Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver transplantation (LT) should not be considered a procedure that resolves the problem of a native liver at once but rather a “long-term care plan.” Indeed, the surgical procedure is only the first step of a complex management program that is almost invariably characterized by complications of various types and severity.
Post-transplant complications are commonly classified as “surgical” or “medical,” but there is commonly no clear-cut distinction between these two fields. The etiology of complications in most cases is multifactorial; for instance, vascular thrombosis is the possible consequence of an anastomotic defect but also of an abnormal hypercoagulable state or of immunological phenomena favoring thrombosis. Similarly, bowel perforation, common in children transplanted for biliary atresia who have abdominal adhesions, may also be the consequence of cytomegalovirus (CMV) infection. In general, several complications, more or less related to surgery, may arise after transplant and are summarized in Figs. 22.1 and 22.2 .
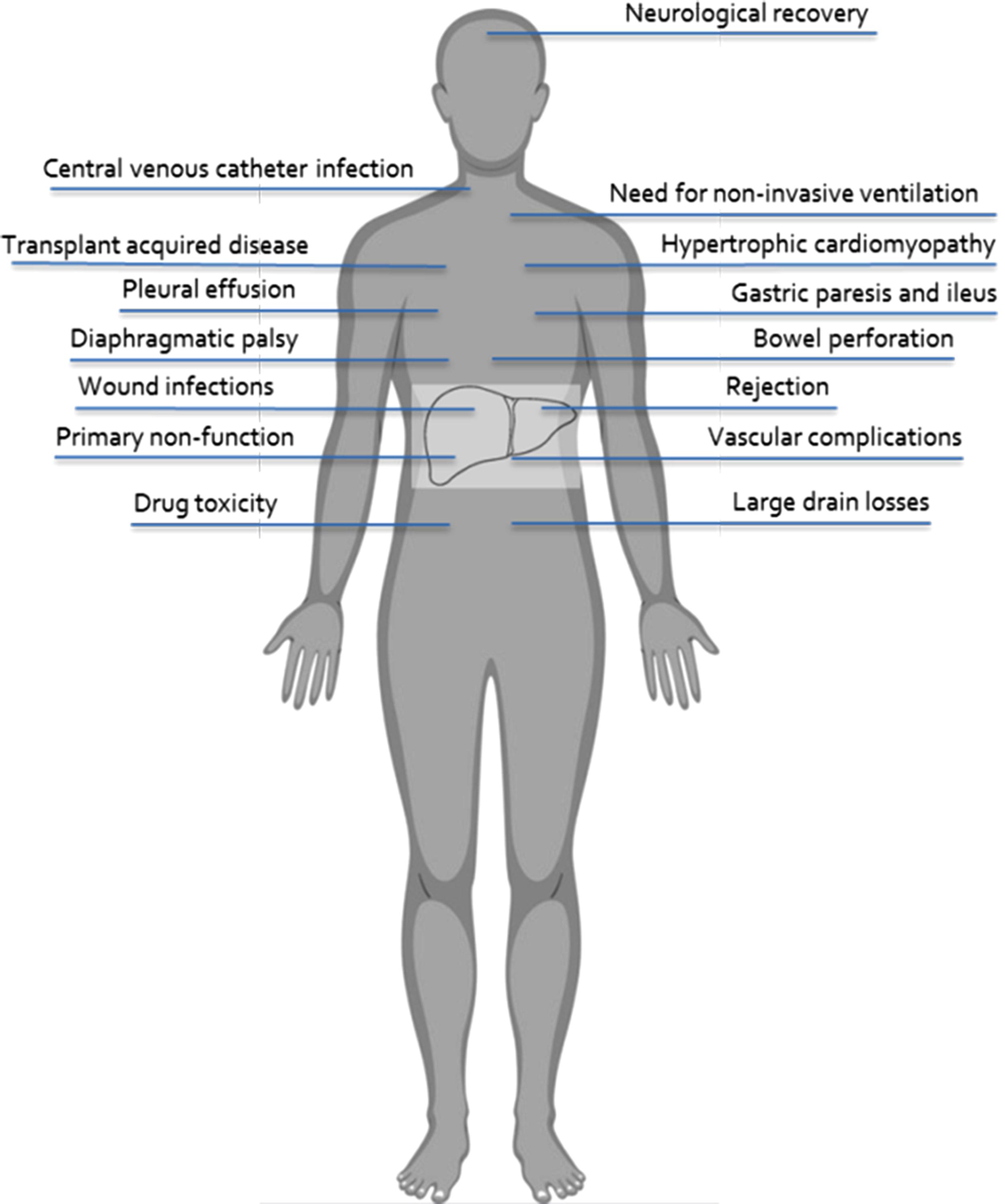
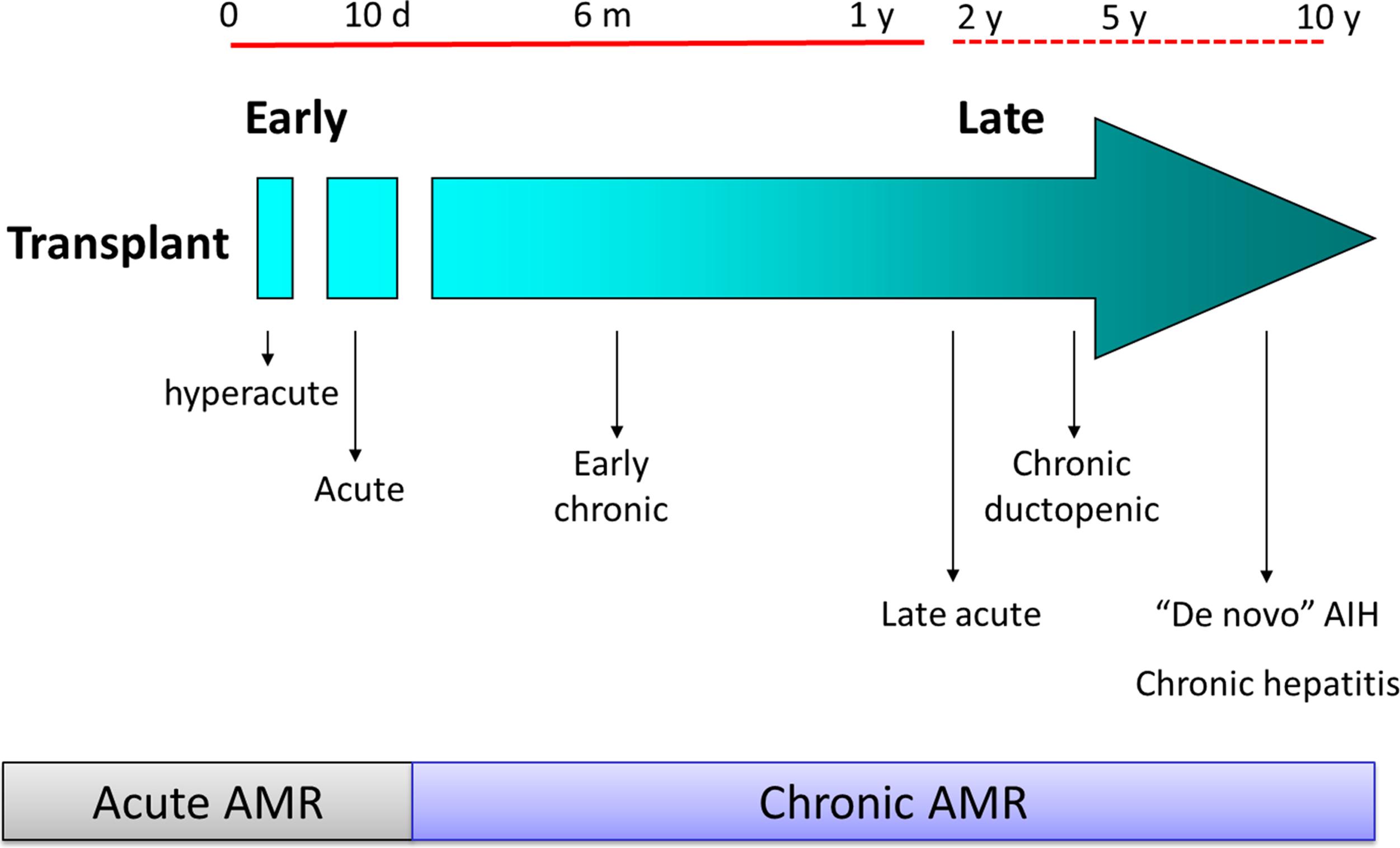
The most severe complications may require surgery, yet data on morbidity and mortality are often controversial because there is no uniform diagnostic or reporting mechanism. The Clavien-Dindo classification of surgical complications has been adapted to the field of LT. This tool attempts to classify and grade the severity of the events based on the management required. Although considered a reference in the surgical field, the Clavien-Dindo classification has major limitations when applied to children. For instance, the need for general anesthesia is considered an element of severity in adults, whereas in children it is required for most procedures regardless of severity, thereby limiting the value of that criterion to evaluate severity.
In general, complications have an impact on the final outcome of LT. The North American Studies of Pediatric Liver Transplantation (SPLIT) multicenter database was analyzed to weigh the impact of complications on patient and graft survival. In the final model, reoperation for any cause increased the risk of patient and graft loss by 11-fold. Vascular thrombosis, bowel perforation, septicemia, and retransplantation each independently increased the risk of patient and graft loss by three- to fourfold. Table 22.1 summarizes the Clavien-Dindo classification adapted to LT.
| Grade 1 |
| An alteration from ideal post-operative course with complete recovery, or which can be easily controlled and which fulfills the general characteristics, namely: (1) not life-threatening; (2) not requiring use of drugs other than immunosuppressors, analgesics, antipyretic, antiinflammatory and antiemetic, drugs required for urinary retention or lower urinary tract infection, arterial hypertension, hyperlipidemia, or transient hyperglycemia; (3) requiring only therapeutic procedures that can be performed at the bedside; (4) post-operative bleeding requiring ≤ 3 units of blood; and (5) never associated with a prolongation of the intensive care unit (ICU) stay ≥ 5 days or total hospital stay ≥ 4 weeks. |
| Examples |
|
| Grade 2 |
| Any complication that is potentially life-threatening or results in ICU stay > 5 days or hospital stay > 4 weeks, but that does not result in residual disability or persistent diseases |
| Grade 2a |
| Complications requiring only the use of drug therapy, or post-operative bleeding requiring > 3 units of blood |
| Examples |
|
| Grade 2b |
| Complications requiring therapeutic invasive interventions, readmission in the ICU, or prolongation in the ICU stay > 5 days, but which do not result in residual disability |
| Examples |
|
| Grade 3 |
| Any complication with residual or lasting functional disability or development of malignant disease (except squamous and basocellular cutaneous malignancies) |
| Grade 3a |
| Complication with lasting disability that shows no evidence of progression and that has a relatively low risk of graft failure and/or death |
| Examples |
|
| Grade 3b |
| Complications with lasting disability that are either difficult to control or have a significant risk of leading to graft failure and/or death |
| Examples |
|
| Grade 4 |
| Complications that lead to retransplantation (Grade 4a) or death (Grade 4b) |
The most common complications following pediatric LT are discussed in depth in other chapters of this section. The aim of this chapter is to provide a quick overview of postoperative complications, focusing on those that are less common but relevant for the real-life management of the patient.
Following pediatric transplant surgery, the development of drain losses is almost universal. Post-operative ascites is usually drained out by means of an abdominal drainage tube placed before abdominal wall closure. However, the amount of drained ascites varies in different organ recipients, ranging from no production to large losses requiring supportive treatment (i.e., colloids replacement, use of diuretics), often prolonging hospitalization. Importantly, large drain losses may be a marker of several postoperative complications, such as stenosis at the hepatic vein anastomosis (covered in another chapter) or small-for-size syndrome (SFS) ( Fig. 22.3 ).
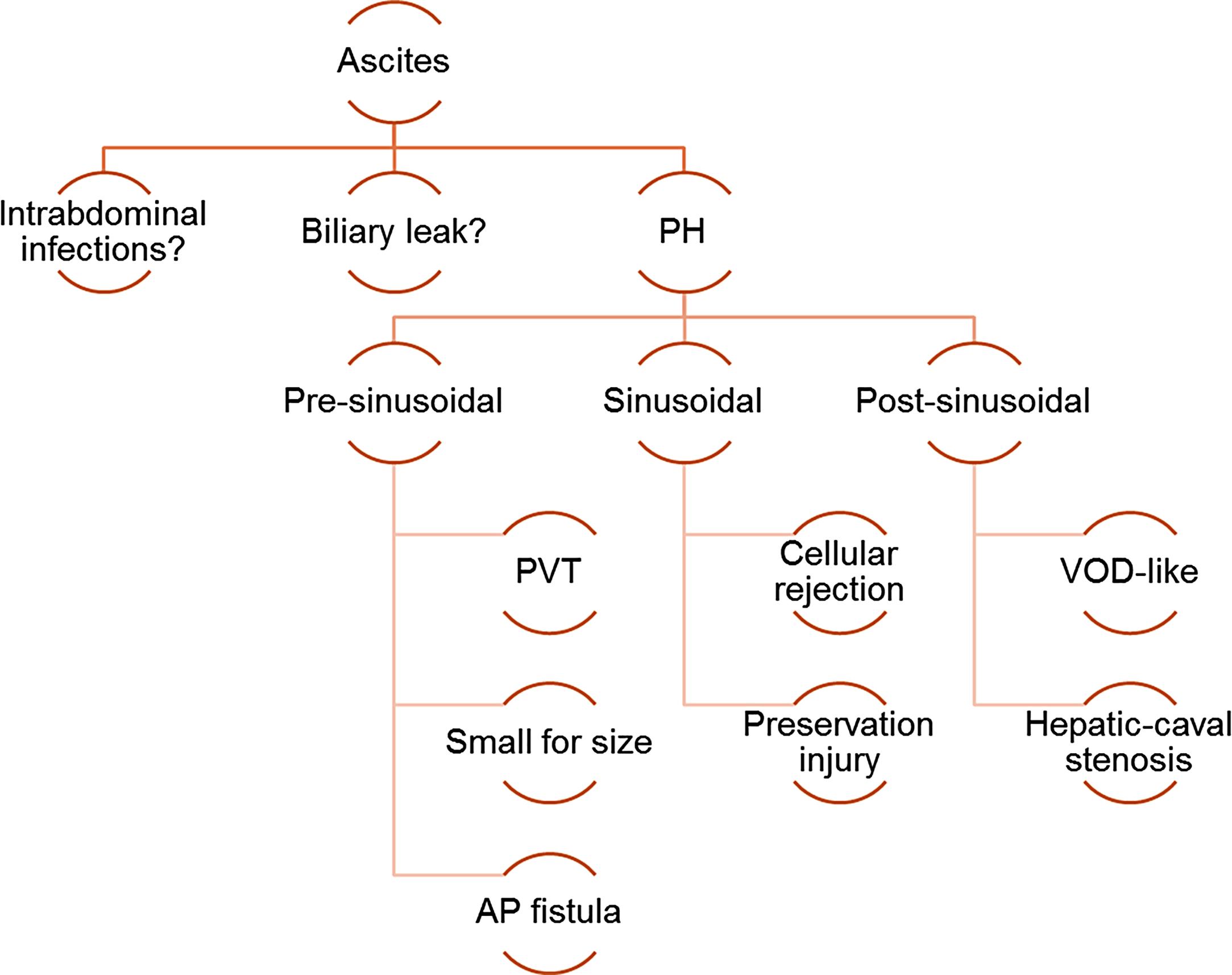
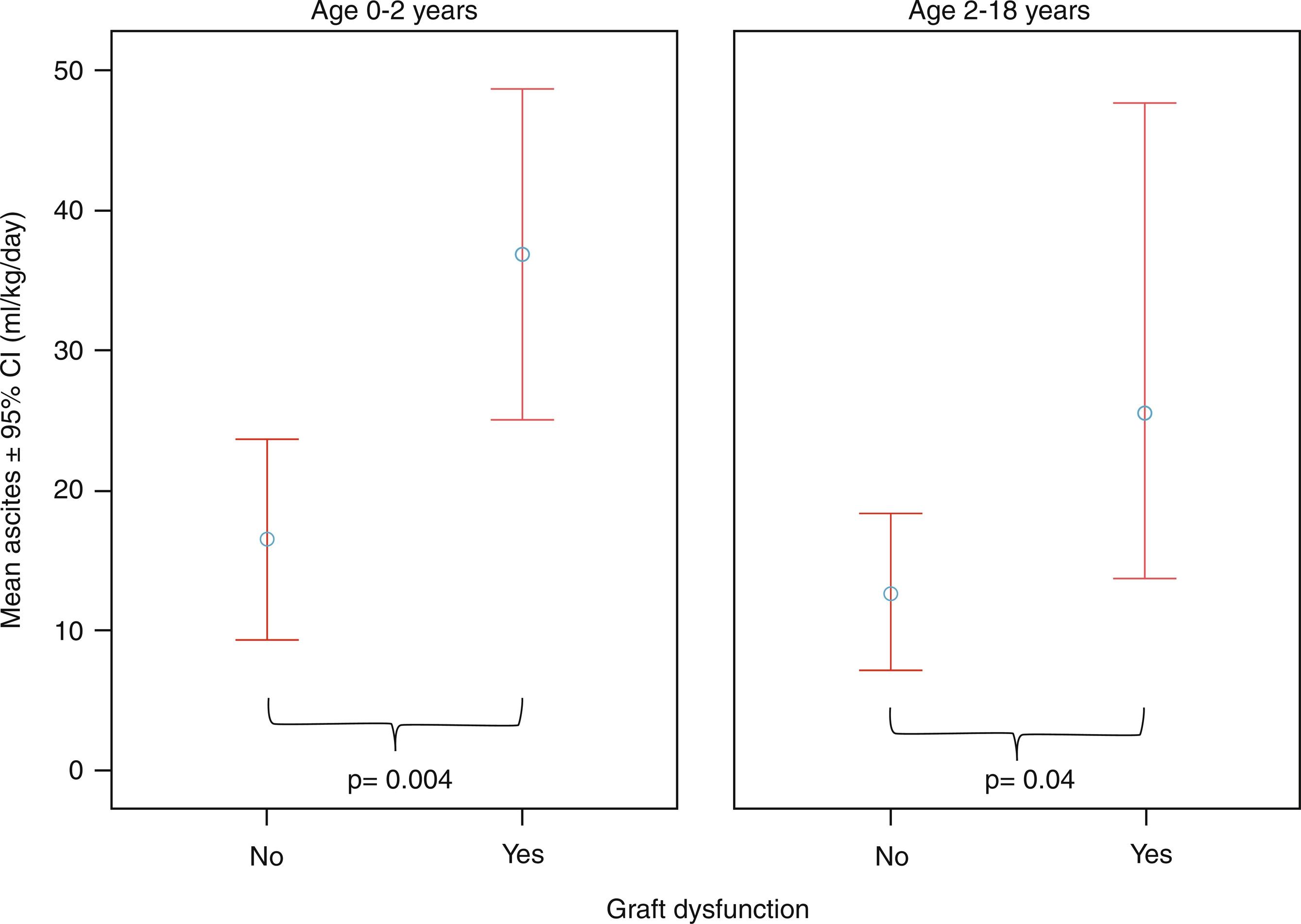
Once these major complications have been ruled out, other factors that may contribute to post-operative ascites should be sought. In some publications, patients with biliary atresia, pre-operative portal hypertension (PH), post-operative pleural effusion, retransplantation, and any post-operative complications had a greater risk of having large drain losses after LT. Our group speculated that large drain losses may represent a marker of graft dysfunction through the mechanism of increased portal pressures resulting from any sort of early graft injury. As in our center, almost all patients come out of the operating theater with an abdominal drain, allowing us to easily measure losses; we conducted a study aimed at correlating ascites volume with recipient and graft features. The number of ascites was calculated as the average of ml/kg/day produced in the first 10 days after the operation, and chylous ascites was recorded separately. Outflow obstruction was always excluded with angiography when the losses were persistently large. Upon statistical analysis, large ascites was associated with the presence of graft abnormalities confirmed by liver histology and low patient and graft weight. Patients with graft dysfunction had a mean ascites of 34 ± 32 mL/kg/d, whereas those without graft dysfunction had a mean ascites volume of 14 ± 19 mL/kg/d. Patient and graft weight were also inversely correlated with the number of daily drain losses, suggesting that younger children tend to produce larger amounts of ascites ( Fig. 22.4 ). The conclusion of this study was that graft dysfunction was predicted by greater than 20 mL/kg/d of ascites at age 0 to 2 years (area under the receiver operating characteristic [AUROC], 0.671), and more than 10 mL/kg/d above 2 years (AUROC, 0.710). The measurement of drain losses after pediatric LT seemed to be useful as a noninvasive marker of any type of graft dysfunction.
The SFS is one of the causes of large-volume drain losses after LT. Although this situation is uncommon in pediatric recipients, SFS occurs when the graft volume and capillary bed are insufficient to accommodate the splanchnic blood reaching the portal vein, making the liver unable to meet the recipient’s metabolic demands.
The SFS has been well defined in adult liver recipients, and in this setting it is clinically characterized by a post-operative prolonged hyperbilirubinemia, presence of severe ascites, prolonged coagulopathy, and encephalopathy in patients receiving an organ with a graft-to-recipient weight ratio (GRWR) less than 0.8%. Nowadays, SFS occurs most commonly following adult-to-adult living-donor LT. The main mechanism for the development of SFS is an excessive portal inflow through a small-size graft, together with arterial hypoperfusion and outflow obstruction. Nonetheless, several other factors, such as quality of the graft, donor age, surgical technique, recipient pre-transplant clinical status, and degree of PH, also contribute to the pathogenesis of SFS.
The hemodynamic changes occurring after reperfusion underlie the pathophysiology of SFS. It is believed that increased shear stress results in disruption of the sinusoidal endothelial lining, leading to septal edema and bleeding in the connective tissue. Furthermore, because of the portal hyperperfusion, the hepatic arterial buffer response is activated in excess, resulting in a decreased hepatic artery flow, which induces ischemic injury.
As previously stated, SFS is very uncommon in pediatric LT because usually the pediatric recipient weighs between 5 and 15 kg, and the graft is a left lateral segment (LLS) from an adult donor; in this situation, the GRWR turns to be usually around 3 but is often higher. However, in older children weighing between 20 and 30 kg, an LLS from an adult donor may remain the best chance, but the GRWR can then decrease to around 1, making SFS a possible post-operative complication.
The management of SFS involves reducing portal blood flow, either by pharmacological treatment or interventional radiology. Several experimental pharmacological treatment strategies have been described for the prevention of SFS. All of them share the common mechanism of action of the attenuation of portal shear stress. However, the drugs that can be used in the clinical setting include somatostatin and octreotide. In an experimental model, somatostatin had a protective effect on pigs receiving extreme hepatectomy, with reduction of portal flow and normalization of the hepatic venous pressure gradient. A similar study was conducted in rats treated with octreotide following extreme hepatectomy, and demonstrated a protective effect through the improvement of hepatic histology and reduction of inflammatory cytokines. However, studies in humans are lacking and impeded by the risks of administering a vasoconstrictor in a patient with fresh anastomoses.
Probably the best choice to prevent or treat SFS is splenectomy or splenic artery ligation/embolization to reduce portal flow or to improve the arterial blood supply to the liver. Reduction of the portal venous flow usually reverts the mechanism of injury through the increase of hepatic oxygen delivery that follows the increased arterial blood flow. Splenic artery modulation can be performed pre-operatively, intra-operatively (as prophylaxis of SFS), or during the early post-operative period once SFS occurs. The procedure bears the risks of septic complications by encapsulated organisms, bleeding, and an increased incidence of PVT. In our center, delayed splenic artery embolization by interventional radiology represents a valid strategy for the treatment of SFS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here