Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The study of many different organisms has contributed to our understanding of cancer at the molecular, cellular, and organismal levels. Considerable effort is focused on the rational design and use of mouse models, including spatially and temporally controlled genetic modifications to recapitulate human cancers. Long before the development of genetically engineered animal models, research on mice, rats, rabbits, and chickens led to major discoveries directly related to cancer, including the discovery of oncogenes and the biochemical purification of tumor suppressor proteins. In addition, many key regulators of proliferation, differentiation, and cell death have been characterized by studying developmental processes in mice. The knowledge of pathways that regulate organ development is an important framework on which to build our understanding of all aspects of tumor initiation, progression, and metastasis.
In this chapter, we discuss mouse models of cancer, emphasizing the techniques used to create genetically engineered mouse models and the application of these models to cancer research. Several fundamental discoveries resulting from the use of mouse models are also highlighted, as well as the important role of these models in the future of cancer research.
Our understanding of the genetic alterations in human tumors and the ability to manipulate the mouse genome has allowed for the development of models of human cancer. Mice are the preferred model organism with which to study the complex processes of tumor development and progression for many reasons, including their short generation time, small size, availability of inbred strains, and the close genetic relationship between mice and humans. Fish, flies, and worms have also been successfully used to investigate tumorigenesis, and the unique genetic tools available in these species have allowed for a range of informative experiments to be performed.
Observational and correlative studies of human cancer combined with in vitro experiments on human cancer cell lines have contributed a great deal to the foundation of our knowledge of tumorigenesis. The dissection of cancer development and progression in humans is greatly limited by the difficulty in accessing lesions at various stages of development and the inability to test gene function in vivo except by pharmacologic means. The interrogation of gene function in vitro is limited to genes that control the intrinsic processes of cancer cells, including proliferation, differentiation, and cell death. Moreover, the complex interactions between different cell types within the tumor are poorly recapitulated in vitro, and the selective pressure of in vitro growth may significantly alter the genotype and phenotype of cultured cells. For all these reasons, animal models in which the entire developmental progression of the disease from tumor initiation to metastatic outgrowth occur in vivo are of paramount importance.
The underlying genetic heterogeneity of the human population, the existence of subtypes of different malignancies, and the genetic and genomic heterogeneity within tumors of the same type complicate studies of human tumor gene expression and mutational analysis. The induction of tumors with specific oncogenic alterations in mice on inbred backgrounds can overcome many of these limitations. Mouse models also offer the ability to assign causality to genetic alteration and to assess the roles of certain genes and pathways in vivo.
Modeling human cancer in mice has evolved as techniques to modify the mouse genome have been developed. Combinations of the approaches described in the following sections have been used to model many human cancers. The plethora of options to create these models has been used to address many fundamental questions in tumor biology.
Mice spontaneously develop a spectrum of cancers. The observation that different inbred strains of mice develop cancer at different frequencies gave early support to the idea that the genetic background of a mouse (or person) can predispose them to cancer. Spontaneous tumor formation is often assessed in non-genetically engineered mouse lines to determine whether the specific gene mutation influences the prevalence, progression, or types of cancers that arise ( Figure 9-1 , A ).
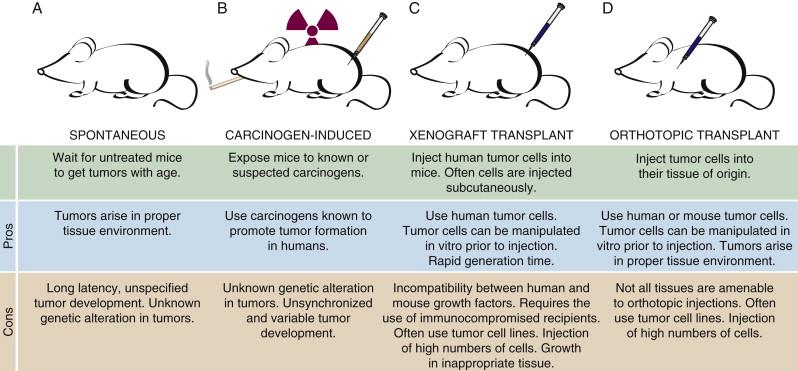
Many known or suspected carcinogens have been used to create mouse models of cancer (see Figure 9-1 , B ). These models rely on the treatment of mice with chemical or physical mutagens, which most often leads to the development of genetically undefined cancers. Carcinogen-induced cancer protocols can be used with genetic techniques to create combined carcinogen/genetic models of human cancer.
The transplantation of human and mouse tumor cells into recipient mice has been used extensively to investigate tumor development in vivo (see Figure 9-1 , C and D ). Human tumor cell lines can be injected orthotopically into the organ from which the tumor originated, intravenously (to mimic the metastatic spread of cancer cells) or subcutaneously (to simply allow the tumor to grow in vivo). Mouse tumor cell lines can be transplanted into syngeneic immunocompetent recipients, whereas human cell lines must be transplanted into immunocompromised recipients. This in vivo tumor growth requires many of the proper tumor-host interactions, including the development of vasculature and recruitment of supportive stromal cells. However, these procedures often involve the injection of high numbers of cells and do not recapitulate the series of events that lead to human cancer. Nonetheless, the ability to manipulate tumor cell lines in vitro before transplantation and the speed and reproducibility of tumor growth are major advantages of these approaches.
Gene expression and genomic analyses of human cancers have uncovered many of the important genetic changes in different tumor types. The knowledge gleaned from these studies coupled with the ability to create germline and somatic alterations in the mouse genome has allowed the creation of genetically defined mouse models of cancer that approximate human cancer at the genetic and histologic levels. Transgenic overexpression was the first genetic technology used to create mouse tumor models ( Figure 9-2 , A ). Tissue-specific promoters can be used to drive the expression of genes of interest in the desired cell type or tissue, and the tumorigenic consequences can be determined. More elaborate transgenic approaches also allow transgene expression to be controlled temporally.
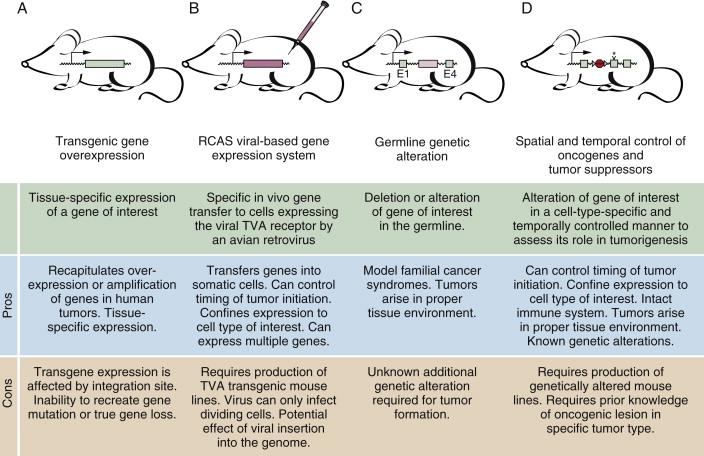
An interesting system for the delivery of genes to somatic cells in vivo uses avian retroviral vectors. Transgenic expression of the cell surface receptor for the RCAS virus (tva) allows the specific and stable infection of a cell type of interest (see Figure 9-2 , B ). The viral vectors can be engineered to express a gene of interest, and the effect of these genes on tumorigenesis can be determined after in vivo infection of the tva-expressing permissive cell type.
Techniques to alter the germline of mice allow the deletion or alteration of genomic loci (see Figure 9-2 , C and D ). These alterations can also be induced in cell-type and temporally regulated fashions. Such powerful approaches allow mouse models to be created that mimic the loss of tumor suppressor genes and activation of oncogenes that occur in different human cancers, resulting in mouse models that closely resemble the human disease. These genetically engineered mouse models are being used in a myriad of research settings to further our understanding of tumor biology.
Different genetic strategies can be used to overexpress, alter, or reduce the expression of genes that affect tumor incidence or progression. Genetic mouse models begin to recapitulate the selected human cancer when the genetic alterations are consistent with those detected in human cancers and when those alterations produce a tumor lesion that appears histologically similar to the human disease. Transgenic overexpression, induced and germline gene deletion, and conditional expression of activated oncogenes allow most of the genetic alterations found in human cancers to be modeled in mice.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here