Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
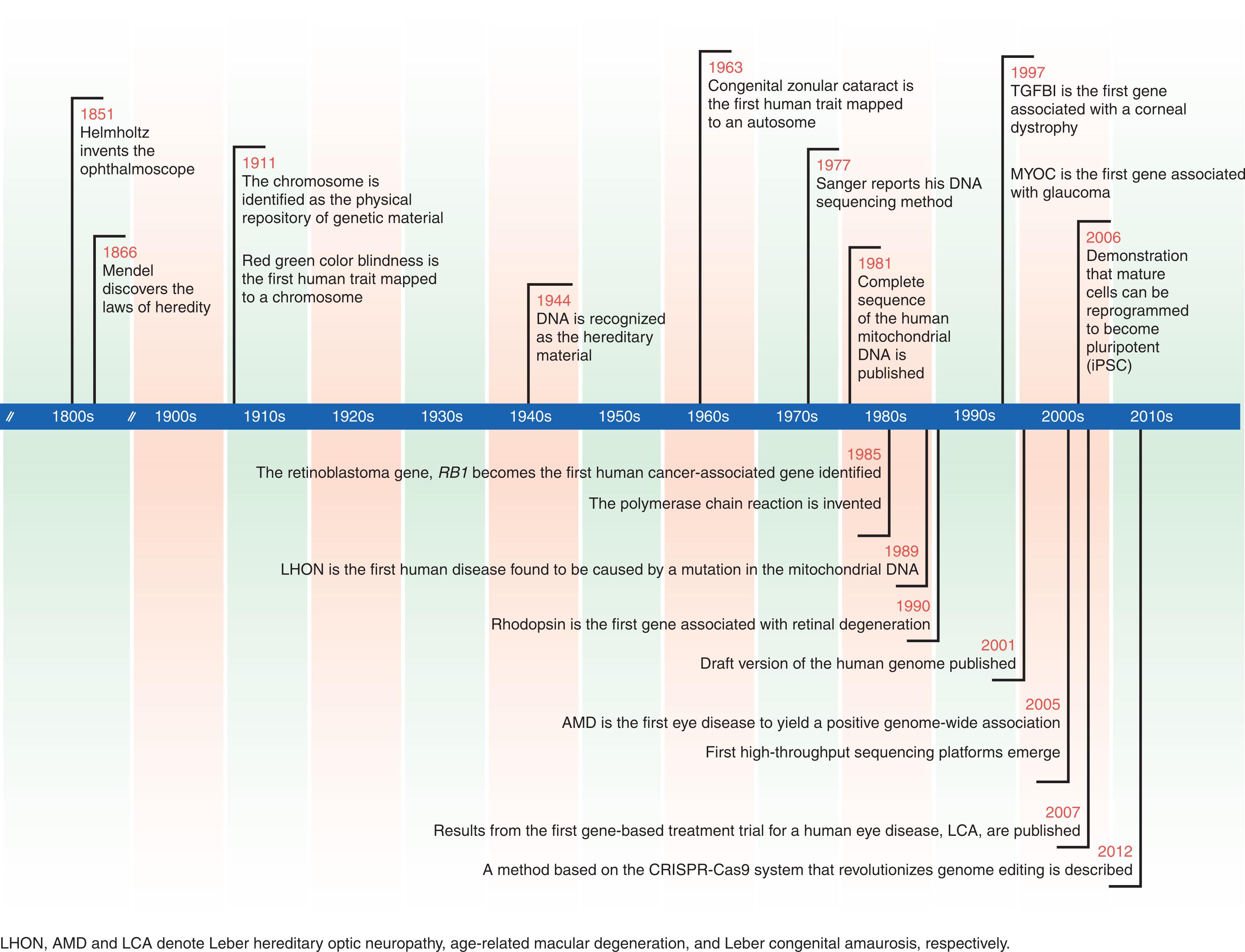
The modern era of genetic ophthalmology began in 1985 with the discovery of the retinoblastoma gene. Exciting progress in deciphering the genetic basis of eye disease has been made since that time ( Fig. 10.1 gives a timeline of historic discoveries). Hundreds of genes associated with a wide variety of common and rare disorders have been discovered leading to clinically valuable diagnostic tests (e.g. for pediatric cataract ), meaningful disease classifications (e.g. for corneal dystrophies ), and novel therapeutic strategies (e.g. for retinal dystrophies ). This chapter focuses on genetics in pediatric ophthalmic practice. The different types of genetic disorders and modes of inheritance, and the principles of genetic testing and counseling are discussed.
Genetic conditions account for a third of childhood blindness worldwide, a figure that is likely to be an underestimate, especially in medium- and high-income countries. Disorders referred to in this context are defined by their predominantly monogenic inheritance. This means that each of these monogenic or Mendelian disorders is caused by a fault or mutation in a single gene.
The human genome (i.e. the totality of one's DNA) is divided among 46 physically distinct chromosomes: 22 pairs of autosomes (non-sex chromosomes) plus two sex chromosomes (two X chromosomes in female, one X and one Y chromosome in male). Monogenic disorders can be associated with genes on autosomes (chromosomes 1–22) or on the X or Y chromosomes. Autosomal characters in both genders and X-linked characters in females can be dominant or recessive. This section will focus on the three main Mendelian pedigree patterns: autosomal dominant, autosomal recessive, and X-linked recessive ( Fig. 10.2A–D ). Mitochondrial inheritance, an atypical mode of inheritance that can be viewed as an expansion of Mendelian concepts, is also described ( Fig. 10.2E ). A more detailed discussion of pedigree patterns, including the rare X-linked dominant, Y-linked, and digenic inheritance, is given by Strachan and Read.
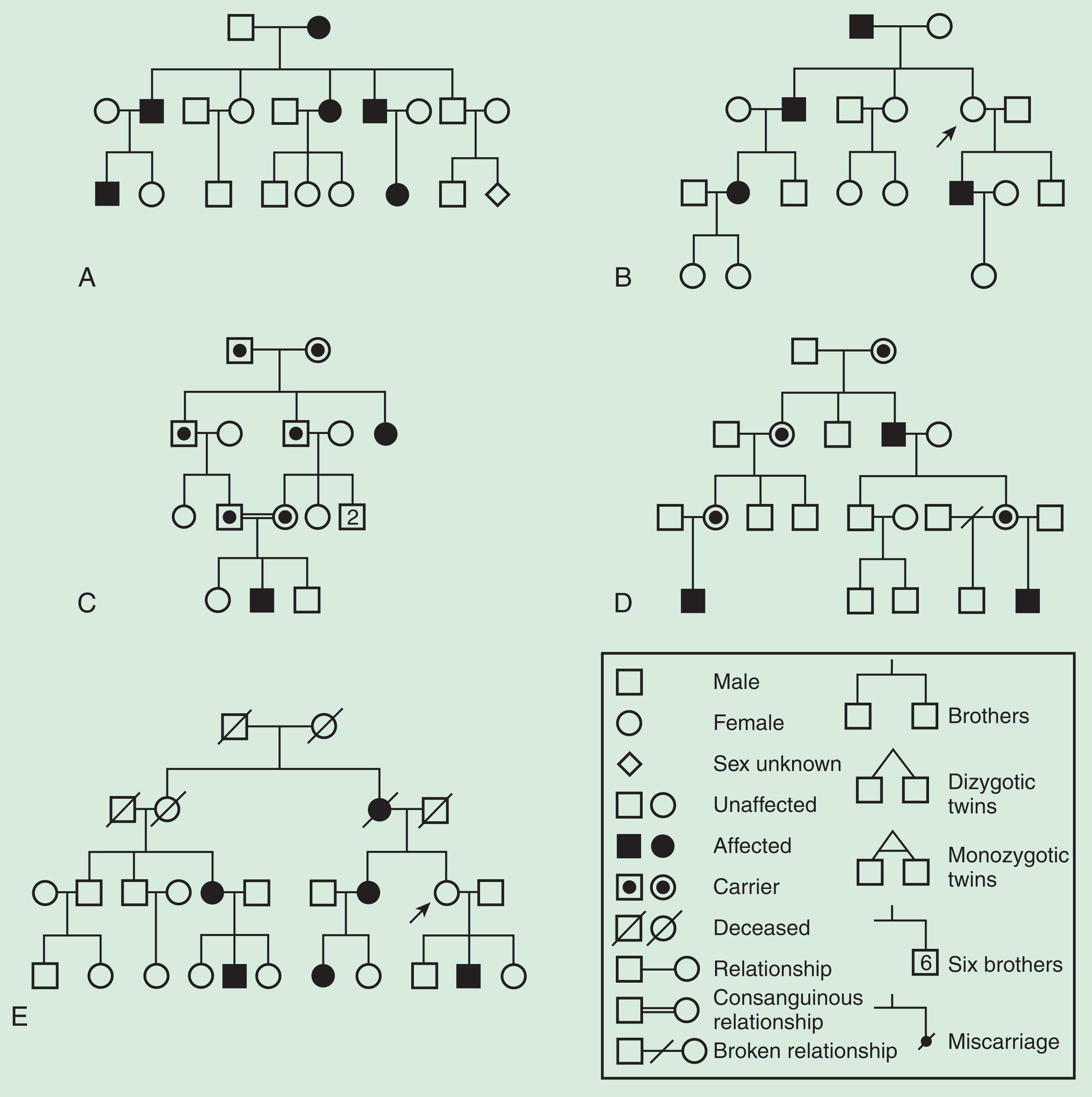
In autosomal dominant conditions, affected individuals carry one normal and one mutated copy of a gene. This means that a single copy of the mutation is sufficient to cause disease (i.e. the condition is expressed in the heterozygous state). Typically, an affected person has at least one affected parent and there are affected individuals in multiple generations. A person with an autosomal dominant disorder has a 1 in 2 chance of passing the mutated gene to an offspring (i.e. of having an affected child). This basic Mendelian pattern can be disguised by phenomena such as non-penetrance (see Fig. 10.2B ), variable expressivity, and new/ de novo mutations (see below).
In autosomal recessive conditions, affected individuals carry defects on both copies of a given gene. They can be either homozygotes , if both copies harbor the same mutation, or compound heterozygotes , if each copy carries a different mutation. Typically, affected individuals are born to asymptomatic carrier parents; this means that the parents carry one normal and one mutant gene copy but are not affected, as the normal copy is sufficient to produce normal function and structure. After the birth of a child with an autosomal recessive condition, each subsequent child has a 1 in 4 chance of being affected.
Autosomal recessive disorders can appear as “sporadic” in a family where all parents and siblings are healthy; this is particularly common in small families. In real life, predicting autosomal recessive inheritance can be challenging and may be inferred on the basis of unaffected parents (“lack of vertical transmission”) and exclusion of X-linked inheritance.
A major risk factor for autosomal recessive disorders is parental consanguinity. A consanguineous marriage is in general defined as a union between a couple related as second cousins or closer. Although these unions increase the likelihood that spouses carry an identical gene mutation, in many ethnic groups they are an important part of family culture. Personalized genetic counseling should be offered to consanguineous couples, but this must be done with sensitivity and appreciation of cultural issues.
An X-linked condition is caused by a mutation in a gene carried on the X chromosome. Males have only one X chromosome and, consequently, such a mutation will be manifest. Females have two copies of each X chromosome gene and the presence of a normal copy typically leads to partially or completely preserved structure and function.
The essential features of X-linked inheritance are the presence of affected males (of greater severity than females) and the lack of father-to-son (male-to-male) transmission. The mothers of affected males are obligate carriers and have a 1 in 2 chance of passing the mutation to their offspring; each son has a 1 in 2 chance of being affected and each daughter has a 1 in 2 chance of being a carrier.
Classically, it is assumed that X-linked recessive conditions only affect males. This is true for conditions such as X-linked retinoschisis and Norrie disease. However, for others, such as X-linked retinitis pigmentosa, there may be phenotypic manifestations in heterozygous females, although these will usually be milder and of later onset than in males. In these cases, it might be difficult to distinguish X-linked from autosomal dominant inheritance, especially in the absence of a detailed family history. Notably, phenotypic manifestations of X-linked disorders in females are highly variable; this is mainly due to a genetic phenomenon termed X chromosome inactivation or lyonization (for more information see the work of Galupa and Head ). It is of interest that in a number of X-linked conditions, including choroideremia and Lowe syndrome, females may show characteristic ocular signs despite the absence of symptoms. These signs can help the identification of apparently unaffected female carriers.
Various clinical phenomena can often disguise an underlying Mendelian inheritance pattern. An example of such a complication is reduced penetrance . Reduced penetrance is common in dominant disorders where heterozygous mutation carriers are classically expected to be affected. However, in reality, the probability of a mutation carrier developing symptoms is rarely 100% – that is, mutations often show reduced penetrance. In practice, this means that for many conditions including certain forms of pediatric cataract and dominant retinitis pigmentosa, individuals may carry a mutation but have no signs of the disorder. An extreme example is an asymptomatic individual with an affected parent whose children are also affected; such a pedigree structure would suggest that he/she certainly carries the mutation but there is non-penetrance (see Fig. 10.2B ).
Another genetic phenomenon with similarities to reduced penetrance is variable expressivity, also frequently seen in the context of dominant disease. A mutation is said to demonstrate variable expressivity if, within a family, affected individuals carry the same genetic defect but the manifestations of the condition vary widely. The cause for variable expressivity is the same as for reduced penetrance: environmental factors or other genes can have some influence on the development of symptoms. Notable examples of variable expressivity include albinism and Stickler syndrome, in which ocular and extraocular manifestations vary among those who carry identical mutations. In these cases, disease severity in one individual may give little or no indication of the likely disease severity for siblings or offspring. Reduced penetrance and variable expressivity are important reasons for examining apparently unaffected parents of children with conditions such as pediatric cataract or anterior segment dysgenesis. Importantly, the presence of mild features can help refine the risk for future offspring.
Many cases of severe dominant disease are the result of new mutations. These so-called de novo mutational events arise as a result of a copying error from a parent's DNA and manifest without warning in families with no previous history of the disorder. This phenomenon is observed in many individuals with aniridia ( Fig. 10.3 ) or retinoblastoma. In such cases, the recurrence risk for future siblings is much lower than 1 in 2. However, the figure will not be zero due to the risk of gonadal mosaicism (i.e. one parent carrying the mutation only in a proportion of his sperm or her eggs).
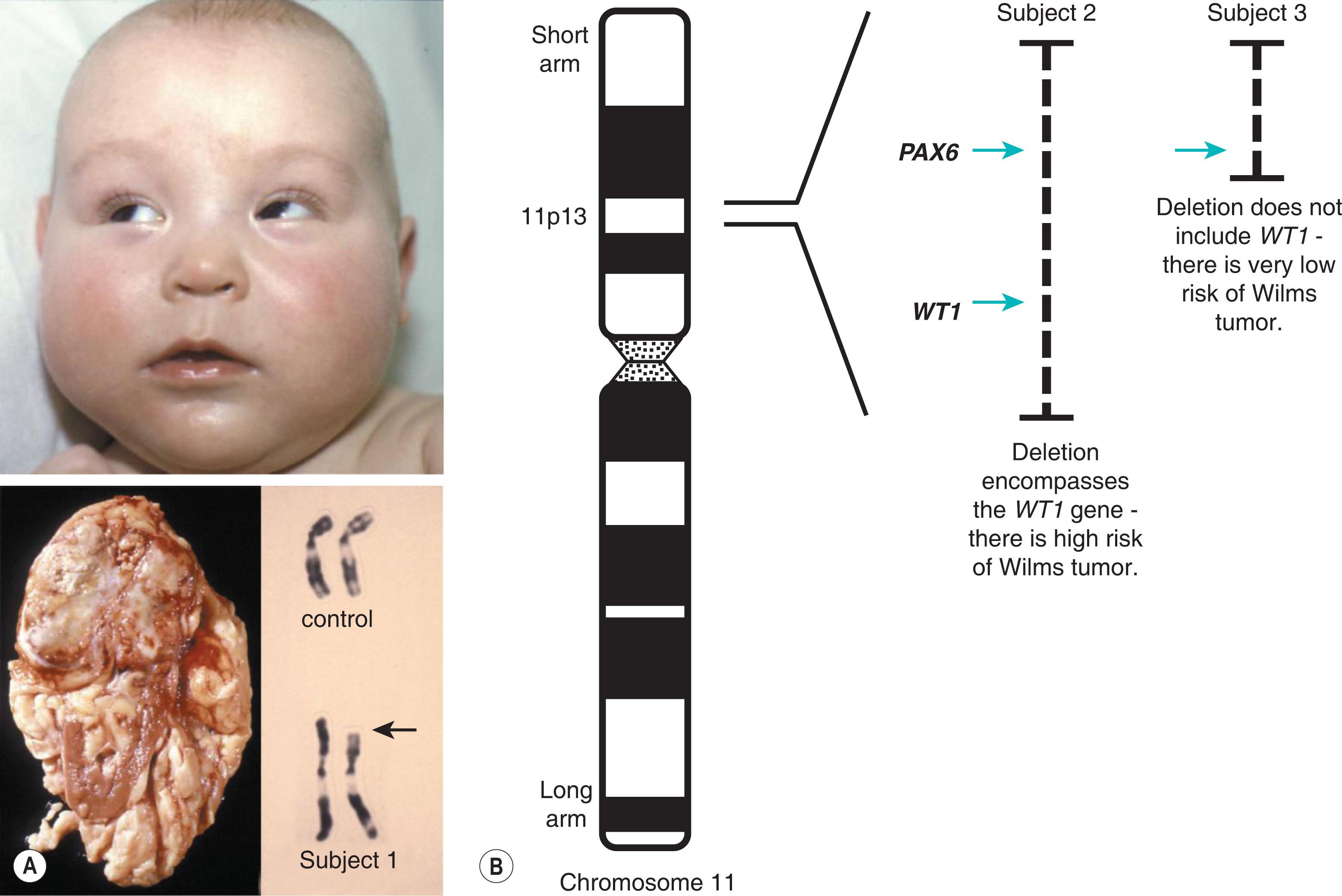
The exact nature of a de novo mutation is difficult to predict. For cases of sporadic/non-familial aniridia, a deletion can remove other neighboring genes; this is seen in WAGR syndrome (Wilms tumor, aniridia, genitourinary abnormalities, and intellectual retardation). These contiguous gene deletions are the reason why individuals with sporadic aniridia require either renal ultrasound screening or molecular evidence that the Wilms tumor gene, WT1 , is unaffected by the mutation (see Fig. 10.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here