrophages, mast cells, neutrophils, intraepithelial cells (IEL), and the recently discovered innate lymphoid cells (ILC). In addition, there is a growing recognition that both innate and adaptive immune processes are involved in maintaining homeostasis. Common features of these immune cells are (1) they reside in healthy gut mucosa; (2) they respond to mediators released from nonhematopoietic cells including epithelial cells; (3) they release products that bind to receptors on both hematopoietic and nonhematopoietic cells; and (4) their phenotype is influenced by the microenvironment, which ultimately modulates their activation, receptor expression, phenotype, and function.
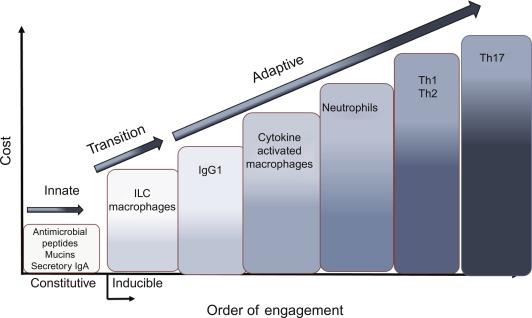
Mucosal homeostasis is a goal rather than an achievable state and is maintained by epithelial surveillance, low levels of epithelial release of cytokines such as IL-25, TSLP, and IL-33, and the relative tolerance of resident immune cells, dendritic cells, mast cells, and macrophages, to stimulation of toll-like receptors (TLR). Epithelial cells and resident immune cells sense the presence of deleterious stimuli in the lumen or damage to epithelial cells. In evolutionary terms, there is a high cost of mounting and maintaining an adaptive immune response and the life span of the animal must be long enough to develop an adaptive immune system. The low-cost options such as secretion of IgA, elaboration of antimicrobial peptides (AMPs), and mucins are produced constitutively as part of the innate response. When this is insufficient, there is increased release of epithelial-derived cytokines that are appropriate to the nature of the stimuli ( Fig. 26.2 ). The cost takes into consideration the risk of immunopathology as well as the metabolic demands activated immune cells. The fact that only 1% of all multicellular animals can mount an adaptive immune responses may be due to the limited ability to shift energy demands from growth and repair to immune responses. This suggests that the adaptive immune response may be confined to species that can handle the metabolic demands of this response. The enhanced mucosal permeability associated with adaptive immune responses also provides a mechanism for increased passage of nutrients for immune cells. This is part of the higher cost of the adaptive immune response (see Fig. 26.2 ).
In the GI tract, infection, inflammation, and injury all activate adaptive immune responses. Although often used interchangeably, there is a distinction between epithelial permeability and mucosal barrier function. The first line of defense is the lining of surface epithelial cells that communicate directly with subepithelial stromal cells, neural structures, enteric glial cells (EGC), and immune cells, which collectively form the mucosal barrier. In vitro cell cultures of intestinal epithelial cells have contributed greatly to our current understanding of the receptors, channels, and signaling pathways involved in epithelial responses to adaptive immune cells and pathogen-derived products. Epithelial cell lines represent a valuable tool for studying the impact of adaptive immune mediators on epithelial permeability. Regional differences in the microenvironment along the GI tract; however, as well as the complexity of interactions among the constituents of mucosal barrier function, support the importance of in vivo and ex vivo analyses.
Permeability in the inverse of resistance, an electrical property that reflects the net movement of ions. There are a variety of techniques that can be used to determine permeability in confluent cultures of intestinal epithelial cells or barrier function in sections of muscularis externa-free intestinal mucosae. The most common use mounted cells or mucosae in Ussing chambers or use of modified microsnap wells to determine transepithelial electrical resistance (TEER). In Ussing chambers, resistance is calculated under short-circuited conditions (net fluxes of all actively transported ions) using Ohm’s law ( V = I / R ) by determining the change in current ( I ) generated in response known voltage ( V ). In TEER, resistance is measured directly under open-circuit conditions (net ion transport). Transcellular resistance is attributed to the passage across the apical and basolateral plasma membrane, while paracellular resistance relies on the integrity of cell-cell contacts. It is important to note that resistance is an electrical measure of the net ion flux and does not distinguish between ions moving via transcellularly or paracellularly. Thus, changes in the net flux of ions (I) due to increased secretion or absorption will affect resistance. TEER measures the net flux of small ions across the mucosa, while paracellular flux uses sized labeled dextran molecules is an index of the leak pathway. Recent studies have concluded there is a size selectivity along the villus with distinct paracellular routes based on the presence of two distinct flux pathways: a high capacity “pore” pathway that is permeable to small solutes (≤ 4 A radius) and a low-capacity leak pathway that allows passage of larger molecules (reviewed in Ref. ). Therefore, the paracellular flux of molecules, which may not carry a charge, are not necessarily consistent with electrical measurements of resistance.
Measurements of resistance in vivo are used clinically and rely on the passage of labeled molecules such as dextran or different sized absorbable and nonabsorbable probe sugars such as lactulose, mannitol, and sucrose. This technique allows an estimation of changes in intestinal permeability by measuring absorption from the gut and recovery in urine. There are a number of factors that can affect interpretation of these in vivo permeability tests such as the rate of GI transit and changes in the vascular endothelium. Changes in GI transit may be associated with an extended or abbreviated residence time of the marker. In addition, altered endothelial permeability will impact the passage of the marker or the sugar, independently of changes in epithelial permeability.
The mucosal barrier is a complex and highly integrated functional system that involves a cross talk among epithelial cells and luminal factors as well as the immune cells in the lamina propria. Its major function is to limit passage of pathogens, pathogen products, or deleterious luminal stimuli as this impacts the balance between tolerance and immunity to self and nonself antigens. Importantly, mucosal barrier function maintains homeostasis as the majority of physiological functions are optimal under a limited range of homeostatic conditions. Disruption or dysregulation of homeostatic conditions is a feature of a number of pathologies and may lead to a new “homeostasis.” The microenvironment along the gut is unique, perhaps, in that there are regional differences in the presence of luminal factors such as nutrients, commensal microbes, and other factors (e.g., bile acids) that play an integral role in maintaining mucosal homeostasis. In response to pathogenic activation of pattern recognition receptors (PRR) or tissue injury-mediated release of damage-associated molecular pattern molecules (DAMPs), there is a release of chemokines and cytokines that interact with resident and recruited immune cells ( Fig. 26.3 ). These have significant effects on barrier function allowing passage of pathogens and/or their products across the mucosal barrier in large numbers to initiate adaptive immune responses.
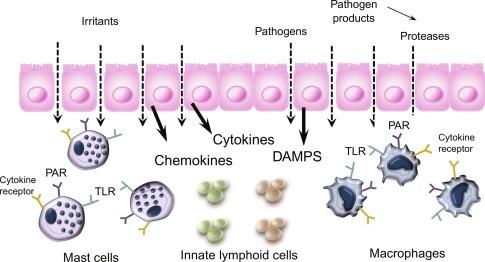
Immune cells play a critical role in the immune regulation of mucosal barrier. The number and type of resident immune cells present along the GI tract, which differ by region, play a critical role in mucosal homeostasis. In response to infection, inflammation, and or injury there is an increased in the numbers of these “innate” immune cells many of which participate actively in the “adaptive” immune response. The resulting profile of recruited immune cells leads to a release of mediators that affects epithelial cell permeability by direct or by indirect effects that mediated by their actions on nearby cells including enteric nerves and other immune cells.
The intestinal tract is considered the largest single immunologic organ in the body, containing upward of 40% of all lymphocytes. Lamina propria lymphocytes are one of two major mucosal T cell populations in the GI tract. As critical components of the adaptive immune response, T cells are the workhorses of the adaptive immune system. Naïve T cells are activated by antigen-presenting cell (APC), such as DC, undergo clonal expansion, and then differentiate into effector cells. Effector T cells migrate to peripheral tissue and develop different phenotypes that can be distinguished by specific cell markers to include CD4 + T helper cells, CD8 + T cells, CD4 +, or CD8 + CD45RO cytotoxic memory cells and CD25 + T regulatory cells (Treg). The two major subsets of peripheral T cells express either the CD4 or CD8 coreceptor molecules with CD4 + T cells predominating in the lamina propria and CD8 cells in the gut epithelium. The CD4-expressing T cells population are the major MHC class II-restricted Th cells and most of the CD4 + T cells in the mucosal immune CCR5 +, activated memory CD4 + T cells. The CD8 expressing cells are the MHC class I-restricted cytotoxic T cell lineage and as part of their effector mechanism, cytotoxic T cells have the “license to kill,” thereby eliminating virus-infected cells. The fate of CD4/CD8 is controlled by various transcription factors with ThPOK, c-Myb, Tox, and GATA-3, directing the development of the CD4 + T cell lineage. It was thought that CD4 + and CD8 + cells retained their identity; however, recent evidence suggests CD4 + cells have the potential to become intraepithelial cytotoxic CD4 + lymphocyte by a process involving loss of ThPOK that is required to maintain the CD4 + lineage.
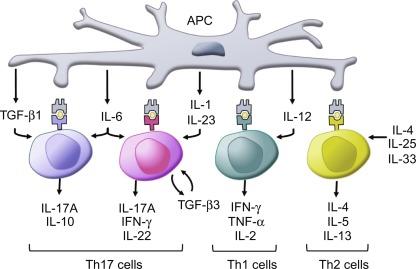
CD4 + T cells can be subdivided further into helper subsets including Th1, Th2, and Th17 ( Fig. 26.4 ) all of which produce cytokines that modulate gut function. Mosmann and Coffman were the first to report that Th cells orchestrate immune responses primarily against pathogens by producing polarized cytokine profiles, which are developed in the context of innate immune responses that are cued by host pathogen interactions. The classical paradigm of host defense is based on the CD4 + Th1/Th2 lineages characterized by polarized cytokine profiles that coordinate the host response to bacterial and helminthic pathogens, respectively. The Th17 subset is distinct from the Th1 and Th2 lineages and is the third T effector cell lineage. First associated with autoimmune diseases including IBD, Th17 cells contribute to the immune response to microbial pathogens and to the development of chronic inflammatory pathologies. TGF-β and with other inflammatory mediators, including IL-1, IL-6, IL-21, or IL-23 cells, are prerequisites for differentiation into Th17 cells. Both Th1 cytokines and members of the Th17 family, especially IL-17A and IL-17F, are implicated in the pathogenesis of IBD. The final major population of CD4 + T cells are T regulatory cells (Treg), which are important in maintaining immunological tolerance to self-antigens and in controlling excessive immune responses. The most characterized populations of Tregs are the forkhead box protein 3 (Foxp3) + Tregs and the interleukin-10 (IL-10)-producing type 1 Tregs (Tr1 cells). A reciprocal regulation among the Th1, Th2, and Th7 cells facilitates the delicate balance of immunologic responses, and expansion of Th1 or Th17 populations suppresses the development of Th2 responses and vice versa. An exception is that IL-17E (IL-25) promoted Th2 responses while inhibiting Th1 and Th17 responses. Tregs are an important feature of inflammation in that they regulate all other effector T cells and different populations of Tregs may be involved in inflammation versus repair.
These various profiles of cytokines are also important for creating an environment appropriate for recruitment of other immune cells that maintain the immune response. In this regard, mucosal barrier function plays an equal role by controlling the passage of luminal contents that shape the microenvironment. Appropriate effector functions are an integral component of immunity and are sustained in effector and memory lymphocyte pools. The ability of conventional T cells to develop immunological memory is a key feature of the adaptive immune response. Naïve T cells develop into differentiated T cells and can become memory stem cells, then central memory cells, which are located in secondary lymphoid structures, and finally effector memory T cells that reside in sites of inflammation. These resident tissue memory cells help orchestrate rapid responses to subsequent antigenic stimuli. In the gut, CD4 + tissue memory cells express CCR9 while at mucosal sites memory cells express CCR6. This specificity indicates that memory cells for specific pathogens may be enriched at areas of infection.
B cells can also adopt a number of distinct effector and memory fates that are directed by BCR signal strength along with additional signals that are linked to critical transcriptional programs such as antibody production, antigen presentation, costimulatory interactions with CD4 T cells, and cytokine secretion. The quality of the response is related to the type and levels of the immunoglobulin heavy chain isotypes, which determine the scope of effector functions. The heavy chain isotypes provide a link between adaptive humoral responses, including complement fixation, and discrete populations of innate immune cells involved in pathogen elimination.
The intraepithelial lymphocytes (IELs) are the second major population of mucosal T cells. They are a mixed population of T cell subtypes interspersed among intestinal epithelial cells in the small intestine (ratio of about 1/1–10 epithelial cells) and colon (ratio of 1/30–50 epithelial cells). They express cell surface markers important for migration and retention in the mucosal compartment as well as molecules that allow them to tether epithelial cells, a property that distinguishes them phenotypically and functionally from peripheral T cells. Most IEL are CD3 + T cells and can be divided further into two distinct populations. Type a or conventional IEL express TCRαβ and CD4 or the heterodimer CD8αβ coreceptors, increase in number with age in response to antigen stimulation, and function as long-term memory cells. Type b or unconventional (also called natural) IEL express TCRαβ + or TCRγδ + as well as CD8αα, lack CD8αβ/CD4 coreceptors, and express natural killer (NK) receptors. About 60% of the IEL in small intestine and colon in mice express the TCRγδ while only about 10%–14% of human IRL express this receptor. There are also a number of other differences between mouse and humans IEL subpopulations that may have functional importance (reviewed in Ref. ). TCRγδ + IEL are tolerant against commensal bacteria and can be activated in the absence of professional APC. IEL are important in the induction of mucosal tolerance in the epithelial barrier, an effect mediated in part by production of a number of cytokines including interferon (IFN)-γ (IFN-γ), TNF, IL-4, and IL-17, all of which can modulate barrier function. They exhibit remarkable mobility, an important component of mucosal surveillance, which is enhanced by enteric infection thereby allowing a small number of IEL to interact with a large number of enterocytes. The TJP protein, occludin, is expressed by IEL and enterocytes forms the point of interaction between these cells types, and is critical to the recruitment and subepithelial migration of IEL. In addition, IELs contribute to the maintenance of structural integrity of the barrier by providing a source of keratinocyte growth factor (KGF), which promotes proliferation of epithelial stem cells and improves barrier function after injury.
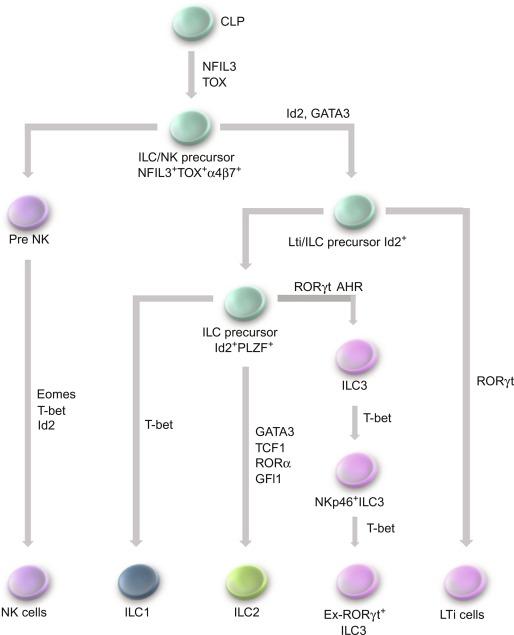
ILC are a relatively rare and distinct population of cells that release cytokines that bind to receptors on both hematopoietic and nonhematopoietic cells. They have a lymphoid-like morphology, but lack surface lymphoid lineage markers as well as antigen specificity (reviewed in Refs. ). Similar to CD4 + T cells, ILC can be subdivided further into functional subsets, ILC1, ILC2, and ILC3, which produce cytokines that parallel their respective adaptive Th1, Th2, and Th17 counterparts ( Fig. 26.5 ). Most of the information on ILC is derived from studies in mice where they are critical to host defense against enteric pathogens in experimental models and participate in restitution of the mucosa in response to DSS-induced injury. Their role in human pathologies is not as well established. The function of ILC is modulated by the local environment and there is an enriched concentration of ILC2 and ILC3 at mucosal surfaces. At these sites, they play a key role in innate immunity and help shape adaptive immune responses by providing an early source of cytokines following simulation by luminal pathogens and their products. These ILC are self-renewed by local tissue-resident progenitor cells. ILC1 are Lin − Id2 + IL-7Rα + CD25 − α4β7 + Flt3 − cells that produce IFN-γ and are induced by viral infection. The major activators of ILC2 cells are the epithelial-derived cytokines IL-25, IL-33, and TSLP. TSLP stimulates the production of IL-5 and IL-13 by ILC2 cells. ILC3 are critical in maintaining mucosal integrity in response to mucosal injury.
PMN are a short-lived population of cells that represents approximately 35%–75% of circulating leukocytes. They are classified as granulocytes because of their cytoplasmic granule content and are characterized by a multilobular nucleus. Neutrophils play an essential role in innate immunity and are part of the first line of defense against enteric pathogens. The immune functions of neutrophils involve (1) phagocytosis; (2) production and release of granules with antimicrobial activity; (3) generation and release reactive oxygen species (ROS); and (4) release of neutrophil extracellular traps (NETs). NETs are composed of nuclear materials, such as chromatin as well proteases that are contained in granules, and are released from neutrophils. These structures can bind to gram-positive and gram-negative bacteria as well as fungi and protozoa. NETs form web-like structures that entrap microorganisms thereby preventing their dissemination. Antimicrobial activity is also present in the chromatin released by dying or activated living neutrophils, which are covered with granular enzymes such as elastase and MPOs that target pathogens. While PMN play a well-recognized role in initiating the inflammatory response, like other immune cells, their phenotype and function are modulated by the microenvironment. They appear to have a major role in limiting bacterial dissemination during enteric infection or inflammation when mucosal barrier function is impaired.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here