Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Introduction to Chapter 54, Guide to Preoperative Parathyroid Localization Testing.
The skill of an experienced parathyroid surgeon remains the most important asset in identifying abnormal parathyroid glands. Results of initial operations for primary hyperparathyroidism (PHPT) are similar with or without preoperative imaging. Historically, more than 95% of patients have been cured after bilateral cervical exploration by experienced parathyroid surgeons without the help of localization studies and was not felt to shorten operative time in experienced hands. However, in contrast to those who undergo a first-time operation, reoperative parathyroidectomy has higher failure and complication rates. Thus localization studies are crucial for patients undergoing reoperation.
Additionally, the incidence of minimally symptomatic primary hyperparathyroidism (PHPT) rose dramatically as a result of routine measurements of serum calcium and the determination of serum parathyroid hormone (PTH) by radioimmunoassay. For this reason, more patients were referred for surgery, and some surgeons began to reassess the morbidity, indications for surgery, and the necessity of bilateral neck exploration. These are some of the driving factors in the study and subsequent use of preoperative parathyroid localization.
Over the past 15 years, trends in the approach to surgery have changed substantially from the majority of surgeons performing four-gland exploration to now, when most surgeons perform focused explorations. Today, the most commonly used noninvasive imaging studies are cervical ultrasound (US), nuclear scintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI).
Initial attempts at parathyroid localization dating to the 1960s included 75 Se-selenomethionine scanning; 131 I scintiscan; thermography; cine cervical esophagography; intravenous methylene blue injection; angiography via injection of the thyrocervical trunk or inferior thyroid artery; pneumomediastinal radiographic evaluation of glands located in the mediastinum; neck massage for PTH measurement; and thyroid lymphography. However, these methods are no longer used. The role of preoperative localization studies is to assist the surgeon in surgical planning, including identifying ectopic glands and enabling a focused or targeted exploration.
It should be emphasized that imaging is not used for diagnosis. The diagnosis of PHPT is made by the concurrent elevation of serum calcium and parathyroid hormone (PTH). Positive imaging does not always confirm the diagnosis, and negative findings are not uncommon, especially with multigland disease. Invasive studies, usually reserved for reoperative cases or ectopic glands, include US or CT-guided fine-needle aspiration (FNA) with concomitant PTH assay, parathyroid angiography, and/or selective venous sampling for PTH gradient.
PHPT is most likely caused by solitary adenomas (75% to 85%); whereas multigland disease (hyperplasia: 10% to 15%; double adenomas: 2% to 12%) and carcinoma (1%) are less common. Preoperative localization by imaging studies identifies candidates for minimally invasive parathyroidectomy in the majority of patients with PHPT. In this chapter, we review three types of parathyroid localization studies: radiology-based studies, nuclear medicine-based studies, and invasive procedures.
Ultrasonography to localize parathyroid tumors was first introduced in the late 1970s. Early ultrasound (US) technology was limited to 5 MHz transducers, which gave static gray scale imagery. Currently, higher frequency transducers (10 to 15 MHz), along with color Doppler, allow for real-time sonographic images, and newer machines can perform three-dimensional (3D) computer reconstruction. Many endocrine surgeons and head and neck surgeons now perform US in the office.
The sonographic appearance of a parathyroid adenoma is typically homogeneous, hypoechoic, and frequently a feeding vessel can be visualized ( Figure 54.1 ). Parathyroid glands are most commonly oval shaped, but they can be elongated, bilobed, or multilobed. Other abnormalities include parathyroid cysts, cystic components within larger solid lesions, giant adenomas, and calcifications. Parathyroid cysts are thin-walled structures that show posterior enhancement and have no internal echoes. Patients with parathyroid cysts can have fluctuating and/or normal serum PTH levels. Cystic lesions within large solid adenomas can have anechoic areas with posterior acoustic enhancement. Giant adenomas are defined as those larger than 3 cm. Calcifications, although rarely found in parathyroid adenomas, are hyperechoic and cast discrete acoustic shadows (see Figure 54.1 ). Finally, an inhomogeneous pattern of hyperechoic and hypoechoic images may be found corresponding to fat and hypercellular parathyroid tissue, respectively.
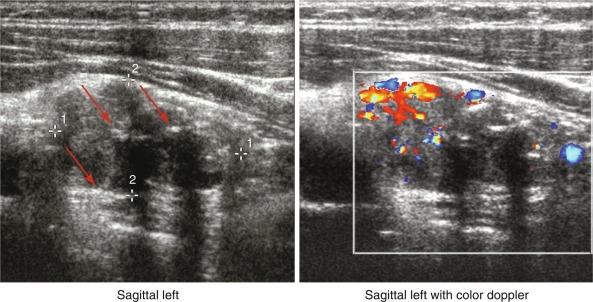
US has several advantages and disadvantages. Advantages include the following: (1) it is inexpensive; (2) it does not involve radiation or contrast exposure; (3) it is noninvasive; (4) it is excellent at detecting parathyroid tumors near the thyroid; (5) it identifies thyroid nodules; (6) it is a diagnostic tool that surgeons can use in their offices; and (7) it can guide needle biopsy. Disadvantages of US include the following: (1) it is operator dependent; (2) a surgeon will find it difficult to detect substernal, retrotracheal, retroesophageal, and deep-seated parathyroid tumors; and (3) it is “nonfunctional” (e.g., it can be difficult to distinguish between small lymph nodes and small abnormal parathyroid glands).
A systematic review showed that the sensitivity and positive predictive value (PPV) of US range from 51% to 96% and 50% to 100%, respectively, and the accuracy of surgeon-performed US in comparison with radiologist-performed is comparable. US is less accurate for localizing multiple adenomas or parathyroid hyperplasia whether it is performed by the radiologist or by the surgeon; sensitivities range from 13% to 24%. However, several factors decrease the sensitivity and PPV. These include concomitant thyroid nodules and multiple parathyroid adenomas or parathyroid hyperplasia. If in doubt, the clinician can consider performing percutaneous FNA of the lesion(s) in question and send the specimen for PTH measurement.
Many surgeons prefer to perform their own neck US, both preoperatively as a complementary addition to the physical examination and intraoperatively to help localize a parathyroid adenoma. US is used both to localize parathyroid adenomas and to evaluate the thyroid for pathology that may require a concurrent operation. Surgeon-performed US in the clinic is convenient for patients because surgical decisions and operative planning can occur without multiple appointments, and the cost likely will decrease by streamlining care. Additionally, the surgeon can use US to determine the localization of the parathyroid in relationship to key anatomic landmarks, such as the thyroid or surrounding vasculature and to place the incision for a minimally invasive parathyroidectomy.
CT is another noninvasive parathyroid localization technique. The sensitivity of CT scans increases with the use of patient-positioning harnesses (providing improved neck extension); intravenous contrast; and high-resolution CT scanners (2.5 to 3 mm cuts). Four-dimensional CT (4D-CT) is a contrast CT protocol format that produces multiplanar images using different perfusion characteristics of parathyroid, thyroid, and lymph nodes over time. 4D-CT can provide both anatomic and physiologic details about the abnormal parathyroid gland in a single study. The parathyroid adenomas have rapid uptake and comparatively early washout of intravenous contrast. 4D-CT scan was found in the initial report study to be more accurate at localizing the parathyroid adenoma to a particular side (sensitivity: 4D-CT 88% versus sestamibi 65% versus US 57%) and quadrant (sensitivity: 4D-CT 70% versus sestamibi 33% versus US 29%) than either US or sestamibi. The expertise of the radiologist increases the accuracy of CT scanning as well.
CT scanning has several advantages and disadvantages in localizing abnormal parathyroid glands. At tertiary referral centers, reported sensitivity of CT scans for lateralizing parathyroid adenomas is > 85% and 66% to 70% for localizing to the correct quadrant. The sensitivity is excellent in cases where US and/or sestamibi are negative, discordant, or ectopic. In cases with negative or discordant US and/or sestamibi, 4D-CT lateralized 73% of parathyroid adenomas and more than 80% in reoperations; cases of four-gland hyperplasia remain challenging. Many clinicians are now using 4D-CT as their initial localization study.
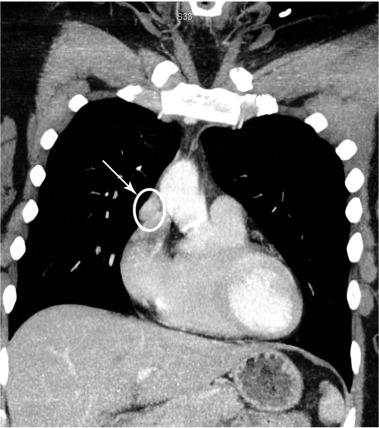
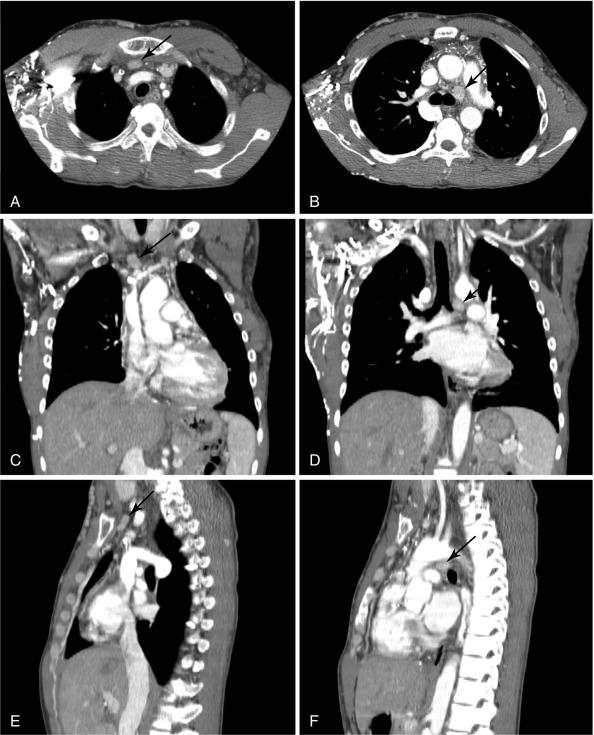
CT is excellent for localizing ectopic glands, such as those in the anterior mediastinum or undescended parathyroid glands ( Figures 54.2 and 54.3 ). CT scanners are readily available in most hospitals, yield reproducible images, take much less time than sestamibi scanning, and can guide needle biopsy; in addition, the interpretation is less subjective than for US. The disadvantages are exposure to contrast, radiation and decreased specificity. Average effective radiation doses are comparable between 4D-CT (~ 10 mSv) and sestamibi scans (~ 7 mSv) with average annual background radiation exposure of 3 mSv/year. Although the dose to the thyroid specifically is reportedly much higher than sestamibi, the lifetime attributable risk of cancer is extremely low for the single scan needed for diagnosis in the vast majority of cases.
Like computed tomography (CT) scanning, a magnetic resonance image (MRI) is primarily used as an adjunct for locating ectopic parathyroid glands and for working with patients who require reoperation because of persistent or recurrent hyperparathyroidism ( Figure 54.4 ). A typical parathyroid adenoma on MRI is an enhancing lesion with increased signal intensity on T2-weighted images and isointensity on T1-weighted images ( Figure 54.5 ). However, parathyroid adenomas may appear isointense or enhanced on both T1- and T2-weighted images. The sensitivity of MRI was comparable to sestamibi and US with ranges from 50% to 88%, depending on etiology, and it is highly sensitive for detecting ectopic parathyroid glands located in the mediastinum. When MRI was combined with 99m Tc MIBI scintigraphy, the sensitivity and positive predictive value (PPV) were 94% and 98%, respectively. There is one specific advantage of MRI over CT scanning: it does not require any ionizing radiation. The disadvantages include cost and motion artifact, which makes interpretation difficult. MRI is not frequently used as the first or only imaging study performed, but it can be helpful when other imaging studies are negative or discordant and when patients have iodinated contrast allergy or concern regarding radiation exposure. Real-time navigation with intraoperative MRI has been reported in a small group of patients. Future studies are needed to better define MRI’s exact role in parathyroid localization.
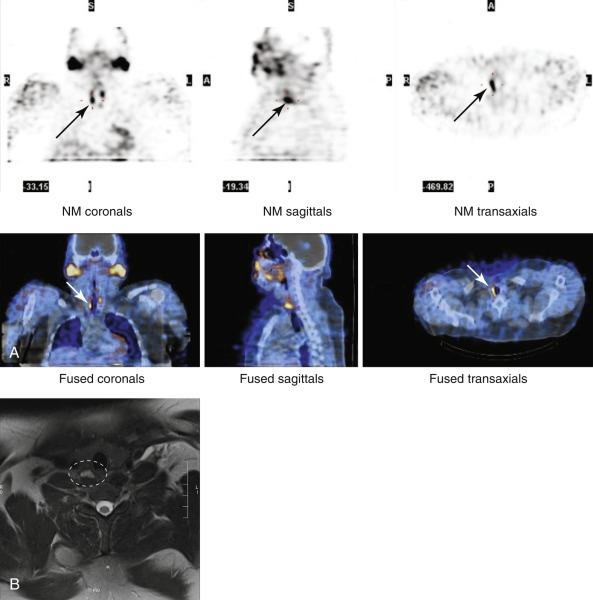
![Fig. 54.5, Magnetic resonance imaging (MRI) of the thyroid in a 61-year-old man with primary hyperparathyroidism and in hypercalcemic crisis (Ca 20 mg/dL). A 2.3 × 1.8 × 1.6 cm circumscribed T1 hypointense and T2 hyperintense enhancing mass is seen in the right paratracheal superior mediastinum corresponding to a descended upper right parathyroid adenoma. This lesion was not seen on ultrasound due to a deep posterior location. MRI views include the following: A, axial T2-weighted image with fat saturation ; B, axial T1-weighted image with no fat saturation; C, axial T1 gadolinium-enhanced image with fat saturation; and D, coronal T1 gadolinium-enhanced image with fat saturation. Through a lateral approach, an upper right gland (3.2 × 1.7 × 1.3 cm) descended into the posterior mediastinum was found (posteriorly located upper gland [PLUG] giant adenoma). Postoperatively, calcium and parathyroid hormone (PTH) levels dropped appropriately. Fig. 54.5, Magnetic resonance imaging (MRI) of the thyroid in a 61-year-old man with primary hyperparathyroidism and in hypercalcemic crisis (Ca 20 mg/dL). A 2.3 × 1.8 × 1.6 cm circumscribed T1 hypointense and T2 hyperintense enhancing mass is seen in the right paratracheal superior mediastinum corresponding to a descended upper right parathyroid adenoma. This lesion was not seen on ultrasound due to a deep posterior location. MRI views include the following: A, axial T2-weighted image with fat saturation ; B, axial T1-weighted image with no fat saturation; C, axial T1 gadolinium-enhanced image with fat saturation; and D, coronal T1 gadolinium-enhanced image with fat saturation. Through a lateral approach, an upper right gland (3.2 × 1.7 × 1.3 cm) descended into the posterior mediastinum was found (posteriorly located upper gland [PLUG] giant adenoma). Postoperatively, calcium and parathyroid hormone (PTH) levels dropped appropriately.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/GuidetoPreoperativeParathyroidLocalizationTesting/4_3s20B9780323661270000545.jpg)
Nuclear medicine imaging of parathyroid glands has continued to improve since its introduction in the 1980s. Many clinicians use nuclear imaging for first-line noninvasive “functional” imaging. It is safe, and it has sensitivity and specificity similar to US in most series. Nuclear imaging techniques can find glands that cannot be seen on US, such as those deep in the neck, posteriorly located or ectopic in the chest and mediastinum.
A basic understanding of how nuclear medicine scans work is important for understanding their advantages and limitations. Nuclear scintigraphy works by either oral or intravenous administration of a radionuclide tracer, which is preferentially taken up by the organ of interest. After the organ accumulates a sufficient amount of the tracer, it can be imaged by a gamma camera that captures gamma rays emitted by the radionuclide (technetium 99m sestamibi). Initially, nuclear medicine imaging was known as scintigraphy, which is now synonymous with traditional two-dimensional (2D), or planar, nuclear medicine images. Planar nuclear images are produced by recording the image with a single gamma camera from a single point of reference ( Figure 54.6 , A ).
The use of 99mTc-sestamibi (also known as MIBI) for parathyroid imaging was first described in 1989. Sestamibi is also used for cardiac perfusion studies. Unlike thallium, sestamibi accumulates in the mitochondria rather than in the intracellular potassium pool. Sestamibi washes out of parathyroid adenomas at different rates, depending on the concentration of mitochondria in the tissue. Parathyroid adenomas often have a high mitochondrial content compared with thyroid tissue, allowing for slower washout from parathyroid adenomas.
Currently, there is no parathyroid-specific radionuclide, which means all radionuclides that accumulate in the parathyroid glands also accumulate in the thyroid, thereby making it difficult to image only the parathyroid gland.
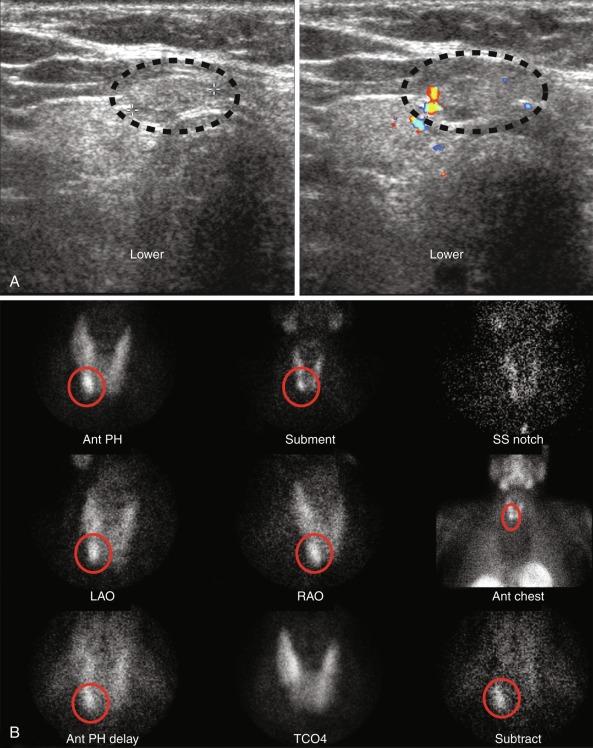
Double-tracer subtraction imaging can be used to solve this problem. In this technique, the patient is given two radionuclides, one that is thyroid specific and one that accumulates in both the thyroid and parathyroid glands. The two thyroid-specific radionuclides most commonly used are radioiodine ( 123 I) and technetium pertechnetate ( 99m Tc-pertechnetate). Images of both radionuclides are taken individually, and a computer is then used to “subtract” the “thyroid-only” image from the combined thyroid/parathyroid image, leaving only the parathyroid image ( Figure 54.7 ). This is technically challenging because it requires the patient to maintain the same position throughout the scan. Because of difficulty with motion artifact, this technique is only useful with planar images, and it cannot be used for single-photon emission computed tomography (SPECT) images. Planar, SPECT, and subtraction imaging can be used for all radionuclide tracers.
Sestamibi can be used to image parathyroid glands by a double-phase technique (using varying washout times) or subtraction imaging (using dual isotopes 123 I or thallium for highlighting the thyroid tissue) with similar accuracy. One study of 246 patients showed that subtraction imaging has a sensitivity of 89% for detecting single parathyroid adenomas; whereas, dual-phase imaging has a sensitivity of 73%. Specificities for both techniques were similar (90% to 95%). In the double-phase technique, images are first taken at 10 minutes and then at 2 to 3 hours.
Dual-phase imaging has advantages over subtraction imaging. Dual-phase imaging is not affected by motion artifact, making it better for SPECT imaging than subtraction imaging.
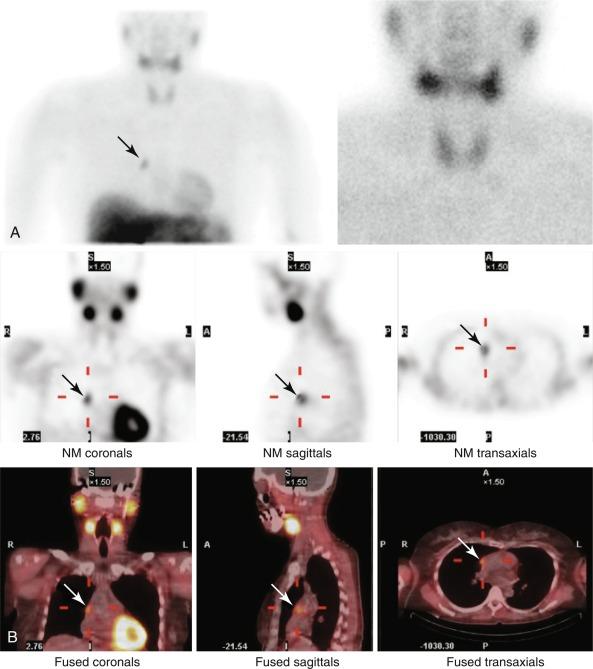
Single-photon emission computed tomography (SPECT) improved on planar images by taking a series of 2D images from multiple angles with either one or more gamma cameras. These images are reconstructed with a computer to produce 3D tomographic images of the organ of interest (see Figure 54.6 , B ). The 3D image adds more structural and anatomic information and improves the accuracy of localization. In expert hands, adenoma lateralization is more than 85%; similar to other modalities, multigland disease remains elusive.
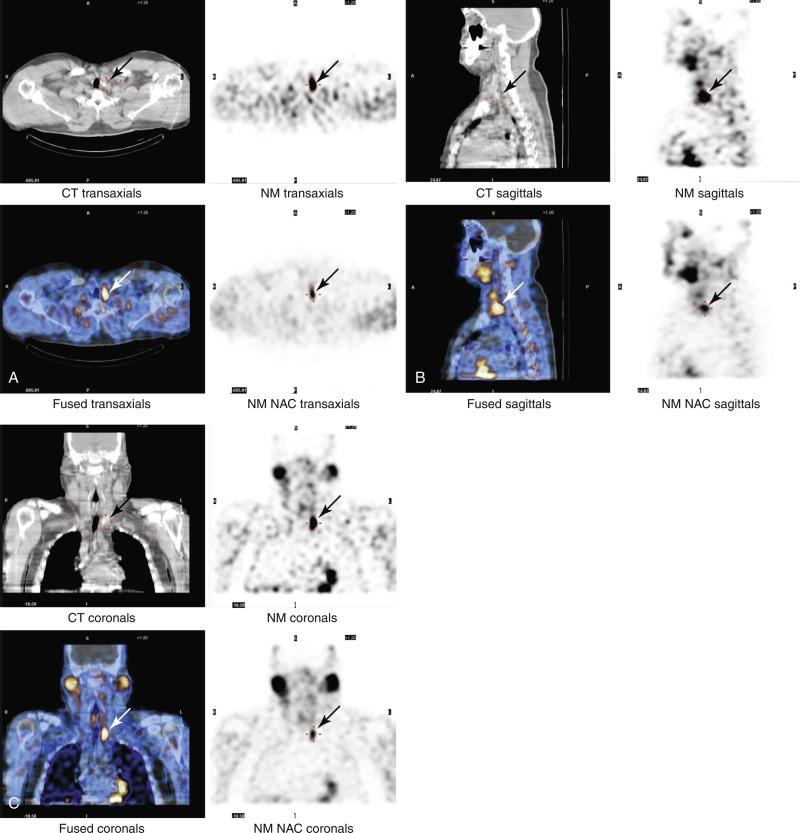
Although SPECT imaging increases the sensitivity of dual-phase planar imaging, it is still less sensitive than subtraction images. A planar subtraction image can be added to a dual-phase SPECT image by injecting 99m Tc-pertechnetate after obtaining the dual-phase delayed images. SPECT images can also be fused with a CT image to provide better imaging and to help plan minimally invasive parathyroidectomies ( Figure 54.8 ) and in multigland disease. Nuclear medicine studies are operator dependent; image acquisition, patient positioning, and protocol greatly affect accuracy.
Sestamibi has several limitations. False positives and false negatives can occur. Some solid thyroid nodules, such as Hürthle cell nodules, have a high oxyphilic content that can cause a false-positive sestamibi scan. This type of false-positive scan can be minimized by the combination of subtraction and dual-phase SPECT imaging. Sestamibi uptake by thyroid carcinomas as well as metastatic disease, lymphadenopathy, sarcoidosis, and brown fat also can cause false-positive results. False-negative sestamibi scans occur from low oxyphilic cell count in the parathyroid adenoma or increased sestamibi metabolism, causing early washout. Low oxyphilic cell count parathyroid tumors are typically associated with parathyroid hyperplasia, double adenomas, and smaller single adenomas.
Sestamibi is good for detecting large single adenomas, but it is less sensitive for hyperplasia, double adenomas, and small single adenomas. In a retrospective review from one institution, sestamibi detected only 30% of double adenomas. A systematic review of more than 20,000 cases found the sensitivity of sestamibi was 45% for multigland hyperplasia and 30% for double adenomas. Small parathyroid adenomas are harder to detect by sestamibi scans. In a meta-analysis of 338 adenomas, sestamibi could not detect adenomas smaller than 480 mg.
In conclusion, sestamibi is more sensitive for detecting single adenomas (sensitivity 89%) than thallium (sensitivity 72%) and is the nuclear imaging test of choice. The best method of sestamibi imaging is debatable; methods range from planar subtraction imaging, dual-phase imaging, and dual-phase SPECT imaging to SPECT/CT fusion imaging. All of these methods can be used in a single sestamibi scan to improve results. The biggest drawbacks of sestamibi are its low sensitivity for multigland disease and its inability to detect small adenomas.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here