Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The classical paradigm of mammalian, including human, sexual development postulates an initially “indifferent” (sexually undifferentiated) state that is transformed, in two major stages, into the sexually dimorphic male and female forms. The two stages are called primary, relating to the gonad, and secondary, referring to all other genital organs. In the first stage, primary sex determination, specific sex-determining genetic factors that are present from conception drive the development of the gonad. In the second stage, secondary sexual differentiation, all other features of sexual dimorphism are established. In this concept, all features of both internal and external sexually dimorphic structures other than the gonads develop from the action of hormones produced in males by the testis, lack of these hormones (or production of other hormones) in females, and independently of the sex-determining gene(s). This paradigm is known as the Jost principle . A large number of genetic pathways interact in a complex and, in part, overlapping and redundant manner to execute the genetic control of sexual development. In this chapter, we will describe this dynamic, sequential program as occurring in six stages as follows:
Chromosomal assignment of sex
Pregonadal sexual development and nongonadal sexual dimorphism
Formation and development of the indifferent gonad
Transformation of the indifferent gonad into a testis or an ovary
Lifelong maintenance of gonadal identity
Secondary sexual differentiation
The primordial, indifferent gonad is situated in the gonadal (genital) ridge region of the intermediate mesoderm of the fetus. In genetic males, the indifferent gonad is induced to become a testis under the influence of male-determining genes. In the female, with no such genes present, the gonad remains uninduced and an ovary develops. The mesenchymal cells of the gonadal ridge and the neighboring metanephros give rise, in males, to androgen-producing Leydig cells of the testis or, in females, to the theca cells of the ovary and to connective tissue. The adjacent coelomic epithelium grows into the mesodermal mass to produce the Sertoli cells of the testicular seminiferous tubules, or the follicle cells of the ovary. In addition, primordial germ cells migrate to the developing gonad to give rise to sperm or egg cells.
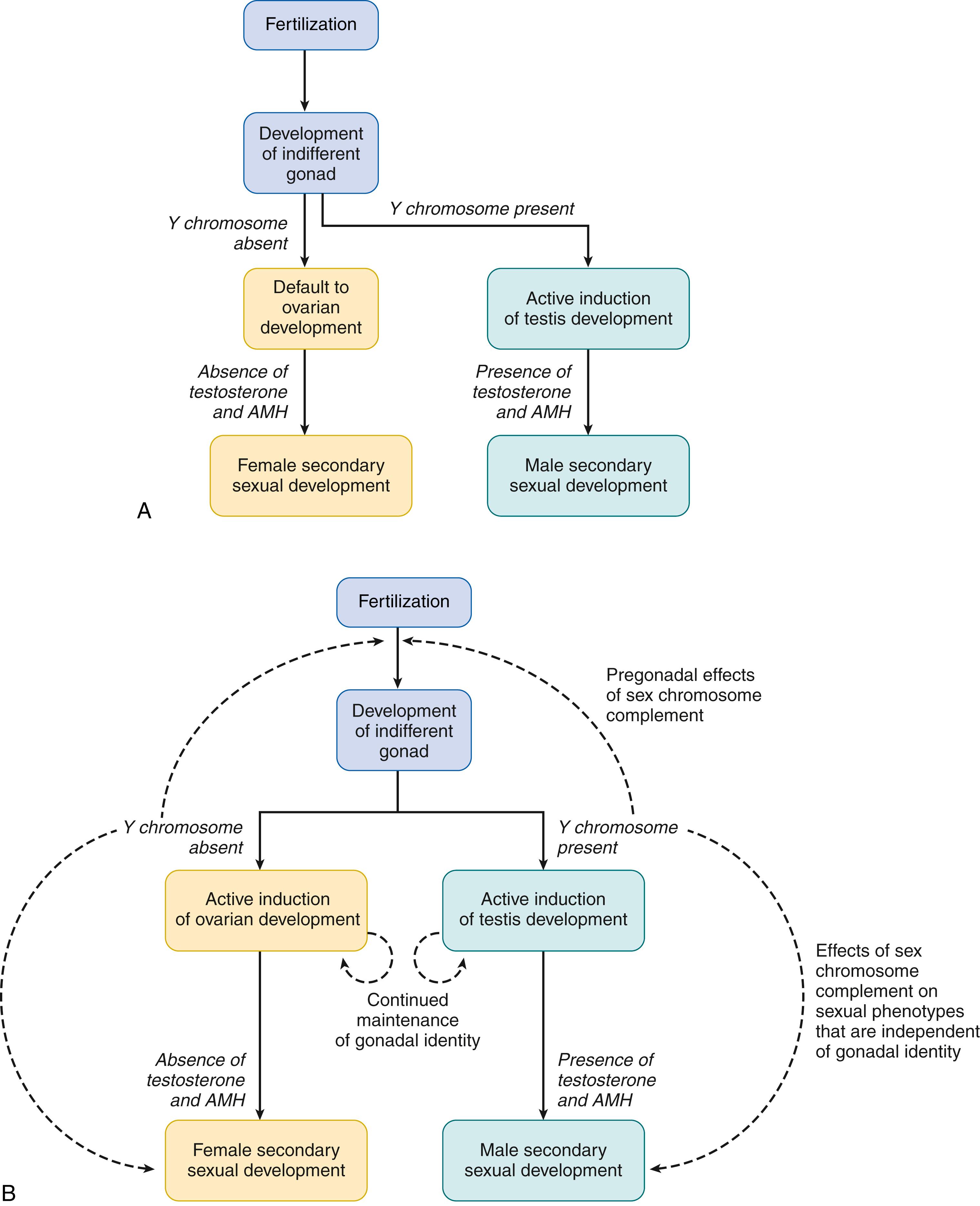
Although current understanding builds on this framework, our knowledge has advanced considerably in recent years, leading to some modifications of the original paradigm. It is now known that “genetic maleness” in mammals is normally dependent on the presence of a sex-determining region of the Y chromosome and “genetic femaleness” is normally dependent on its absence. The identity of the testis-determining gene on the Y chromosome is known, as is the fact that the effect of this gene in formation of the testis is not direct, but rather is mediated by other, so-called downstream genes. Some of the latter have been identified. Also, the action of the Y-chromosomal region is preceded by “upstream” gene action that is evidently a prerequisite for Y-chromosomal function in gonadal determination. Furthermore, some sex-specific gene action, including but not limited to Y-chromosomal (male-specific) gene action, appears to influence the sexual phenotype either before gonadal development (i.e., at a “pregonadal” juncture) or later, but independently of hormonal action. Finally, we now better understand the development of the ovary as being not merely a passive, uninduced state, but also dependent on specific gene action. These findings necessitate modification of the Jost paradigm. Fig. 146.1 summarizes the original Jost paradigm (see Fig. 146.1A ) and the updated version based on current understanding (see Fig. 146.1B ).
In 1923 it was demonstrated cytologically that humans have X and Y chromosomes. By analogy with Drosophila, it was assumed that sex determination in mammals would be mediated by the ratio of the number of X chromosomes to the number of sets of autosomes. However, in 1959, it was discovered that an XO sex chromosome karyotype produced a predominantly female phenotype both in humans (Turner syndrome) and in mice, and XXY produced predominant maleness in humans (Klinefelter syndrome). , These observations demonstrated firstly that the presence of a Y chromosome resulted in the development of a predominantly male phenotype independently of the number of X chromosomes, and secondly that the absence of the Y chromosome resulted in the development of a predominantly female phenotype. Thus the Y chromosome appeared to possess a gene or genes, the presence or absence of which determined the destiny of the bipotential gonad as a testis or ovary, respectively, and, through this, the development of the dimorphic secondary sex characteristics. In the human the hypothetical Y-chromosomal gene was named testis-determining factor (TDF), and in mice it was named testis-determining gene on the Y (Tdy) . Minor deviations of Klinefelter and Turner syndromes from the normal male and female phenotype, respectively, are probably due to the influence of other (non- TDF ) sex-chromosomal factors (see later) on the sex differentiation pathway.
The discovery that 46,XX males usually have translocations (transfers of chromosomal material) of Yp (where p indicates the short arm of a chromosome; q indicates the long arm) to one of their Xps was a major breakthrough in mapping TDF on the Y chromosome. , The X and Y chromosomes have homologous segments at the ends of their respective p arms, that pair during male meiosis. Because of genetic exchange between these segments, allelic variants located in this region appear to be inherited as if on autosomes. Accordingly, these regions are called pseudoautosomal. The occurrence of abnormal crossovers adjacent to the pseudoautosomal region can result in the illegitimate transfer of TDF to the X chromosome. , Molecular analyses in such patients permitted the construction of a deletion map of the Y chromosome , and showed that a small region of Yp adjacent to the pseudoautosomal region is essential for testicular differentiation. Finer mapping continued, and one candidate gene was mistakenly identified: Page and colleagues found a highly conserved sequence that seemed to map with a male-determining Y fragment and named it ZFY . However, one of their XX males did not have ZFY , and the finding of more such patients , suggested that ZFY could not be TDF .
The resumed search for TDF was targeted on 35 kilobases (kb) of DNA between the proximal (i.e., towards the centromere of the chromosome) limit of the pseudoautosomal region of the Y chromosome and the breakpoint in XX males lacking ZFY . A subclone selected for its male specificity in mammals was found to encode a protein containing an 80–amino acid segment that was homologous to the mating-type protein in Schizosaccharomyces pombe and to a conserved 80–amino acid, DNA-binding motif present in the high mobility group (HMG) 1 transcription-regulating proteins. Further analyses of a variety of tissues disclosed a transcript of this gene only in the testes. It was proposed that this gene, designated sex-determining region, Y chromosome ( SRY ), was TDF. Soon thereafter, mutations in the SRY gene were found in some cases of XY, pure gonadal dysgenesis. The mouse Sry gene was cloned at the same time as human SRY . Some mice transgenic (i.e., in which a gene has been artificially transferred) for a fragment containing Sry showed 40,XX sex reversal—that is, mice otherwise destined to be female had a male appearance (although they were sterile). Testes of these mice were histologically similar to those of human XX males. However, sex reversal did not always occur, and this incomplete penetrance may be related to the influence of genetic background on Sry transgene expression. Genetic modifiers may also be the explanation for the multiple phenotypes seen with particular mutations in SRY . Interestingly, human SRY does not cause sex reversal in transgenic mice; it is not nearly as conserved in function as some human genes, a few of which can work even in Drosophila . The HMG boxes of SRY and its mouse homologue, Sry , are examples of so-called generalized HMG boxes. It has been demonstrated that the SRY HMG box not only binds to synthetic DNA fragments of the sequence AACAAAG but also to cruciform DNA structures irrespective of their sequence. Both targets adopt similar conformations in solution, in which state the binding of SRY induces a large conformational change in the DNA. Some mutations in the SRY HMG box alter the DNA contact sites in such a way as to preclude the usual deformation of the DNA.
The principal evidence in favor of SRY / Sry being TDF / Tdy is the following:
It is localized in the smallest region of chromosome Y capable of inducing testicular differentiation in humans and mice.
Some cases of XY, pure gonadal dysgenesis, have de novo mutations in the SRY gene.
In the mouse, Sry expression correlates with the initiation of testicular determination in the gonadal ridge, and in both the mouse and the human, its expression in the blastocyst corresponds to the more rapid growth of the Y-bearing blastocyst (see later).
A fragment of DNA that includes the mouse gene is able to induce male-type development in some transgenic XX mice.
It is an evolutionarily conserved gene on the Y chromosome of metatherian and eutherian mammals. ,
Pregonadal and nongonadal sexual dimorphism comprises those differences between individuals that are related to sex chromosome complement, but that either arise before (pregonadal) or are otherwise independent of (nongonadal) the presence of testes/ovaries and associated sex hormones. Nongonadal sexually dimorphic phenotypes are outside the scope of this chapter, but interested readers are referred to a recent review.
Marsupial mammals clearly have pregonadal sexual differentiation. In the tammar wallaby, some features of sexual dimorphism, including the scrotum in the male and the mammary glands in the female, appear before development of the gonads and independently of hormones. Although these organs develop mainly under hormonal influence in placental (eutherian) mammals, evolutionary conservation of some pregonadal sex-determining gene expression has also occurred in mice. The anogenital distance, which is a measure of masculinization (and which is also the region in which the scrotum develops), is smaller in prepubertal and XX, Sxr pseudomales than in their normal, XY brothers. These data suggest an effect of an effect of sex chromosome dosage on this secondary sex characteristic in mice, independent of any hormonal action. , These and other pregonadal sex differences might be controlled by sex chromosome to autosome or X to Y ratios: “Re-examination of the few known marsupial intersexes suggested that a gene on the X chromosome is responsible, with one X chromosome coding for a scrotum, and two X chromosomes for a pouch, regardless of the presence or absence of testes or ovaries.”
Since the technology was developed to identify the sex of preimplantation embryos by polymerase chain reaction, many studies on the differential rates of early embryo development have been performed. Conflicting results on which sex develops faster, and whether in vitro or in vivo, have been obtained. However, there is abundant evidence of sexual dimorphism. Yadav and colleagues, studying in vitro fertilized and cultured bovine embryos, found that the first embryos performing the one cell division were significantly likelier to be male than female. This faster division rate has been confirmed in humans. Preimplantation XY mouse embryos have been reported to be more advanced than XX embryos, as have XY cattle embryos. , In contrast, Peippo and Bredbacka found that among day 3 mouse embryos in vivo, females were more advanced, but during a subsequent 24-hour in vitro culture period the increase in cell numbers was greater in male embryos. They proposed that previous results suggesting increased rates of cell division in male embryos might be an artifact of in vitro culture. Larson and colleagues showed no difference in the rates of development of male and female bovine embryos in vitro, but observed a sexual dimorphism in interferon-tau expression and a delay in expanded blastocyst formation of female embryos when using a rich culture medium. However, gene expression is altered when embryos are cultured in vitro. This has been particularly shown for Igf1 , the expression of which is very significantly delayed with in vitro culture.
On balance, therefore, most studies indicate that male embryos grow faster than female embryos throughout development, including before gonad formation. This faster growth is reflected at the transcriptional level: there are sexually dimorphic gene expression patterns in the pre–sex-specific gonadal embryo, as early as day 7.5.
These differences between male and female embryos naturally raise the question as to whether they are mediated by an extragonadal role of Sry . Some studies on the expression of Sry did not show expression until day 10.5 (reviewed by Kanai and colleagues ); however, other studies have shown much earlier expression in preimplantation embryos. Polymerase chain reaction studies of mouse embryos from embryonic day 1.5 to embryonic day 4.5 found abundant evidence for the transcription of Sry from the two-cell stage to the blastocyst stage, and this result was confirmed in human embryos. Quantitation disclosed that there are approximately 40 to 100 copies of the messenger RNA (mRNA) per cell in a male blastocyst. This is a much larger amount than could have been carried in with sperm. An effect of SRY on preimplantation growth rate could potentially explain its rapid evolution. ,
However, SRY/Sry is not the only potential candidate gene. One especially fruitful line of inquiry has been the comparison of chromosomally variant and/or consomic mice, in order to tease out the interacting effects of Y chromosome presence/absence, X chromosome dosage, and X chromosome parent of origin. Thornhill and Burgoyne showed via comparison of X m O and X p O embryos that the presence of a paternal X (and absence of a maternal X) has a retarding effect on early embryonic growth, particularly of the ectoplacental cone, in XO mouse embryos, a finding that was subsequently confirmed by Jamieson and colleagues. Although this was initially proposed as an explanation for growth rate differences between XY males and XX females (because only the latter have a paternal X), it is now believed that the effect is specific to XO conceptuses and reflects the paternal imprinting of Xp in rodent preimplantation embryos and extraembryonic tissue.
In a comparison of consomic strains with different Y chromosomes, Burgoyne found an accelerating effect of the Y chromosome on preimplantation growth in mouse embryos, which was polymorphic among different Y chromosomes. Further analyses by Burgoyne and colleagues showed that for mid-gestation embryos (10.5 days after coitus), there are two competing effects on embryo size: an Sry -independent Y chromosome effect promotes faster growth of male embryos relative to female embryos, whereas a dosage-dependent effect of the X chromosome promotes faster female growth relative to male growth. This suggests that the gene modulating early embryonic growth may be an X-Y homologous gene escaping X inactivation, and that the variability in experimental data for male and female comparisons may relate to allelic differences segregating in different populations. An attractive candidate gene in this case is Zfy, because this has also been shown to be expressed in preimplantation embryos, and the X-linked homologue Zfx is known to play a role in stem cell maintenance. Set against this, in the mouse (although not in the human), Zfx is subject to X inactivation, and thus it is challenging to explain the effects of X dosage in this species.
Turning to later stages of gestation, Ishikawa and colleagues showed that in late-gestation embryos there is a specific effect of X dosage on placental size, with a single X chromosome promoting late-onset placental hyperplasia independently of the parental origin of the X chromosome, fetal androgens, and the presence or absence of a Y chromosome. This effect is likely to be mediated by X-specific genes that escape X inactivation. These genes will be selected for female benefit and therefore may act to restrict fetal growth and reduce the demand on the mother, in accordance with the fetal-maternal conflict hypothesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here