Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the fully developed organism, growth hormone (GH), a 22-kDa molecule of 191 amino acids, is synthesized by acidophil cells in the anterior pituitary gland under the direction of hypothalamic GH-releasing hormone, which binds to its G protein–coupled receptor to stimulate GH secretion, and via somatotropin release–inhibiting factor, also known as somatostatin, an inhibitor of GH release in a “dominant negative” manner. The interplay of positive and negative signals results in a pulsatile release of GH. In addition, the anterior pituitary contains a distinct and separate GH secretagogue receptor. The endogenous ligand for GH secretagogue receptor is the gastric hormone ghrelin, which is released by the sensing of hunger, and acts to stimulate GH release to mobilize fuels via metabolic effects on fat, muscle, and liver, and induce insulin resistance to diminish the risk for hypoglycemia. Sleep augments GH release by increasing the amplitude of pulses; increased secretion occurs during the first phase of slow-wave sleep (stages III to IV). A variety of signals can augment GH secretion, including α-adrenergic stimuli, dopa, opioids, sex hormones (primarily estrogen), glucagon, and certain amino acids, especially arginine. Glucocorticoids and hypothyroidism blunt GH secretion.
Therefore, GH secretion is influenced by a variety of physiologic and nonphysiologic states that also form the basis for GH stimulation tests in the evaluation of GH reserves. The maturation of GH-releasing hormone secretion in the fetus precedes that of somatotropin release–inhibiting factor. Hence, GH secretion is stimulated in the absence of restraint by somatotropin release–inhibiting factor, and consequently, GH levels are high in the fetus and newborn infant and range from 20 to 50 ng/mL in the first few weeks of life. These levels gradually decline to below 10 ng/mL. Normal functional maturation of pulsatile GH secretion is not attained until several months of postnatal life in the term infant. High GH levels in the newborn may be partly related to diminished GH sensitivity and growth hormone receptor (GHR) immaturity.
From a diagnostic point of view, this has the advantage that screening for GH deficiency in the newborn does not require stimulation tests. A random GH level of 10 ng/mL or lower in the newborn is virtually diagnostic of GH deficiency. GH deficiency may be associated with hypoglycemia, requires evaluation of other pituitary hormone deficiencies that provide clues to the genetic defect responsible, and requires magnetic resonance imaging for determination of anatomic maldevelopment. Genetic studies using sequencing techniques such as targeted exome sequencing can be utilized to make a specific molecular diagnosis.
The gastric hormone ghrelin is also capable of directly stimulating GH release and increasing the GH response to GH-releasing hormone. The role of ghrelin in fetal GH secretion is not known. GH secretion is also regulated in a negative feedback fashion by insulin-like growth factor 1 (IGF-1), a member of the insulin-like growth factor family of peptides. In the fully mature organism, GH stimulates the production and release of IGF-1 from the liver after interacting with its cognate receptors, and this is the major source of circulating IGF-1; smaller amounts of IGF-1 may be produced after GH interacts with its receptors at local tissue sites, such as bone.
The effects of GH on growth are mediated in part by direct effects on bone and via the generation of IGF-1 ( Fig. 141.1 ). To do so, GH must interact with its cognate receptor, GHR, via a process mediated by the Janus kinase 2–signal transducer and activator of transcription 5 signaling cascade. GHR belongs to the cytokine family of receptors. In humans, after binding GH, the extracellular binding domain of GHR is released into the circulation as GH-binding protein, which can be measured as an indirect reflection of GHR. IGF-1 from the liver is coupled with a binding protein (insulin-like growth factor–binding protein 3) and the acid-labile subunit as a ternary complex transporting IGF-1 in the circulation. IGF-1 also can be generated in bone, muscle, and fat, but the predominant source in the circulation is the liver.
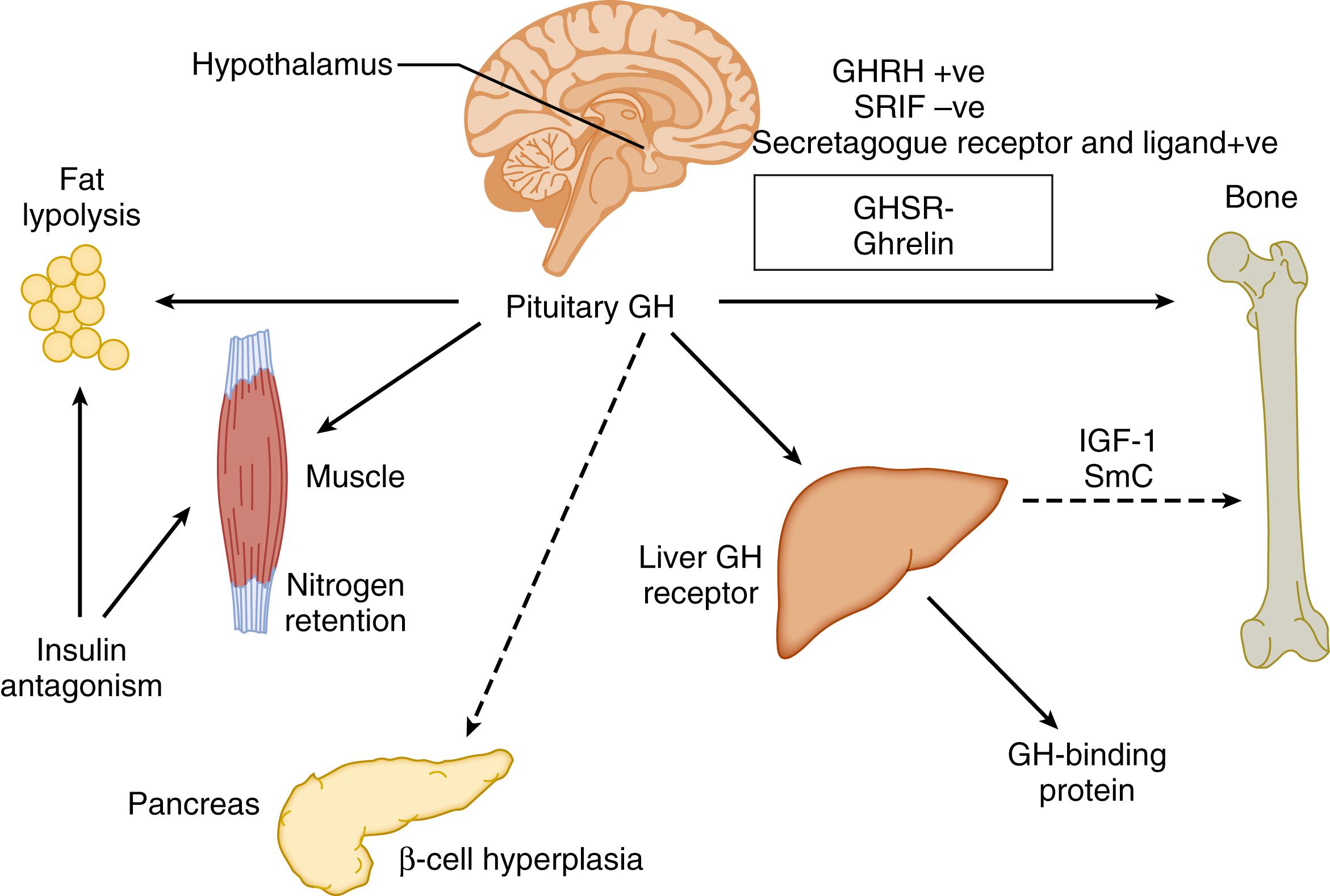
GH is so named because of its dramatic effects in those with excessive GH-secreting conditions, gigantism if onset is before closure of the epiphyses, and acromegaly if onset begins after puberty. This growth is caused in part by direct effects of GH itself and in part by a “second messenger,” IGF-1. However, GH has profound direct effects on metabolic functions. These include lipolysis with release of fatty acids and their subsequent mitochondrial conversion to ketone bodies that can be used as a source of energy, as well as the augmentation of amino acid uptake and nitrogen retention by muscle. In addition, GH induces resistance to insulin’s effects on carbohydrate metabolism, which is compensated by an increase in pancreatic insulin secretion and hyperplasia of pancreatic β cells in young organisms. However, pancreatic “exhaustion” can lead to various degrees of carbohydrate intolerance, including diabetes, in the older organism. Notably, the resistance to insulin’s effects on carbohydrate is not shared by muscle or fat ; GH has synergistic effects on muscle anabolism via insulin, as well as via sex steroids. Thus GH, in addition to its growth-promoting effects, is a major regulator of metabolism, acting to counter insulin effects to avoid hypoglycemia (i.e., it is one of the counterregulatory hormones together with glucagon, catecholamines, and cortisol), promoting lipolysis in response to fasting, and regulating muscle anabolism.
The consequences of deficiency or excess of GH secretion and action become apparent from consideration of the growth-promoting and metabolic effects of this hormone system. Deficiency of GH promotes fasting hypoglycemia, poor muscle development, excessive body fat, poor growth, and diminished bone mass. Conversely, excessive secretion and action of GH result in rapid growth, well-developed muscles with large bones, increased lean body mass, and possibly impaired carbohydrate tolerance. The degree and severity of these features depend on the degree of deficiency/excess of the hormone and timing. The developmental aspects of these coordinated actions form the basis of this chapter.
Growth of the human is fastest in fetal life and continues at a fast but rapidly decelerating rate in the neonatal period. Fetal growth is determined by genetic, nutritional, and environmental factors. Maternal environmental influences are the most important determinant of fetal growth. However, several genetic conditions causing growth factor deficiencies may play a role in intrauterine growth retardation. IGF1 mutations and deletions have been associated with intrauterine growth failure. Genetic defects in IGF2 have also been identified to cause both prenatal and postnatal growth failure due to insulin-like growth factor 2 (IGF-2) deficiency. External environmental factors such as nutrition and good general health are the key factors in normal growth in early infancy. Although the GH-IGF-1 axis has significant roles in childhood growth, growth in the fetus and the newborn is not determined by GH because GHR is poorly expressed in fetal life and appears to become functional at approximately 3 to 6 months of life. However, GH levels are high in the newborn, and although they assist in limiting hypoglycemia in the newborn, the full functional significance of these high concentrations is not clear. Understanding the natural patterns of developmental and functional changes in pituitary hormone-producing cells during the fetal and perinatal period will help to differentiate normal and adverse development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here