Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
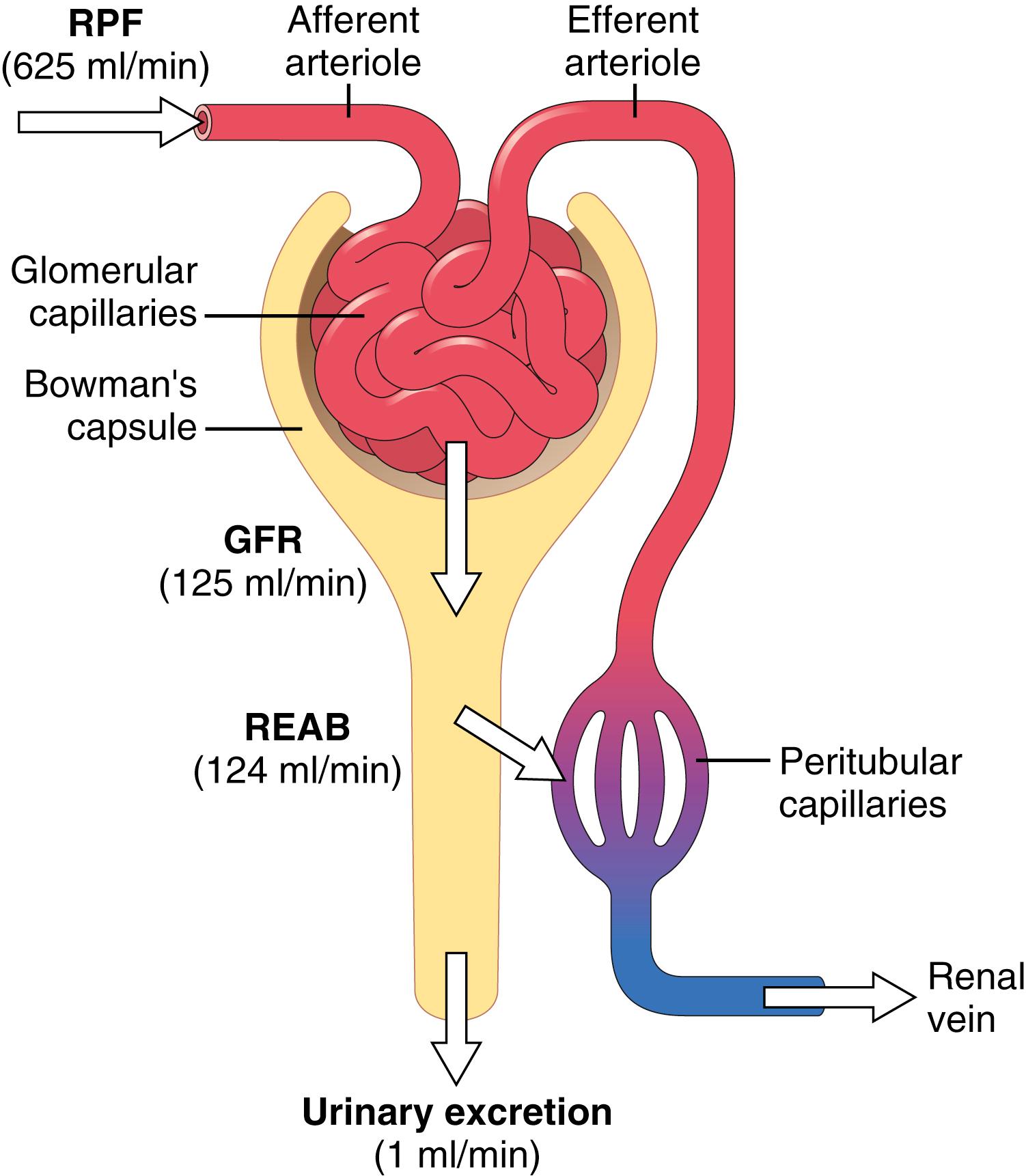
The first step in urine formation is filtration of large amounts of fluid through the glomerular capillaries into Bowman’s capsule—almost 180 L/day. Most of this filtrate is reabsorbed, leaving only about 1 liter of fluid to be excreted each day, although the renal fluid excretion rate is highly variable, depending on fluid intake. The high rate of glomerular filtration depends on a high rate of kidney blood flow, as well as the special properties of the glomerular capillary membranes. In this chapter, we discuss the physical forces that determine the glomerular filtration rate (GFR), as well as the physiological mechanisms that regulate GFR and renal blood flow.
Like most capillaries, the glomerular capillaries are relatively impermeable to proteins, so the filtered fluid (called the glomerular filtrate ) is essentially protein-free and devoid of cellular elements, including red blood cells. The concentrations of other constituents of the glomerular filtrate, including most salts and organic molecules, are similar to the concentrations in the plasma. Exceptions to this generalization include a few low-molecular-weight substances such as calcium and fatty acids that are not freely filtered because they are partially bound to the plasma proteins. For example, almost half of the plasma calcium and most of the plasma fatty acids are bound to proteins, and these bound portions are not filtered through the glomerular capillaries.
Similar to other capillaries, the glomerular capillaries filter fluid at a rate that is determined by the following: (1) the balance of hydrostatic and colloid osmotic forces acting across the capillary membrane; and (2) the capillary filtration coefficient (K f ), the product of the permeability and filtering surface area of the capillaries. The glomerular capillaries have a much higher rate of filtration than most other capillaries because of a high glomerular hydrostatic pressure and a large K f . In the average adult human, the GFR is about 125 ml/min, or 180 L/day. The fraction of the renal plasma flow that is filtered (the filtration fraction) averages about 0.2, which means that about 20% of the plasma flowing through the kidney is filtered through the glomerular capillaries ( Figure 27-1 ). The filtration fraction is calculated as follows:
The glomerular capillary membrane is similar to that of other capillaries, except that it has three (instead of the usual two) major layers: (1) the endothelium of the capillary; (2) a basement membrane ; and (3) a layer of epithelial cells (podocytes) surrounding the outer surface of the capillary basement membrane ( Figure 27-2 ). Together, these layers make up the filtration barrier, which, despite the three layers, filters several hundred times as much water and solutes as the usual capillary membrane. Even with this high rate of filtration, the glomerular capillary membrane normally filters only a small amount of plasma proteins.
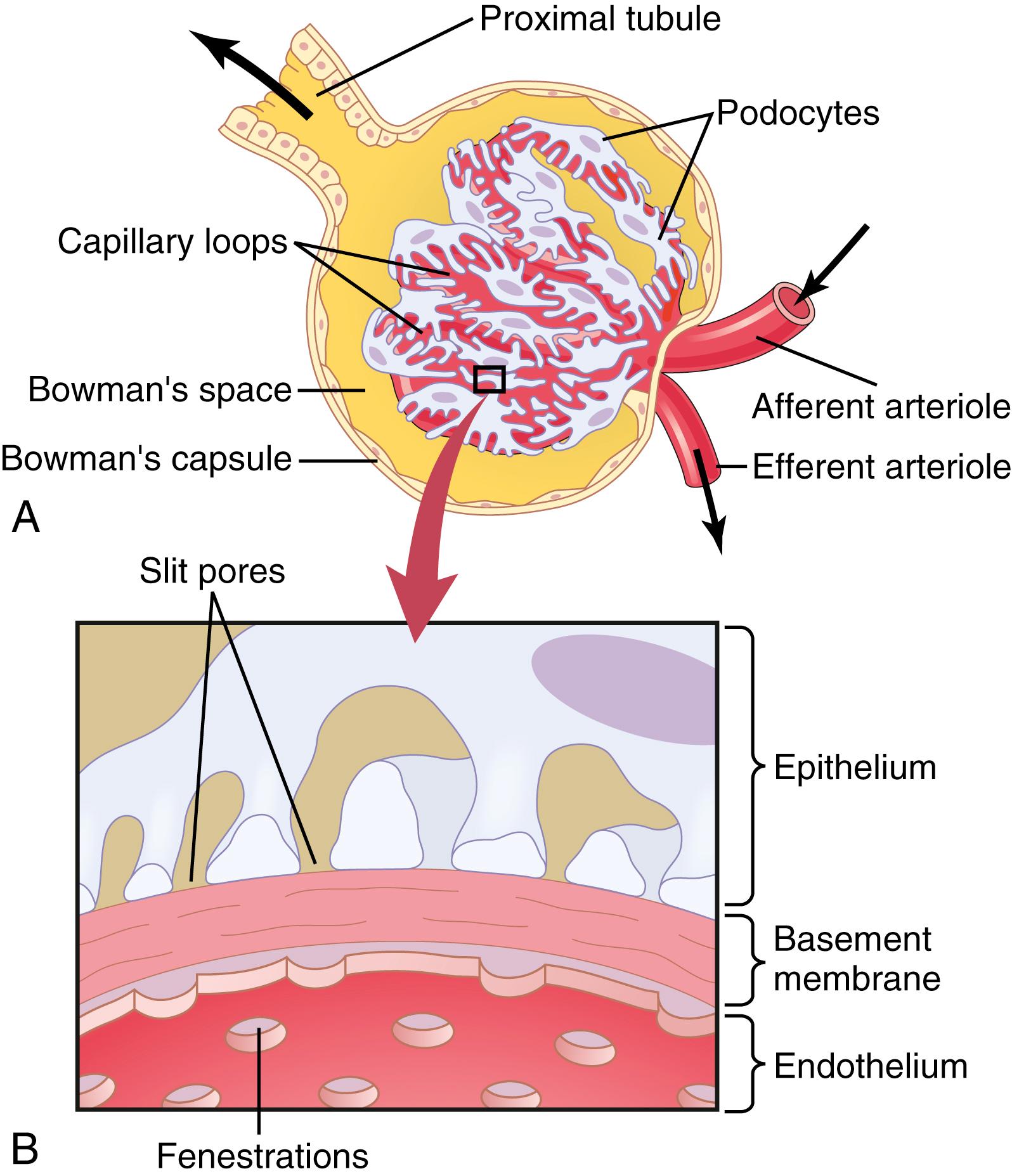
The high filtration rate across the glomerular capillary membrane is due partly to its special characteristics. The capillary endothelium is perforated by thousands of small holes called fenestrae , similar to the fenestrated capillaries found in the liver, although smaller than the fenestrae of the liver. Although the fenestrations are relatively large, endothelial cell proteins are richly endowed with fixed negative charges that hinder the passage of plasma proteins.
Surrounding the endothelium is the basement membrane , which consists of a meshwork of collagen and proteoglycan fibrillae that have large spaces through which large amounts of water and small solutes can filter. The basement membrane greatly hinders filtration of plasma proteins, partly because of strong negative electrical charges associated with the proteoglycans.
The final part of the glomerular membrane is a layer of epithelial cells (podocytes) that line the outer surface of the glomerulus. These podocytes are not continuous but have long footlike processes (pedicels) that encircle the outer surface of the capillaries (see Figure 27-2 ). The foot processes are separated by gaps called slit pores through which the glomerular filtrate moves. The epithelial cells, which also have negative charges, provide additional restriction to filtration of plasma proteins. Thus, all layers of the glomerular capillary wall provide a barrier to the filtration of plasma proteins but permit rapid filtration of water and most solutes in the plasma.
The glomerular capillary membrane is thicker than most other capillaries, but it is also much more porous and therefore filters fluid at a high rate. Despite the high filtration rate, the glomerular filtration barrier is selective in determining which molecules will be filtered, based on their size and electrical charge.
Table 27-1 lists the effect of molecular size on filterability of different molecules. A filterability of 1.0 means that the substance is filtered as freely as water, whereas a filterability of 0.75 means that the substance is filtered only 75% as rapidly as water. Note that electrolytes such as sodium and small organic compounds such as glucose are freely filtered. As the molecular weight of the molecule approaches that of albumin, the filterability rapidly decreases, approaching zero.
| Substance | Molecular Weight | Filterability |
|---|---|---|
| Water | 18 | 1.0 |
| Sodium | 23 | 1.0 |
| Glucose | 180 | 1.0 |
| Inulin | 5500 | 1.0 |
| Myoglobin | 17,000 | 0.75 |
| Albumin | 69,000 | 0.005 |
The molecular diameter of the plasma protein albumin is only about 6 nanometers, whereas the pores of the glomerular membrane are thought to be about 8 nanometers (80 angstroms [Å]). Albumin is restricted from filtration, however, because of its negative charge and the electrostatic repulsion exerted by negative charges of the glomerular capillary wall proteoglycans.
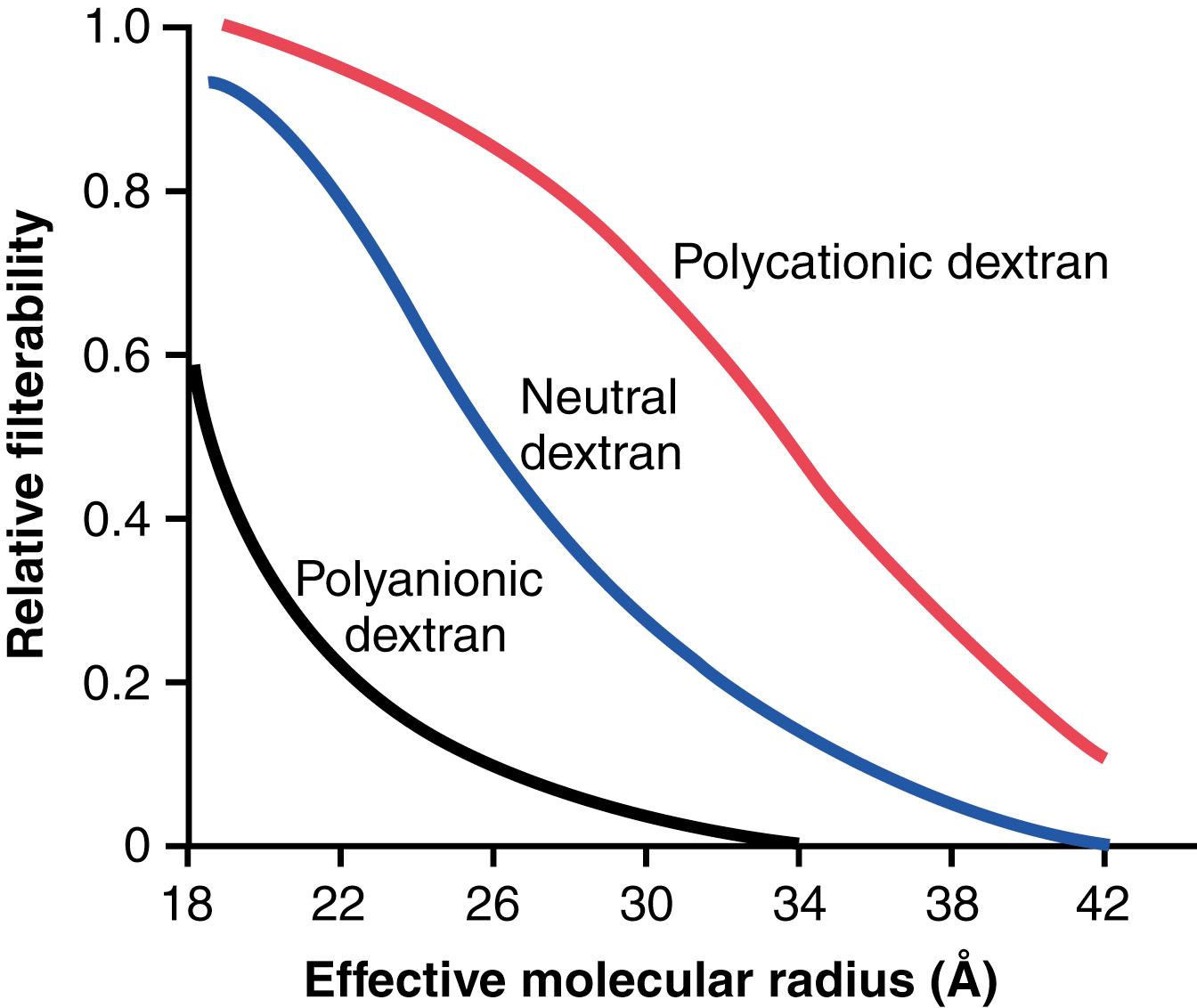
Figure 27-3 shows how electrical charge affects the filtration of different molecular weight dextrans by the glomerulus. Dextrans are polysaccharides that can be manufactured as neutral molecules or with negative or positive charges. Note that for any given molecular radius, positively charged molecules are filtered much more readily than negatively charged molecules. Neutral dextrans are also filtered more readily than negatively charged dextrans of equal molecular weight. The reason for these differences in filterability is that the negative charges of the basement membrane and podocytes provide an important means for restricting large negatively charged molecules, including the plasma proteins.
In minimal-change nephropathy , the glomeruli become more permeable to plasma proteins, even though they may look normal when viewed with a standard light microscope. However, when viewed at high magnification with an electron microscope, the glomeruli usually display flattened podocytes with foot processes that may be detached from the glomerular basement membrane (podocyte effacement) .
The causes of minimal change nephropathy are unclear but may be at least partly related to an immunological response and abnormal T-cell secretion of cytokines that injure the podocytes and increase their permeability to some of the lower molecular weight proteins, especially albumin. This increased permeability permits the proteins to be filtered by the glomerular capillaries and excreted in the urine, a condition known as proteinuria or albuminuria . Minimal change nephropathy is most common in young children but can also occur in adults, especially in those who have autoimmune disorders.
The GFR is determined by the following: (1) the sum of the hydrostatic and colloid osmotic forces across the glomerular membrane, which gives the net filtration pressure ; and (2) the glomerular K f . Expressed mathematically, the GFR equals the product of K f and the net filtration pressure:
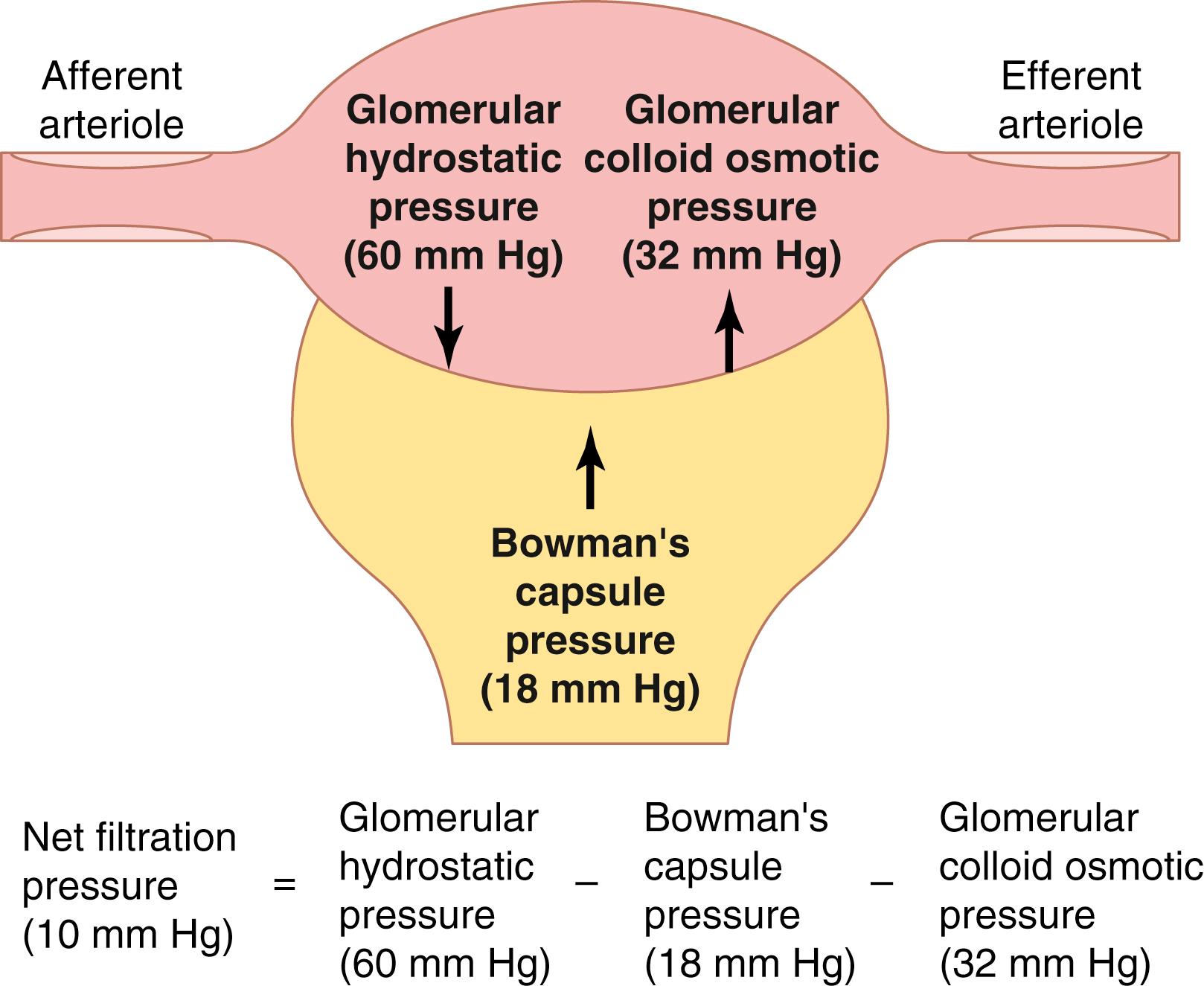
The net filtration pressure represents the sum of the hydrostatic and colloid osmotic forces that favor or oppose filtration across the glomerular capillaries ( Figure 27-4 ). These forces include the following: (1) hydrostatic pressure inside the glomerular capillaries (glomerular hydrostatic pressure, P G ), which promotes filtration; (2) the hydrostatic pressure in Bowman’s capsule (P B ) outside the capillaries, which opposes filtration; (3) the colloid osmotic pressure of the glomerular capillary plasma proteins (π G ), which opposes filtration; and (4) the colloid osmotic pressure of the proteins in Bowman’s capsule (π B ), which promotes filtration. Under normal conditions, the concentration of protein in the glomerular filtrate is so low that the colloid osmotic pressure of the Bowman’s capsule fluid is considered to be zero.
The GFR can therefore be expressed as follows:
Although the normal values for the determinants of GFR have not been measured directly in humans, they have been estimated in animals such as dogs and rats. Based on the results in experimental animals, the approximate normal forces favoring and opposing glomerular filtration in humans are believed to be as follows (see Figure 27-4 ):
| Forces Favoring Filtration (mm Hg) | |
| Glomerular hydrostatic pressure | 60 |
| Bowman’s capsule colloid osmotic pressure | 0 |
| Forces Opposing Filtration (mm Hg) | |
| Bowman’s capsule hydrostatic pressure | 18 |
| Glomerular capillary colloid osmotic pressure | 32 |
Thus, the net filtration pressure = 60 − 18 − 32 = +10 mm Hg.
Some of these values can change markedly under different physiological conditions, whereas others are altered mainly in disease states, as discussed later.
The K f is a measure of the product of the hydraulic conductivity and surface area of the glomerular capillaries. The K f cannot be measured directly, but can be is estimated experimentally by dividing the GFR by the net filtration pressure:
Because the total GFR for both kidneys is about 125 ml/min, and the net filtration pressure is 10 mm Hg, the normal K f is calculated to be about 12.5 ml/min per mm Hg of filtration pressure. When K f is expressed per 100 grams of kidney weight, it averages about 4.2 ml/min per mm Hg, a value about 400 times as high as the K f of most other capillary systems of the body. The average K f of many other tissues in the body is only about 0.01 ml/min per mm Hg/100 g. This high K f for the glomerular capillaries contributes to their rapid rate of fluid filtration.
Although increased K f raises the GFR and decreased K f reduces the GFR, changes in K f probably do not provide a primary mechanism for the normal daily regulation of GFR. Some diseases, however, lower K f by reducing the number of functional glomerular capillaries (thereby reducing the surface area for filtration) or by increasing the thickness of the glomerular capillary membrane and reducing its hydraulic conductivity. For example, chronic uncontrolled hypertension may gradually reduce K f by increasing the thickness of the glomerular capillary basement membrane and, eventually, by damaging the capillaries so severely that there is loss of capillary function.
Direct measurements of hydrostatic pressure in Bowman’s capsule and at different points in the proximal tubule in experimental animals using micropipettes have suggested that a reasonable estimate for Bowman’s capsule pressure in humans is about 18 mm Hg under normal conditions. Increasing the hydrostatic pressure in Bowman’s capsule reduces GFR, whereas decreasing this pressure raises GFR. However, changes in Bowman’s capsule pressure normally do not serve as a primary means for regulating GFR.
In certain pathological states associated with obstruction of the urinary tract, Bowman’s capsule pressure can increase markedly, causing serious reduction of GFR. For example, precipitation of calcium or of uric acid may lead to formation of stones that lodge in the urinary tract, often in the ureter, thereby obstructing outflow of the urinary tract and raising Bowman’s capsule pressure. This situation reduces GFR and eventually can cause hydronephrosis (distention and dilation of the renal pelvis and calyces) and can damage or even destroy the kidney unless the obstruction is relieved.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here