Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The ability of allogeneic hematopoietic cell transplantation (HCT) to cure certain hematologic malignancies is widely recognized. An important therapeutic aspect of HCT in eradicating malignant cells is the graft-versus-leukemia (GVL) effect. The importance of the GVL effect in allogeneic HCT has been recognized since the earliest experiments in stem cell transplantation. Forty years ago, Barnes and colleagues noted that leukemic mice treated with a subtherapeutic dose of radiation and a syngeneic (identical twin) graft transplant were more likely to relapse than mice given an allogeneic stem cell transplant. They hypothesized that the allogeneic graft contained cells with immune reactivity necessary for eradicating residual leukemia cells. They also noted that recipients of allogeneic grafts, although less likely to relapse, died of a “wasting syndrome” now recognized as graft-versus-host disease (GVHD). Thus, in addition to describing GVL, these experiments highlighted for the first time the intricate relationship between GVL and GVHD. Since these early experiments, both GVHD and the GVL effect have been studied extensively. This chapter reviews the pathophysiology, clinical features, and treatment of GVHD and summarizes current understanding of the relationships between GVHD and the GVL effect.
Ten years after the work of Barnes and Loutit, Billingham formulated the requirements for the development of GVHD: the graft must contain immunologically competent cells, the recipient must express tissue antigens that are not present in the transplant donor, and the recipient must be incapable of mounting an effective response to destroy the transplanted cells. According to these criteria, GVHD can develop in various clinical settings when tissues containing immunocompetent cells (blood products, bone marrow, and some solid organs) are transferred between persons. The most common setting for the development of GVHD is following allogeneic HCT; without prophylactic immunosuppression, most allogeneic HCTs will be complicated by GVHD. GVHD is induced by mismatches between histocompatibility antigens between the donor and recipient. Matching of the major histocompatibility complex (MHC) antigens hastens engraftment and reduces the severity of GVHD. In humans, the MHC region lies on the short arm of chromosome 6 and is called the human leukocyte antigen (HLA) region. The HLA region is divided into two classes, class I and class II, each containing numerous gene loci that encode a large number of polymorphic alleles. MHC class I molecules are involved in the presentation of peptides to CD8 + T cells, and class II molecules present peptides to CD4 + T cells.
The determination of HLA types has become much more accurate with molecular techniques that replace earlier serologic or cellular methods. The average patient has a 25% chance of having an HLA match within his or her immediate family. Patients who lack an HLA-identical family member donor must seek an unrelated haploidentical or cord blood donor. In patients whose ancestry involves extensive interracial mixing, the chances of identifying an HLA identical donor are diminished. Using a population-based genetic model, it is estimated that 75% of Whites of European decent will have a high-resolution matched unrelated donor at the key major class 1 and class II loci; however, patients of African or South Asian ancestry have a less than 30% chance of identifying a similar pairing. Advances in the clinical application of haploidentical (partial HLA match) HCT have now expanded alternate options for ethnic groups disproportionately affected by limited access to unrelated donors.
Despite HLA identity between a patient and donor, substantial numbers of patients still develop GVHD because of differences in minor histocompatibility antigens (MiHAs) that lie outside the HLA loci. Genome-wide arrays demonstrate that single nucleotide polymorphisms (SNPs), reflecting amino-acid coding differences between donor-recipient pairs, are themselves sufficient for producing clinical GVHD. For example, each 1% difference in SNPs resulted in 20% relative risk of developing grade III to IV GVHD. Nonetheless, the identity of the relevant minor antigens that comprise differences in peptides that are expressed on the cell surface bound to specific HLA molecules has yet to be precisely elucidated.
Acute GVHD typically occurs within 30 days of transplantation (in HLA-matched recipients receiving standard GVHD prophylaxis) but occurs in diminishing frequencies for up to 6 months. The incidence ranges from less than 10% to more than 80%, depending on the degree of histoincompatibility between donor and recipient, the number of T cells in the graft, the patient’s age, and the GVHD prophylactic regimen. The principal target organs include the immune system, skin, liver, and intestine. GVHD occurs first and most commonly in the skin as a pruritic maculopapular rash, often involving the palms, soles, and ears; it can progress to total-body erythroderma, with bullae formation, rupture along the epidermal-dermal border, and desquamation in severe cases. Gastrointestinal (GI) and liver manifestations often appear later and rarely represent the first and only findings. Intestinal symptoms include anorexia, nausea, diarrhea (sometimes bloody), abdominal pain, and paralytic ileus. Liver dysfunction includes hyperbilirubinemia and increased serum alkaline phosphatase and aminotransferase values. Coagulation studies may become abnormal, and hepatic failure with ascites and encephalopathy may develop in severe cases. Hepatic GVHD can be distinguished from hepatic venoocclusive disease (VOD) by weight gain or pain in the right upper quadrant in the latter. Acute GVHD also results in the delayed recovery of immunocompetence. The clinical result is profound immunodeficiency and susceptibility to infections, often further accentuated by the immunosuppressive agents used to treat GVHD.
Pathologically, the sine qua non of acute GVHD is selective epithelial damage of target organs. The epidermis and hair follicles are damaged and sometimes destroyed. Small bile ducts are profoundly affected, with segmental disruption. The destruction of intestinal crypts results in mucosal ulcerations that may be either patchy or diffuse. Other epithelial surfaces, such as the conjunctivae, vagina, and esophagus, are less commonly involved. A peculiarity of GVHD histology is the early paucity of mononuclear cell infiltrates; however, as the disease progresses, the inflammatory component may be substantial. Studies that identified inflammatory cytokines as soluble mediators of GVHD have suggested that direct contact between target cells and lymphocytes is not always required (see following sections). GVHD lesions are not evenly distributed: in the skin, damage is prominent at the tip of rete ridges; in the intestine, at the base of the crypts; and in the liver, in the periductular epithelium. These areas all contain a high proportion of stem cells, giving rise to the idea that GVHD targets may be undifferentiated epithelial cells with primitive surface antigens.
The histologic severity of GVHD is at best semiquantitative in part due to inability to sample tissues in the small intestine, and consequently pathologic scores are not used to grade GVHD. Because it is often difficult to obtain an adequate tissue biopsy, and because it can be very difficult to distinguish GVHD from other post-HCT complications such as drug eruptions or infectious complications, the physician is left to use clinical judgment.
Standard grading systems generally include clinical changes in the skin, GI tract, liver, and performance status ( Table 109.1 ). Although the severity of GVHD is often difficult to quantify, the overall maximal grade correlates with disease outcome: mild GVHD (grade I or II) is associated with little mortality, whereas higher grades are associated with significantly decreased survival. Refined staging methods that standardize diagnosis and clinical grading (Mount Sinai Acute GVHD International Consortium [MAGIC]) are becoming more widely adopted in clinical practice and research. However, consistent across all clinical staging systems is the association between high-grade organ involvement (grade III to IV) or disease affecting multiple organs and increased mortality. Once severe GVHD is established, the likelihood of responding to treatment is less than 50% and patients are at twice the risk of succumbing to GVHD or its sequela than mild to moderate forms of disease. Although GVHD remains a clinical diagnosis, recent advances in the use of biomarkers may improve diagnostic sensitivity or provide early prognostic information to inform primary therapy.
| Organ | Clinical Manifestations | Staging |
|---|---|---|
| Skin |
|
|
| Liver | Painless jaundice with conjugated hyperbilirubinemia and increased alkaline phosphatase |
|
| Gastrointestinal tract |
|
|
The clinical features, staging, and grading of acute GVHD are summarized in Tables 109.1 and 109.2 . In a comprehensive review of patients receiving therapy for acute GVHD, Martin and colleagues found that 81% had skin involvement, 54% had GI involvement, and 50% had liver involvement at the initiation of therapy. After high-intensity (myeloablative) conditioning, acute GVHD generally occurs within 14 to 35 days of stem cell infusion. A rapid and severe form of GVHD may occur in patients with severe HLA mismatches and in patients who receive T cell–replete transplants without or with inadequate in vivo GVHD prophylaxis. Although such GVHD is sometimes called “hyperacute,” this term is misleading because it is pathophysiologically distinct from hyperacute rejection after solid organ allografting, which is due to preformed antibodies. This form of GVHD, which is manifested by fever, generalized erythroderma and desquamation, and often edema, typically occurs about 1 week after stem cell infusion and may be rapidly fatal. In patients receiving standard (in vivo) GVHD prophylaxis such as a combination of calcineurin inhibitors, such as cyclosporine (CSP) or tacrolimus, and methotrexate, the median onset of GVHD is typically 21 to 25 days after transplantation; however, after in vitro T-cell depletion of the graft, the onset of GVHD symptoms may be much later. Thus the findings of rash and diarrhea by 1 week after transplantation would very likely be due to ineffective prophylaxis and would be very unlikely with the use of calcineurin inhibitors or in vitro T-cell depletion of the stem cell inoculum. A less ominous syndrome of fever, rash, and fluid retention occurring in the first 1 to 2 weeks after stem cell infusion is the “engraftment syndrome.” These manifestations may be seen with either allogeneic or autologous transplantation. Although this syndrome’s pathophysiology is poorly understood, it is thought to be due to a wave of cytokine production as the graft starts to recover. These symptoms are related to, but distinct from, the “cytokine storm” of acute GVHD because there is no concomitant T cell–mediated attack. This syndrome responds immediately to steroids in most patients, and it typically presents earlier than acute GVHD.
| Overall Grade | Skin | Liver | Gut | |
|---|---|---|---|---|
| I | 1–2 | 0 | 0 | |
| II | 1–3 | 1 | and/or | 1 |
| III | 2–3 | 2–4 | and/or | 2–3 |
| IV | 2–4 | 2–4 | and/or | 2–4 |
a See Table 109.1 for individual organ staging. Traditionally, individual organs are staged without regard to attribution. However, the overall grade of graft-versus-host disease (GVHD) reflects the actual extent of GVHD. To achieve each overall grade, skin disease plus liver and/or gut involvement are required.
Skin is the most commonly affected organ ( Fig. 109.1 ). In patients receiving transplants after myeloablative conditioning, the skin is usually the first organ involved, and GVHD often coincides with engraftment. However, the presentation of GVHD is more varied following nonmyeloablative transplants or donor lymphocyte infusions. The characteristic maculopapular rash can spread throughout the rest of the body but usually spares the scalp; it is often described as feeling like a sunburn, tight or pruritic. In severe cases the skin may blister and ulcerate. Histologic confirmation is critical to rule out drug reactions, viral infections, etc. but is optimally performed 1 to 2 days after the onset of rash in cases of patchy involvement. Pathologic findings that describe apoptosis at the base of dermal crypts is characteristic. Other features include dyskeratosis, exocytosis of lymphocytes, satellite lymphocytes adjacent to dyskeratotic epidermal keratinocytes, and dermal perivascular lymphocytic infiltration.
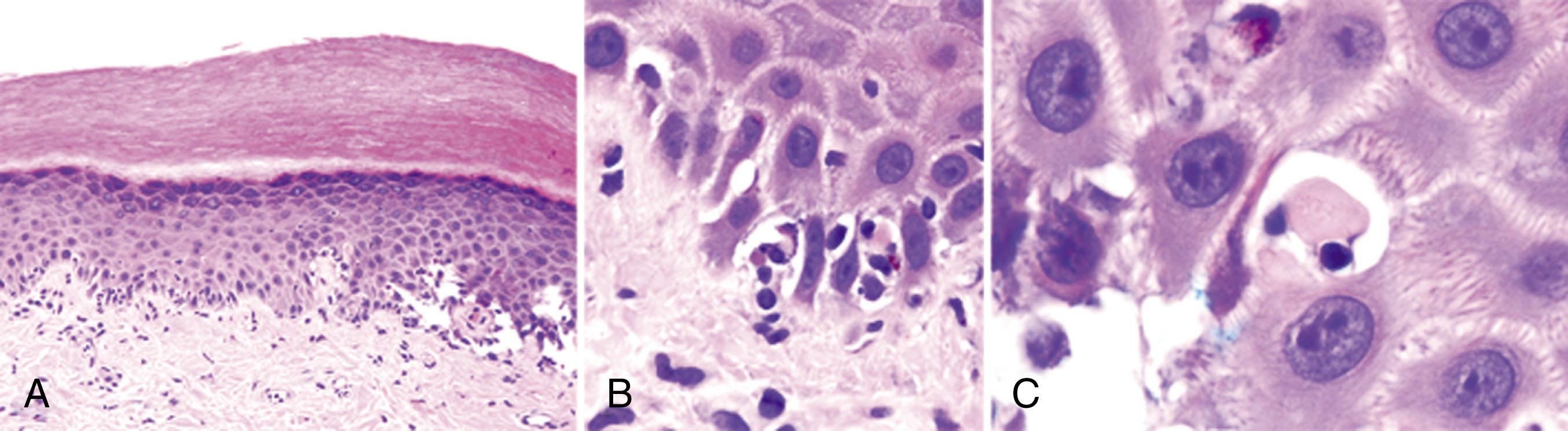
GI tract involvement of GVHD may present as nausea, vomiting, anorexia, diarrhea, and/or abdominal pain. It is a panintestinal process, often with differences in severity between the upper and lower GI tracts. Although gastroparesis is seen after bone marrow transplant, it is usually not associated with GVHD. The diarrhea of GVHD is secretory; in extreme cases significant GI blood loss may occur as a result of mucosal ulceration and is associated with a poor prognosis. In advanced disease, diffuse, severe abdominal pain and distension is accompanied by voluminous diarrhea (>2 L/day).
Radiologic findings of the GI tract include luminal dilatation with thickening of the wall of the small bowel and air/fluid levels suggestive of an ileus on abdominal flat plates or small bowel series. Abdominal computed tomography may show the “ribbon” sign of diffuse thickening of the small bowel wall. Little correlation exists between the extent of disease and the appearance of mucosa on endoscopy, but mucosal sloughing is pathognomonic for severe disease. Nevertheless, some studies have shown that antral biopsies correlate well with the severity of GVHD in the duodenum and in the colon even when the presenting symptom is diarrhea. Histologic analysis of tissue is imperative to establish the diagnosis. The histologic features of GI GVHD are the presence of apoptotic bodies in the base of crypts, crypt abscesses, crypt loss, and flattening of the surface epithelium.
Liver function test abnormalities are common after bone marrow transplant and occur secondary to VOD, drug toxicity, viral infection, sepsis, iron overload, and other causes of extrahepatic biliary obstruction. The exact incidence of hepatic GVHD is unknown because many patients do not undergo liver biopsies. The development of jaundice or an increase in the alkaline phosphatase and bilirubin may be the initial features of acute GVHD of the liver. The histologic features of hepatic GVHD are endothelialitis, lymphocytic infiltration of the portal areas, pericholangitis, and bile duct destruction and loss.
Whether GVHD affects organs other than the classic triad of skin, liver, and gut has remained a matter of debate, although numerous reports suggest additional organ manifestations. The most likely candidate is the lung. Lung toxicity, including interstitial pneumonitis and diffuse alveolar hemorrhage, may occur in 20% to 60% of allogeneic transplant recipients but in fewer autologous transplant recipients. Causes of pulmonary damage other than GVHD include engraftment syndrome (see earlier), infection, radiation pneumonitis, and chemotherapy-related toxicity (e.g., methotrexate, busulfan). One retrospective analysis failed to link severe pulmonary complications to clinical acute GVHD per se. The mortality due to pneumonia increases with the severity of GVHD, but this association may be related to increased immunosuppressive therapy.
Despite the ability of kidneys and hearts to serve as targets of transplant rejection, there is no convincing evidence for direct renal or cardiac damage from acute GVHD that is not secondary to drugs or infection. Similarly, neurologic complications are also common after transplantation but most can be attributed to drug toxicity, infection, or vascular insults.
Acute GVHD ought to be distinguished from any process that causes a constellation of fever, erythematous skin rash, and pulmonary edema that may occur during neutrophil recovery and has been termed engraftment or capillary leak syndrome. In allogeneic transplant recipients, distinction from acute GVHD is difficult. Engraftment syndrome is thought to reflect cellular and cytokine activities during early recovery of (donor-derived) blood cell counts and/or homeostatic proliferation of lymphocytes, but a precise delineation of the activated cells and mechanisms has not been demonstrated. Engraftment syndrome may be associated with increased mortality, primarily but not exclusively from pulmonary failure. Corticosteroid therapy may be effective particularly for the treatment of pulmonary manifestations. Skin rashes may reflect delayed reactions to the conditioning regimen, antibiotics, or infections; furthermore, histopathologic skin changes consistent with acute GVHD can be mimicked by chemoradiotherapy and drug reactions. Diarrhea can be a consequence of total body irradiation (TBI), viral infection (especially with cytomegalovirus [CMV] and other herpes viruses), parasitic infection, Clostridioides difficile infection, nonspecific gastritis, narcotic withdrawal, and drug reactions—all of which mimic GVHD of the gut. Liver dysfunction can be due to parenteral nutrition, VOD, and viral- or drug-induced hepatitis. Thus establishing a diagnosis of GVHD requires consideration of the timing of events in relation to HCT conditioning as well as exclusion of relevant infectious etiologies in HCT patients.
The graft-versus-host (GVH) reaction was first noted when irradiated mice were infused with allogeneic marrow and spleen cells. Although mice recovered from radiation-induced injury and marrow aplasia, they subsequently died with “secondary disease,” a phenomenon subsequently recognized as acute GVHD. Three requirements for the development of GVHD were formulated by Billingham. First, the graft must contain immunologically competent cells, now recognized as mature T cells. In both experimental and clinical allogeneic HCT, the severity of GVHD correlates with the number of donor T cells transfused. The precise nature of these cells and the mechanisms they use are now understood in greater detail (see later). Second, the recipient must be incapable of rejecting the transplanted cells (i.e., immunocompromised). After allogeneic HCT, the recipient is typically immunosuppressed by chemotherapy and/or radiotherapy before the hematopoietic cell infusion. Third, the recipient must express tissue antigens that are not present in the transplant donor. Thus Billingham’s third postulate stipulates that the GVH reaction occurs when donor immune cells recognize disparate host antigens. These differences are governed by the genetic polymorphisms.
Recognition of alloantigens depends on the match with the presenting major histocompatibility molecule. In humans, the MHC is governed by the HLA antigens that are encoded by the MHC gene complex on the short arm of chromosome 6 and can be categorized as class I, II, and III. Class I antigens (HLA-A, B, and C) are expressed on almost all cells of the body. Class II antigens (DR, DQ, and DP) are primarily expressed on hematopoietic cells, although their expression can also be induced on other cell types following inflammation. The incidence of acute GVHD is directly related to the degree of MHC mismatch. For example, recipients of single loci mismatched unrelated donors have higher rates of grade II to IV GVHD, higher transplant-related mortality, and inferior survival compared with 8/8 HLA-matched unrelated donors. In the context of 8/8 HLA matching, mismatching that was nonpermissive (based on T-cell epitope grouping) was associated with increased mortality compared with permissive mismatches. In alternative donor HCT the degree of HLA disparities also correlates with GVHD risk; however, there appears to be greater permissiveness as compared with unrelated donor transplant. However, it should be acknowledged that direct comparisons between various donor source are challenging because conditioning regimens and GVHD prevention platforms may differ significantly.
In most clinical allogeneic transplants where the MHC of donor and recipient are matched, donor T cells recognize MHC-bound peptides derived from the protein products of polymorphic genes (MiHAs) that are present in the host but not in the donor. Substantial numbers (50%) of patients will develop acute GVHD despite receiving HLA-identical grafts as well as optimal postgrafting immune suppression. MiHAs are widely but variably expressed in different tissue, which is one possible explanation for the unique target organ distribution in GVHD. Many MiHAs such as HA-1 and HA-2 are expressed on hematopoietic cells, which may be one reason for the host immune system to be a primary target for the GVH response, and help to explain the critical role of direct presentation by professional recipient antigen-presenting cells (APCs) in the GVH response. By contrast, other MiHAs such as H-Y and HA-3 are expressed ubiquitously. MiHAs do not all equally induce lethal GVHD but show hierarchic immunodominance. Furthermore, the difference in a single immunodominant MiHA is insufficient to elicit GVHD in murine models, even though a single MiHA can elicit T cell–mediated damage in a skin explant model. However, the role of specific MiHAs that are able to induce clinical GVHD has not been systematically evaluated in large groups of patients.
Genetic polymorphisms in several non-HLA genes such as in killer-cell immunoglobulin (Ig)-like receptors (KIRs), cytokines, and nucleotide-binding oligomerization domain containing 2 (NOD2) genes have been shown to modulate the severity and incidence of GVHD.
KIRs on natural killer (NK) cells that bind to the HLA class I gene products are encoded on chromosome 19. Polymorphisms in the transmembrane and cytoplasmic domains of KIRs govern whether the receptor has inhibitory (e.g., KIR2DL1, -2DL2, -2DL3, and -3DL1) or activating potential. Two competing models have been proposed for HLA-KIR allorecognition by donor NK cells following allogeneic HCT: the “mismatched ligand” and the “missing ligand” models. Both models are supported by several clinical observations, albeit in patients receiving very different transplant and immunosuppressive regimens (see Chapter 21, Chapter 107 ).
Proinflammatory cytokines involved in the classic cytokine storm of GVHD cause pathologic damage to target organs, such as the skin, liver, and GI tract (see later). Several cytokine gene polymorphisms in both recipients and donors have been implicated. Specifically, tumor necrosis factor (TNF) polymorphisms (TNFd3/d3 in the recipient, TNF863 and TNF857 in donors and/or recipients, and TNFd4, TNF-α-1031C, and TNFRII-196R in the donors) have been associated with an increased risk for acute GVHD and transplant-related mortality (TRM). The three common haplotypes of the interleukin (IL)-10 gene promoter region in recipients, representing high, intermediate, and low production of IL-10, have been associated with severity of acute GVHD following HLA-matched sibling donor allogeneic HCT. By contrast, smaller studies have found neither IL-10 nor TNF-α polymorphisms to be associated with GVHD following HLA-mismatched cord blood transplant (CBT). Interferon-γ (IFN-γ ) polymorphisms of the 2/2 genotype (high IFN-γ production) and 3/3 genotype (low IFN-γ) have been associated with decreased or increased acute GVHD, respectively.
NOD2/caspase-activating recruitment domain 15 (CARD15) gene polymorphisms in both the donors and recipients were recently shown to have a striking association between GI GVHD and overall mortality following related and unrelated donor allogeneic HCT. It is likely that the importance of non-HLA gene polymorphisms in GVHD will differ depending on the donor source (related vs. unrelated), HLA disparity (matched vs. mismatched), graft source (cord blood vs. bone marrow vs. peripheral blood stem cells), and the intensity of the conditioning.
It is helpful to remember two important principles when considering the pathophysiology of acute GVHD. First, acute GVHD represents exaggerated but normal inflammatory responses against foreign antigens (alloantigens) that are ubiquitously expressed in a setting where they are undesirable. The donor lymphocytes that have been infused into the recipient function appropriately, given the foreign environment they encounter. Second, donor lymphocytes encounter tissues in the recipient that have been often profoundly damaged. The effects of the underlying disease, prior infections, and the intensity of conditioning regimen all result in substantial changes not only in the immune cells but also in the endothelial and epithelial cells. Thus the allogeneic donor cells rapidly encounter not simply a foreign environment, but one that has been altered to promote the activation and proliferation of inflammatory cells. Therefore the pathophysiology of acute GVHD may be considered a distortion of the normal inflammatory cellular responses that, in addition to the absolute requirement of donor T cells, involves multiple other innate and adaptive cells and mediators. The development and evolution of acute GVHD can be conceptualized in three sequential phases ( Fig. 109.2 ) to provide a unified perspective on the complex cellular interactions and inflammatory cascades that lead to acute GVHD: (1) activation of the APCs; (2) donor T-cell activation, differentiation, and migration; and (3) effector phase. It is important to note that this three-phase description permits a unified perspective on GVHD biology, but it is not meant to suggest that all three phases are of equal importance or that GVHD occurs in a stepwise and sequential manner. The spatiotemporal relationships among these biologic processes, depending on the context, are likely to of vary, and their relevance to the induction, severity, and maintenance of GVHD may depend on the factors cited earlier.
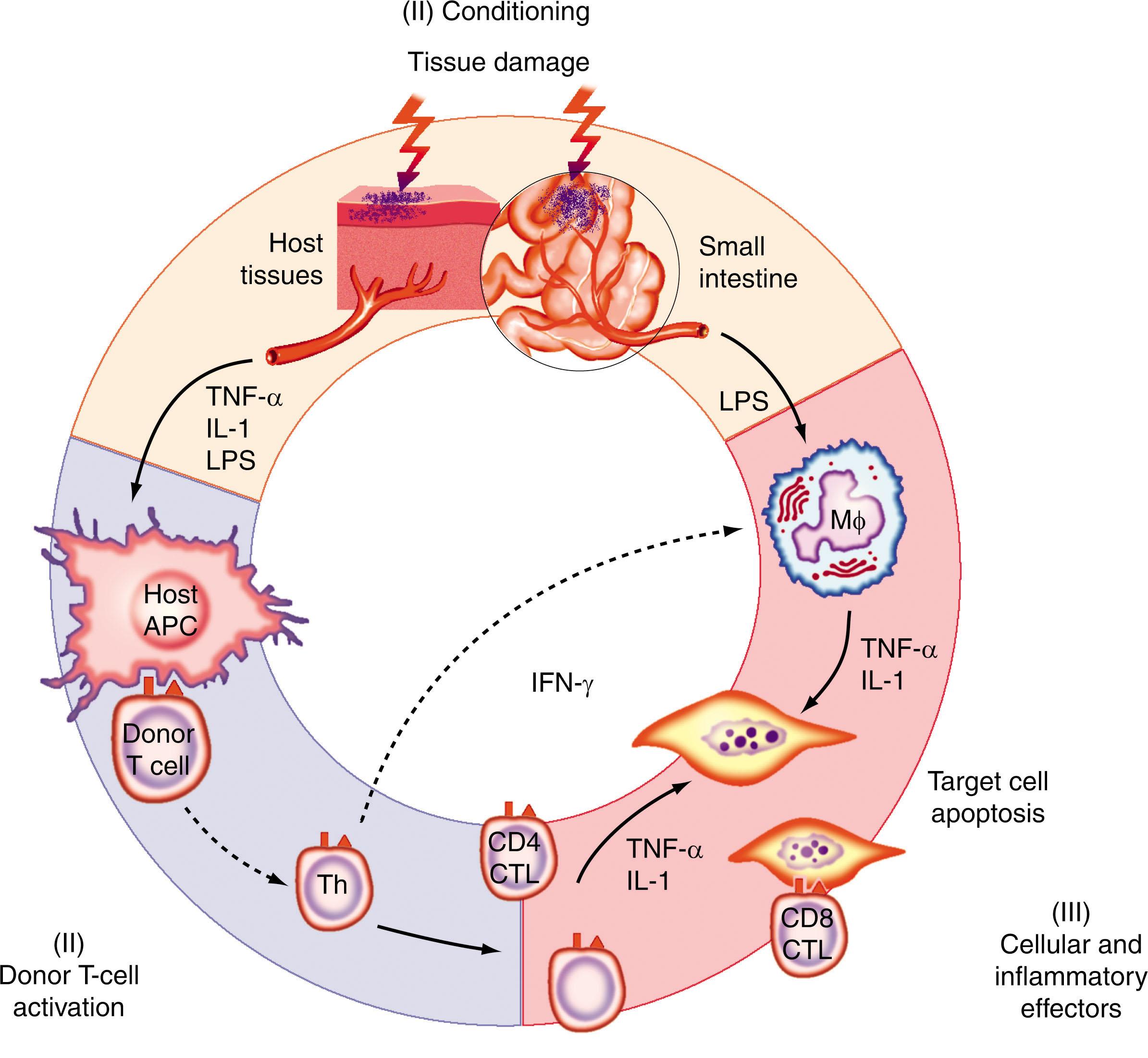
The earliest phase of acute GVHD is initiated by the profound damage caused by the underlying disease and infections and further exacerbated by BMT conditioning regimens (which include TBI and chemotherapy) that are administered even before the infusion of donor cells. This first step results in activation of the APCs. Specifically, secretion of proinflammatory cytokines from APCs, such as TNF-α and IL-1, described as the “cytokine storm.” More recently, HCT models have revealed an emerging role for damage-associated molecular patterns (DAMPs), released from injured host tissues, and pathogen-associated molecular patterns (PAMPs), from microbes, in amplifying inflammation and GVHD. These “danger signals” engage TLRs and other pattern recognition receptors to activate host APCs. Damage to the GI tract from the conditioning is particularly important in this process because it allows for systemic translocation of immunostimulatory PAMPs such as lipopolysaccharide (LPS) that further enhance the activation of host APCs, and the secondary lymphoid tissue in the GI tract is likely the initial site of interaction between activated APCs and donor T cells. This scenario accords with the observation that an increased risk for GVHD is associated with intensive conditioning regimens that cause extensive injury to epithelial and endothelial surfaces with a subsequent release of inflammatory cytokines and increases in expression of cell surface adhesion molecules. The relationship among conditioning intensity, inflammatory cytokine, and GVHD severity has been supported by elegant murine studies. Furthermore, the observations from these experimental studies have led to two recent clinical innovations to reduce clinical acute GVHD: (1) reduced intensity conditioning to decrease the damage to host tissues and thus limit activation of host APC and (2) KIR mismatches between donor and recipients to eliminate the host APCs by the alloreactive NK cells.
Host-type APCs that are present and have been primed by conditioning are critical for the induction of this phase; recent evidence suggests that donor-type APCs exacerbate GVHD, but in certain experimental models, donor-type APC chimeras also induce GVHD. In clinical situations, if donor-type APCs are present in sufficient quantity and have been appropriately primed, they too might play a role in the initiation and exacerbation of GVHD. Among the cells with antigen-presenting capability, dendritic cells (DCs) are the most potent and play an important role in the induction of GVHD. Experimental data suggest that GVHD can be regulated by qualitatively or quantitatively modulating distinct DC subsets. Langerhans cells were also shown to be sufficient for the induction of GVHD when all other APCs were unable to prime donor T cells, although the role for Langerhans cells when all APCs are intact is dispensable. Studies have yet to define roles for other DC subsets. In one clinical study, persistence of host DC after day 100 correlated with the severity of acute GVHD, whereas elimination of host DCs was associated with reduced severity of acute GVHD. The allostimulatory capacity of mature monocyte-derived DCs (mDCs) after reduced intensity transplants was lower for up to 6 months compared with the mDCs from myeloablative transplant recipients, thus suggesting a role for host DCs and the reduction in danger signals secondary to less intense conditioning in acute GVHD. Nonetheless this concept of enhanced host APC activation explains a number of clinical observations such as increased risks for acute GVHD associated with advanced-stage malignancy, conditioning intensity, and histories of viral infections.
Other professional APCs such as monocytes/macrophages or semiprofessional APCs might also play a role in this phase. For example, recent data suggest that host-type B cells might play a regulatory role under certain contexts, whereas in other contexts, they were dispensable for GVHD induction. Similarly, host basophils have been shown not to affect the induction or severity of acute GVHD. Recent data suggest that radiosensitive hematopoietic-derived APCs may not be obligatory for induction of APCs. In addition, host- or donor-type nonhematopoietic stem cells (e.g., mesenchymal stem cells, endothelial cells, epithelial cells, or stromal cells) can function as APCs in the context of inflammation. The role of these cells in the presence and absence of professional hematopoietic-derived APCs remains to be elucidated.
GI tract cells such as intestinal epithelial cells (IECs), intestinal stem cells (ISCs), and Paneth cells are targeted in GI GVHD. GI tract damage from conditioning along with subsequent disruptions in intestinal homeostasis, which are further compounded by the use of broad-spectrum antibiotics and alterations in nutrition that occur during the process of allogeneic HCT, are now understood to play important roles in augmenting GVHD.
Maintenance of intestinal homeostasis is in part regulated by the intestinal microbiota and its interactions with the host immune system. The host immune system has been shown to modulate the intestinal microbiota and its disturbances result in intestinal microbiota dysbiosis. For example, retinoic acid receptor–related orphan receptor-γt (RORγt) + group 3 innate lymphoid cells (ILC3s) regulate intestinal bacteria by limiting local and systemic inflammation, and the absence of intestinal immune cells, particularly isolated lymphoid follicles (ILFs), has been shown to result in alteration of the intestinal microbiota of mice by over representation of gram-negative bacteria. The intestinal microbiota also modulates host immune responses. Germ-free mice developed an increase in IFN-γ after colonization with Escherichia coli . In another study, germ-free mice colonized with conventional intestinal microbiota developed structural changes in the depth of the intestinal epithelial crypts as well as expansion in the lamina propria with an increase in immune cells after exposure to intestinal bacteria. Multiple studies have linked the intestinal microbiota to regulation of T cells. In one study, it was shown that colonization of mice with segmented filamentous bacteria (SFB) induces CD4 + T helper cells in the lamina propria. In other studies, several intestinal microbes have been shown to induce forkhead box protein (Foxp3)-expressing regulatory T cells (Tregs). Furthermore, interactions between the intestinal microbiota and the host promote the functions of nonimmune cells such as IECs and goblet cells which play important roles in regulating the barrier function of the GI tract. Thus emerging data now strongly point to intestinal microbiota as playing an important role in intestinal homeostasis and augmenting GVHD.
After allogeneic HCT there is loss of microbial diversity with a shift towards Enterococci and fewer obligate anaerobic bacteria, such as Frimicutes . This change can be pronounced with routine clinical use of antibiotics and the development of GVHD. Acute GVHD itself has been associated with major fluctuations in the intestinal microbiota, including a general loss of diversity and expansion of Enterobacteriales (including Escherichia, Klebsiella , and Enterobacter ), Lactobacillales (including Lactobacillus, Enterococcus , and Streptococcus ), Proteobacteria , and Akkermansia . This expansion is accompanied with loss of beneficial microbes including Firmicutes (including Clostridia and Blautia ). Accompanying these shifts is Paneth cell loss in experimental GVHD that results in decreased secretion of α-defensins, leading to further intestinal dysbiosis, and that loss of Paneth cells and dysbiosis were associated with nonrelapse mortality (NRM) in clinical GVHD. Several studies have noted that expansion of specific pathogenic bacteria (VRE, Enterococcus) often preceded bloodstream infections by the same organisms and identified treatment with certain antibiotics with anaerobic coverage such as metronidazole as a risk factors for bacterial expansion. The intestinal microbiota can also modulate acute GVHD severity. In the 1970s, investigators paradoxically found less GVHD in mice transplanted in germ-free conditions or receiving gut decontamination antibiotics. These studies have not been confirmed in modern day germ-free mice facilities. However, early clinical studies also showed similar beneficial effects of bacterial decontamination in allogeneic HCT patients, although subsequent studies did not replicate these benefits. More recent research made major strides in investigating the specific alterations in the composition of the intestinal microbiota and their association with acute GVHD. In one study, high intestinal abundance of the anaerobic bacteria Blautia was correlated with lower rates of GVHD and improved survival. Loss of diversity of the intestinal microbiota was associated with increased mortality in allogeneic HCT patients which was attributed to increased death due to GVHD or infection rather than relapse. Another study showed associations between the expansion of certain bacteria Lactobacillales as well as general loss of diversity and acute GVHD severity. This study also showed that expansion of Lactobacillales was not the cause for the development of GVHD because eliminating Lactobacillales from the intestinal flora in mice before allogeneic HCT caused more severe GVHD and reintroducing Lactobacillales alleviated this effect. However, another study found that that modifying the intestinal microbiota using the probiotic microorganism Lactobacillus rhamnosus GG reduced experimental GVHD. Similarly, administration of a cocktail of 17 species of Clostridia reduced experimental GVHD and improved survival. These data suggest that probiotic therapy might become a rational strategy for ameliorating GVHD. As such, limited published early clinical experiences explored the use of probiotics, including via fecal microbiota transplantation (FMT) in allogeneic HCT patients. However, such approaches require rigorous assessments of safety in immunocompromised populations, as well as challenges related to scalability for widespread application in large prospective trials.
A recent retrospective study showed that broad-spectrum antibiotic treatment with imipenem-cilastatin and piperacillin-tazobactam was associated with increased GVHD-related mortality in patients and that treatment with imipenem-cilastatin was associated with loss of the protective mucus lining of the colon and a shift toward Akkermansia in mice. Antibiotic agents with more limited spectra of activity such as rifaximin, cefepime, and aztreonam were associated with reduced GVHD severity. The use of rifaximin correlated with a lower enterococcal load after allogeneic HCT and the use of cefepime and aztreonam correlated with less alterations to the composition of the intestinal microbiota post allogeneic HCT when compared with broader-spectrum antibiotics with increased anaerobic activity.
The intestinal microbiota metabolize nutrients ingested by the host and produce microbial metabolites which play critical roles in the microbiota's interactions with the host and in maintaining intestinal homeostasis. Microbial metabolites include essential fatty acids and amino acids for the host. Short-chain fatty acids (SCFAs) are the most studied microbial metabolites. Examples of other microbial metabolites are bile acids, polyamines, and aryl hydrocarbon receptor (AhR) ligands. These metabolites impact both nonimmune and immune intestinal cells as well as the microbiome and have varying functions in maintaining the barrier surface of the GI tract, as well as modulating the innate and adaptive host immune responses.
SCFAs (e.g., butyrate, proprionate, and acetate) are produced from fermentation of indigestible carbohydrates by intestinal anaerobic commensal bacteria such as Clostridia species and impact both nonimmune and immune intestinal cells. SCFAs serve as an energy source for the intestinal microbiota and IECs and assist in maintaining the integrity of the mucosal barrier. For example, goblet cells upregulated their expression of mucin genes in response to SCFAs and inoculating germ-free rats with SCFA-producing Bacteroides thetaiotaomicron or Faecalibacterium prausnitzii induced goblet cell differentiation and mucus production. Furthermore, colonization with Bifidobacterium longum protected mice against death induced by a lethal infection by increasing production of SCFA acetate, which maintained the barrier integrity of IECs and inhibited translocation of the E. coli O157:H7 Shiga toxin from the gut lumen into the blood. Finally, SCFAs play an important part in innate immunity because they are histone deacetylase (HDAC) inhibitors with antiinflammatory effects. These observations reveal a crucial protective function of the microbiome in regulating the response of host tissues to immunologic and/or cytotoxic insults that occur after HCT.
One potential approach for stimulating SCFA production could be via dietary supplementation with carbohydrates that are resistant to degradation by human enzymes but can be metabolized by select microbes in the gut. Multiple studies have reported increased fecal SCFAs, particularly butyrate, by dietary supplementation with different formulations of resistant starch (RS). Another study showed that one such RS prepared from potatoes (RPS) is more potent at increasing average fecal butyrate levels compared with other RS formulations. Butyrate production from RS is a complex multistep process, and understanding all the gut microbes involved in this process is an ongoing challenge. Only a limited number of gut bacteria can degrade RS; however, most primary RS degraders are not among the known butyrate producers. Thus, for dietary supplementation with RS to stimulate butyrate production, the activities of secondary fermenters are required. These secondary fermenters capture degradation and fermentation products from primary degraders and metabolize them into new molecules, including butyrate. Known primary RS degraders include Ruminococcus bromii, Ruminococcus lactaris, Ruminococcus gnavus , and Bifidobacterium spp., and known butyrate producers (secondary fermenters) include Roseburia spp., F. prausnitzii , Eubacterium rectale , and Anaerostipes spp.
A recent murine study explored the role of microbial metabolites, specifically SCFAs, in acute GVHD severity and found that the SCFA butyrate was significantly decreased in the IECs of mice experiencing acute GVHD. Restoring butyrate levels, either by direct administration of butyrate or by increasing intestinal butyrate-producing bacteria, reduced acute GVHD. Two SCFAs, butyrate and propionate, were shown to directly protect IECs and reduce acute GVHD severity in mice. A small clinical study found that fecal butyrate and propionate levels are decreased in patients after allogeneic HCT and are lower with antianaerobic antibiotic exposure. Another recent clinical study examined fecal SCFA concentrations after allogeneic HCT and found that higher levels of butyrate and other SCFAs correlated with abundance of butyrate-producing organisms in the intestinal microbiota and this abundance was associated with resistance against lower tract respiratory infections. However, the impact of SCFA levels on acute GVHD has yet to be examined in patients post allogeneic HCT.
Diet affects the composition of the intestinal microbiome and the associated metabolome is correspondingly altered. In one study, high-protein and low-carbohydrate diet was associated with loss of members of Clostridiales , including Roseburia, Faecalibacterium, Ruminococcus , and Blautia . Similar patterns were seen with diets derived entirely from animal products. Turnbaugh et al. showed that changes in diet from a low-fat, plant polysaccharide–rich diet to a high-fat, high-sugar diet in mice that were colonized with human feces altered the intestinal microbiota composition and this was accompanied by changes in the metabolic milieu. In two other studies, researchers showed that total parenteral nutrition resulted in a proinflammatory pattern of immune responses in mice and humans. These data suggest that diet can alter the intestinal microbiota and the metabolome and thereby regulate intestinal homeostasis.
Specific elements of the diet called prebiotics, which usually refer to indigestible carbohydrates that are metabolized by the intestinal microbiota to produce SCFAs, are particularly influential in modulating the structure and function of the intestinal microbiota. Despite their wide commercial availability, here is lack of published clinical data on the effect of prebiotics in acute GVHD. However, several studies showed that the prebiotics inulin and fructo-oligosaccharides increased intestinal microbiota diversity and decreased disease activity in inflammatory bowel disease patients. These studies, along with the evidence that SCFAs protect IECs and reduce experimental acute GVHD severity, provide a compelling rationale for now ongoing studies of dietary manipulation of the microbiome-metabolome axis using prebiotics to mitigate acute GVHD in patients receiving allogeneic HCT.
The infused donor T cells interact with the primed APCs, leading to the initiation of the second phase of acute GVHD. This phase includes antigen presentation by primed APCs and the subsequent activation, proliferation, differentiation, and migration of alloreactive donor T cells. After allogeneic HSC transplants, both host- and donor-derived APCs are present in secondary lymphoid organs. The T-cell receptor (TCR) of the donor T cells can recognize alloantigens either on host APCs (direct presentation) or donor APCs (indirect presentation). In direct presentation, donor T cells recognize either the peptide bound to allogeneic MHC molecules or allogeneic MHC molecules without peptide. During indirect presentation, T cells respond to the peptide generated by degradation of the allogeneic MHC molecules presented on self-MHC. Experimental studies demonstrated that APCs derived from the host, rather than from the donor, are critical in inducing GVHD across MiHA mismatch. Recent data suggest that presentation of distinct target antigens by the host- and donor-type APCs might play a differential role in mediating target organ damage. In humans, most cases of acute GVHD developed when both host DCs and donor DCs were present in peripheral blood after BMT.
The interaction of donor lymphocyte TCR with the host allopeptide presented on the MHC of APCs alone is insufficient to induce T-cell activation. Both TCR ligation and costimulation via a “second” signal through interaction between the T-cell costimulatory molecules and their ligands on APCs are required to achieve T-cell proliferation, differentiation, and survival. The danger signals generated in phase 1 augment these interactions, and significant progress has been made on the nature and impact of these second signals. Costimulatory pathways are now known to deliver both positive and negative signals, and molecules from two major families, the B7 family and the TNF receptor (TNFR) family, play pivotal roles in GVHD. Interruption of the second signal by blockade of various positive costimulatory molecules (CD28, ICOS, CD40, CD30, 4-1BB, and OX40) reduces acute GVHD in several murine models, whereas antagonism of the inhibitory signals (PD-1 and CTLA-4) exacerbates the severity of acute GVHD. Blockade of activating costimulatory signals with the CTLA-4 fusion protein Abatacept has shown initial evidence of efficacy for prevention of GVHD in phase II clinical trials, particularly in settings of mismatched HCT. Various T-cell and APC costimulatory molecules and the impact on acute GVHD are summarized in Table 109.3 . The specific context and the hierarchy in which each of these signals plays a dominant role in the modulation of GVHD remain to be determined.
| T Cell | APC |
|---|---|
| Adhesion | |
| ICAMs | LFA-1 |
| LFA-1 | ICAMs |
| CD2 (LFA-2) | LFA-3 |
| Recognition | |
| TCR/CD4 | MHC II |
| TCR/CD8 | MHC I |
| Costimulation | |
| CD28 | CD80/86 |
| CD152 (CTLA-4) | CD80/86 |
| ICOS | B7H/B7RP-1 |
| PD-1 | PD-L1, PD-L2 |
| Unknown | B7-H3 |
| CD154 (CD40L) | CD40 |
| CD134 (OX 40) | CD134L (OX40L) |
| CD137 (4-1BB) | CD137L (4-1BBL) |
| HVEM | LIGHT |
T cells consist of several subsets whose responses differ based on antigenic stimuli, activation thresholds, and effector functions. The alloantigen composition of the host determines which donor T-cell subsets proliferate and differentiate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here