Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infusion of chimeric antigen receptor (CAR) transduced peripheral blood T-lymphocytes has demonstrated therapeutic activity for patients with B cell lymphomas and leukemia and two of which have received FDA regulatory approval.
Intralesional injection of Talmogene laherparepvec (T-VEC), a herpes simplex type-1 oncolytic virus modified to attenuate viral pathogenicity, increases antigen presentation and induces selective tumor lysis that, together with its GM-CSF transgene, has demonstrated induction of antitumor immunity and therapeutic responses, which received FDA regulatory approval.
Clinical use of a viral vector that incorporates the ability to regulate transgene expression in the context of gene transfer with promoter systems inducible by small molecule drugs are currently part of protocols with CAR-T vector and as suicide gene regulation in allogeneic stem cell transplantation.
Include vectors that can be targeted either physically or via promoter expression and that are nontoxic, noninflammatory, and nonimmunogenic.
Include vectors with the potential to incorporate a large transgene and that result in high levels of both transduction and transgene expression.
Include vectors with the ability to regulate transgene expression and/or genomic integration optimally with the use of small molecular, oral drugs.
Toxicities associated with gene therapy have included the use of an adenovirus vector that was been implicated in the death of at least one patient.
Another toxicity associated with gene therapy has included leukemic transformation by insertional mutagenesis via a retroviral vector.
The choice of disease, clinical implementation, and vector are critically important to the future development of successful gene therapy.
Because of deficiencies in gene delivery and targeting, as well as expression levels, it is critical to pair protocols with specific vector attributes.
Despite the preclinical success of gene therapy, the clinical translation of this therapeutic approach has achieved limited success until recently. However, this base of clinical trials has highlighted and allowed the development of strategies to address the challenges to the clinical development of gene therapeutics. This includes vector targeting, potential toxicities, and the cost of vector production. One additional challenge to gene therapy is the lack of a consensus regarding its definition. The US Food and Drug Administration (FDA) defines gene therapy products as “products that mediate their effects by transcription and/or translation of transferred genetic material and/or by integrating into the host genome and that are administered as nucleic acids, viruses, or genetically engineered microorganisms. The products may be used to modify cells in vivo or transferred to cells ex vivo prior to administration to the recipient.” In practical terms, the objectives of gene therapy are the delivery of a transgene to an adequate number of cells and at an effective level of expression sufficient to result in therapeutic outcomes. Both criteria require the use of a vector, and potentially a formulation such that these objectives can be achieved. Although this approach is both simple and attractive, thus far gene therapy has promised much and delivered little because of the technical hurdles. A number of preclinical studies and clinical trials have been undertaken to improve gene transfer systems. As of August 2016, the Journal of Gene Medicine clinical trials database had reported a total of 2409 gene therapy clinical trials worldwide, the majority of which (64.5%) targeted cancer. Because of the tropism of viral vectors and their superior efficiencies for gene transfer and expression, transgene delivery via viral vectors has been the predominant method, with 75% utility in these clinical trials. Furthermore, naked DNA/RNA comprised 23%, and bacterial and yeast vectors comprised 2%. The primary viral vector used has been adenovirus (Adv) based (21%), with retrovirus vectors a close second (18.6%).
In 2003 the first gene therapeutic was approved in China. This was an Adv serotype 5 vector engineered to express p53 (Gendicine) for treatment of patients with head and neck squamous cell carcinoma (HNSCC). A second gene therapy product, H101 (ONYX-015), an Adv vector modified to replicate in and kill cancer cells with TP53 mutations, was approved in December 2005. In December 2011, Neovasculogen (a plasmid vector with a vascular endothelial growth factor [VEGF] transgene) was approved in Russia for the treatment of peripheral arterial disease. In May 2016, Strimvelis (a retroviral vector used as part of an ex vivo stem cell gene therapy) received European Medicines Agency (EMA) authorization for treatment of adenosine deaminase (ADA) deficiency and severe combined immunodeficiency (SCID). As discussed later, a herpes simplex virus (HSV-1) vector with a granulocyte-macrophage colony-stimulating factor (GM-CSF) transgene was approved in the United States, the European Union, and Australia for the treatment of melanoma. Regardless of these approvals, the primary challenges in gene therapy remain improvements in the targeting of existing vectors and increasing gene transduction efficiency. Overcoming these obstacles will facilitate the development of targetable vectors, and given the systemic nature of most malignancies, will help in the development of vectors that can be administered intravenously. This chapter focuses on strategies to improve efficacy and on ongoing gene therapeutic strategies. It also examines and discusses recent advances and indicates areas that require further development in order for clinical gene therapy to become a widely used treatment modality.
Viral gene delivery has developed from the innate ability of viruses to infect T cells, which offers many intrinsic advantages :
Specific cell-binding and cell-entry properties
Efficient targeting of the transgene to the nucleus of the cell
Ability to avoid intracellular degradation
Most viral vectors are based on the principle that an intact wild-type (wt) virus can be modified for safe and effective gene transfer. In general, the more severely attenuated the viral vector is from the wt, the safer the virus is for use in gene therapy protocols, yet the poorer the yield obtained after propagation. Typically, two (or preferably, three) discontiguous partial or complete gene sequences are deleted to reduce the potential for homologous recombination. Typically, specific genes critical to viral replication are modified or deleted, resulting in a recombinant viral vector that is “replication defective.” The transgene to be delivered by the virus is then inserted into the viral genome at the site created by the removal of the viral replication genes. The transgene must be a smaller size to fit within the available space, which is a critical characteristic because the transgene cannot be packaged into an infectious particle if the new viral genome is too large. Many of the viruses that are used as vectors lack genes for replication in normal cells; therefore the recombinant virus and its transgene must be grown in a packaging cell line that provides the complementary genes required for viral replication. The recombinant viral particles are purified as live infectious viruses and are replication incompetent in the absence of the packaging cell line. Alternatively, the packaging cell line can be used to infect (transduce) cells or tissues in vitro .
The Retroviridae are a large family of RNA viruses including Moloney murine lentivirus–related viruses (e.g., Moloney murine leukemia virus [MMLV]) and lentiviruses (e.g., human immunodeficiency virus [HIV] types 1 and 2). Their genomes consist of two identical positive-sense, single-stranded RNA molecules (~3.5 kb), and are encased in a capsid along with integrase and reverse transcriptase enzymes. Initially, retroviral vectors were the most widely used viral vectors, a distinction that has been replaced by Adv vectors in recent years. Retroviruses can transduce only those cells that are actively undergoing mitosis, limiting their usefulness with certain cell populations, especially hematopoietic stem cells (HSCs). Retroviral vectors provide a reasonable level of gene expression and are technically easy to produce, although the titers obtained are suboptimal. In addition, the production of retroviral vectors needs to be carefully monitored owing to the potential for helper virus contamination.
One retroviral vector, Strimvelis, was approved in July 2016 as part of an ex vivo, gene-modified autologous CD34+ cell therapy for the treatment of ADA-SCID. This approach was developed by scientists at San Raffaele Scientific Institute and licensed to GlaxoSmithKline in 2010. Because of orphan indication, Strimvelis was approved with a clinical trial of only 12 patients, but demonstrated 100% efficacy (survival at 3 years and longer time points) in a pivotal trial.
Most of the retroviral vectors that are used for gene therapy are based on MMLV. Vector replication is prevented by the deletion of the gag, pol, and env gene regions. The gag region encodes the capsid proteins; the pol region encodes reverse transcriptase and integrase; and the env region encodes proteins required for receptor recognition and envelope anchoring ( Fig. 29.1 ). The genome includes long terminal repeats (LTRs) at either end that play a vital role in initiating DNA synthesis and regulating transcription of the viral genes. The gag, pol, and env gene products are supplied by a complementary packaging cell line. When a retroviral vector plasmid is introduced into a packaging cell line, viral RNA is produced, packaged into virions, and secreted into the medium. Each resultant viral particle is able to integrate itself into the genome of the host cell but is unable to produce additional viral particles because it lacks the gag, pol, and env genes. The transduced DNA sequences are stably integrated into the chromosomal DNA of the target T cells, and in this way are transferred to cellular progeny of transduced cells. Highlights of results obtained to date with retroviral vectors include the findings of the therapeutic studies in children with SCID-with an x-linked defect in the intrleukin-2 receptor γ chain (X1), which will be discussed later in this chapter, as well as the gene-marking studies of Malcolm Brenner and others. In the latter studies, it was shown that tumor cells within autologous stem cell transplant products could be responsible for tumor relapse, at least in patients with leukemia.
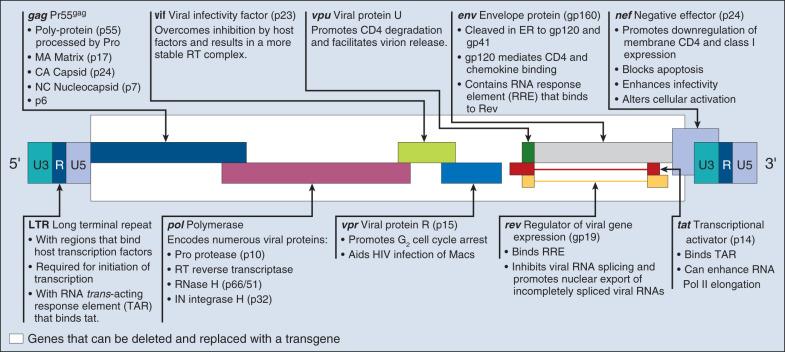
The most recently discovered members of the retrovirus family are HIV and simian immunodeficiency virus (SIV), which belong to a subclass of retroviruses known as lentiviruses. The development of HIV gene therapy vectors has several potential advantages based on the following characteristics:
Transduction of actively dividing and nondividing cells
Long-term, stable transgene expression, which occurs because of genetic integration
Inherent tropism for CD4 T cells, macrophages, and HSCs
Genetic modifications, such as the introduction of vesicular stomatitis virus (VSV) G protein into the lentiviral envelope, can widen the tropism of this vector. The first clinical study using a lentivirus vector was undertaken in HIV-infected patients. This study investigated the safety of infusion of autologous T cells modified with an HIV-1–based lentiviral vector expressing an antisense gene against the HIV envelope. Five patients with HIV infections that were resistant to antiviral therapy and had viral loads of greater than 5000 copies/mL and CD4+ T-cell counts between 200 and 500 cells/mm 3 were treated. The primary end points included adverse events, viral load, CD4+ counts, and the emergence of replication-competent lentivirus derived from the vector. In this phase I study, one subject was reported to have a sustained decrease in viral load. The CD4 counts remained steady or increased in the other four subjects, and sustained gene expression was observed. These preliminary studies support the safety of lentivirus vectors.
Since the initial trials, lentivirus and retrovirus vectors have been used as part of adoptive immunotherapy using engineered expression of chimeric antigen receptors (CARs) on the surface of T cells, enabling the redirection of T-cell specificity. This is a rapidly growing therapeutic strategy for treating patients with refractory cancer. The enthusiasm for CAR T cells has been driven by the clinical success of CD19-targeted CAR T-cell therapy in B-cell acute lymphoblastic leukemia, and the promising data in B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. The successful therapeutic outcomes depend on the long-term expression of the CAR transgene in T cells, which is achieved by delivering the transgene using integrating gammaretrovirus or lentivirus. However, uncontrolled integration in host cell genomes has potential to result in insertional mutagenesis and tumor induction. One approach used to address this is an episomal long-term cell engineering method, using a nonintegrating lentiviral vector containing a scaffold or matrix attachment region element, for either expression of transgenes or silencing of target genes. An alternative approach that is being developed is the use of safety or suicide genes, with a conditional promoter, allowing the elimination or reduction of T-cell toxicity. This approach has been used clinically for CAR T-cell therapy and for the control of graft-versus-host disease (GVHD) after haploidentical stem cell therapy.
Recombinant Adv is a nonenveloped, icosahedral, double-stranded DNA virus with a capsid containing 252 capsomeres (240 hexons and 12 pentons). The large genome of Adv (36 kb) enables large genes to be inserted into an Adv-based vector. Transgenes in Adv vectors are not incorporated into the genome of transduced cells, but rather remain as an extrachromosomal entity in the nucleus. First isolated from US Army recruits who had acute respiratory symptoms, Adv vectors have been found to be common human pathogens. To date, 49 serotypes have been characterized and associated with a variety of symptoms, ranging from a mild cold to acute febrile pharyngitis. Replication-defective recombinant Adv vectors are currently the most commonly used viral vectors in clinical trials. Ad2 and Ad5 are used primarily for gene therapy applications. Recently, however, the Ad11 and Ad35 serotypes were shown to exhibit a unique tropism that includes HSCs, a finding that potentially widens their utility.
The Adv vector's genome ( Fig. 29.2 ) can be divided into two main regions: early (E) and late (L) according to the time at which their genes are expressed during virus replication. There are four regions of early genes that are termed E1, E2, E3, and E4, and one region of late genes composed of the five coding units termed L1, L2, L3, L4, and L5. The E1 region is essential for viral replication; therefore recombinant Advs without the E1 region are considered replication defective. In a replication-defective Adv vector, the E1 region can be replaced with a transgene for expression. Furthermore, removal of genetic material from the vector, such as the E3 and/or the E4 region(s), allows for larger genes to be inserted and reduces the viral immunogenicity. Viruses without the E3 and E4 regions are referred to as “gutless” and have decreased antigenicity.
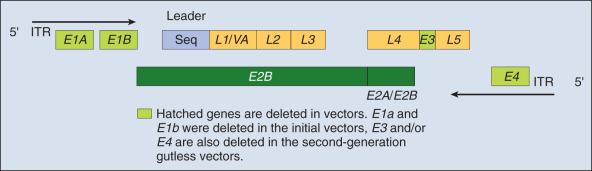
The E1 region of Adv vectors is subdivided into E1A and E1B. The E1A gene product is a viral transcription unit that activates the expression of other Adv transcription units by binding to viral promoters. The E1B region codes for a 55-kD protein that interacts with the cellular p53 tumor suppressor protein and regulates the host T cells' cycle progression supporting viral replication. E1B also binds to viral E4 proteins and to p53, which together act to depress host protein synthesis. The E2 region codes for viral DNA polymerase and the Adv single-stranded DNA-binding protein. The E3 region is not required for in vitro replication; however, it does offer the virus some protection against host defense mechanisms. The E4 region codes for proteins involved in:
Regulation of viral and cellular protein expression
Replication of viral DNA
Switching off of host protein synthesis
The late genes (L1–L5) are expressed at the onset of viral DNA replication and code for structural polypeptides that are needed for virion assembly. This understanding of viral replication has allowed the development of extremely elaborate, conditionally replicative Adv vectors capable of replication only in cancer cells.
The transduction efficiency of Adv vectors is high compared with that of most other viral vectors. Because of the structural stability of the capsid polypeptides of Adv, viral particles can be purified and concentrated to a very high titer of ~1 × 10 12 plaque-forming units (pfu) per milliliter. This is in contrast to retroviral titers, which achieve much lower titers (~1 × 10 7 pfu/mL) because of the instability of their envelopes. Another distinguishing characteristic of Adv vectors is their lack of integration into the human genome. The Adv genome remains in the nucleus of the target T cells as a nonreplicating extrachromosomal entity, thereby avoiding any potential for mutagenic effects caused by random integration into the host.
However, Adv vectors have potential shortcomings, including:
Expression is transient because the viral DNA does not integrate into the host.
Viral protein expression by the Adv vector occurs after administration into a host.
Advs are a common pathogen, resulting in hampered in vivo delivery associated with antibody (Ab) responses.
The period of Adv transgene expression is relatively short; therefore this is a suboptimal vector if expression is desired for longer than 10 to 14 days. This short expression time is due primarily to the induction of a cytotoxic T-lymphocyte (CTL) response to viral polypeptides, as well as potentially to the transgene itself, especially if it is not expressed normally. Because the Adv genome does not integrate into the target cell, only one of the daughter cells (if the target T cells are dividing) will contain the transgene. Manipulation of the immune response can result in longer expression; however, Adv gene delivery is ideally suited to those situations that require only a single period of transgene expression in which transient expression is desired—for example, growth factor therapy. A second major disadvantage of Adv vectors used in vivo is the immune response (CTL and Ab), both endogenous and induced, which can preclude infection and cause the destruction of transduced cells, resulting in local tissue damage and inflammation. This shortcoming was demonstrated in studies with intrabronchial delivery of Adv for the treatment of cystic fibrosis. Host T cells presenting peptides from Adv-encoded transgene products target the host cell for CTL-mediated destruction. A third major disadvantage of Adv vectors is that most humans are primed against at least one serotype because Adv is a naturally occurring virus. Using the same serotype in a gene therapy context will likely result in a rapid and vigorous immune response such that high levels of anti-Adv Ab occur in the sera within days of Adv vector administration. Another similar problem is the potential secondary immune response induced by the readministration of a vector. It must be stressed that transgene expression can occur during a boost, although a shortened duration is observed. The augmentation of a CTL response by an Adv vector suggests the usefulness of Adv vectors as vaccine adjuvants ( Box 29.1 ).
Adenovirus (Adv) vectors have a number of positive and negative attributes. The positive attributes include the transduction of a wide profile of cellular phenotypes, including not only epithelial and carcinoma cells but also hematopoietic cells. Furthermore, the use of Adv vectors results in a high frequency of transduction and high levels of transgene expression. A negative attribute of Adv vectors is transient expression, although for appropriate targets such transitory infection is a positive attribute. The transient expression is due, in part, to the high level of innate vector immunogenicity, which can limit multiple cycles of transduction and chronic transgene expression. The resulting Adv profile of activity is ideal for the transduction of dendritic cells (DCs) as vaccines, the purging of tumor cells from stem cell products, and intralesional injection of carcinomas. In addition, the ability to develop Adv vectors that are conditionally replicative has great potential for the treatment of neoplastic disease. It is noted that two Adv vectors have received regulatory approval in China. These controversial studies have resulted in the treatment of several thousand patients, supporting the safety of these vectors.
Adeno-associated virus (AAV) vectors offer many of the same advantages as Adv vectors, including a wide host-cell range and a relatively high transduction efficiency. AAV vectors stably integrate at specific sites in the host genome, resulting in a longer-lasting transgene expression. In addition, these stable vectors can infect a variety of dividing and nondividing cells without inducing an immune response. AAV vectors cause little damage to target T cells—unlike Adv vectors, which can cause a high degree of cytopathogenicity. There is evidence, however, to suggest that AAV vectors are significantly less efficient than retroviral vectors at transducing primary cells because most of their DNA remains extrachromosomal and does not integrate into the host genome. Furthermore, they cannot incorporate genes larger than 5 kb and must be screened closely for Adv contamination.
HSV vectors are developed primarily for protocols that target neuronal tissue. Similar to Adv vectors, HSV vectors are maintained as an extrachromosomal DNA element in the nucleus of host T cells, but can establish long-lived asymptomatic infections in the sensory neurons of the peripheral and central nervous tissue. HSV vectors also have a wide host range and are similar to Adv vectors in that they allow large gene inserts of up to 20 kb. These vectors are infective even with multiple deletions of immediate-early (IE) genes that are essential for replication, resulting in less cytotoxic vectors, thereby reducing safety concerns. HSV vectors can be produced at high titers and express transgenes for a long period of time in the central nervous system (CNS). The major concern associated with HSV is the potential for wt virus to replicate lytically in the human brain, resulting in encephalitis. Other significant disadvantages with HSV vectors include:
Requirement for additional engineering to increased efficiency
Transient expression associated with lytic infection and viral protein expression
Relatively low transduction efficiency
Despite the inherent deficiencies, an HSV-1 vector was the first gene therapeutic product approved by the FDA in the treatment of neoplasia. This HSV-1 therapeutic agent was developed as an oncolytic virus, which is an approach based on the ability of a virus to replicate in tumor cells and promote T-cell responses. Oncolytic viruses can be native viral particles or can be genetically manipulated to decrease pathogenicity or increase immunogenicity. In general, all oncolytic viruses induce antitumor activity both through direct infection of tumor cells with resultant lysis of the cancer cell and secondarily though induction of antitumor immunity. Talimogene laherparepvec (T-VEC), an HSV-1–based intralesional oncolytic immunotherapy, modified to attenuate viral pathogenicity and increase antigen (Ag) presentation and induce selective tumor lysis, was the first oncolytic gene therapy to demonstrate an improved response in a phase III randomized clinical trial of advanced melanoma patients. It was approved by the FDA, then by the European Commission for this indication. The antitumor response is due to the insertion and expression of a gene encoding human GM-CSF, resulting in local GM-CSF production and the induction of tumor-specific T-cell responses.
The FDA approval of T-VEC was based on the overall intention-to-treat (ITT) population from a randomized, multicenter, open-label phase III study. This trial had 436 patients with unresectable stage IIIB–IVM1c melanoma. In this ITT population, intralesional T-VEC injection significantly improved the durable response rate compared with subcutaneously administered GM-CSF and was well tolerated. Secondary end point analyses included overall survival (OS), and a significant median survival difference was observed with T-VEC versus GM-CSF. Further analysis showed the efficacy of T-VEC to be greater in patients with unresectable melanoma with regional or distant metastases and no visceral disease. In patients with stage IIIB–IVM1a melanoma, median OS was significantly longer in the T-VEC arm compared with the GM-CSF arm. Based on these and other results, T-VEC was approved in Europe for adults with unresectable stage IIIB, IIIC, or IVM1a melanoma with no bone, brain, lung, or other visceral disease. Reports from subsequent studies have included a detailed analysis of the efficacy and safety of T-VEC in patients with stage IIIB/C or IVM1a melanoma in the OPTiM study (Oncovex [GM-CSF] Pivotal Trial in Melanoma), including a benefit-risk analysis ( Box 29.2 ).
Oncolytic viruses comprise a new class of gene therapy that work through multiple and distinct mechanisms including direct killing of tumor cells and the induction of an antitumor immune response.
The first US Food and Drug Administration (FDA)–approved oncolytic virus, talimogene laherparepvec (T-VEC) is a modified herpesvirus that was approved for the treatment of melanoma by regulatory agencies in the United States, Australia, and Europe.
T-VEC is attenuated by the deletion of the herpes neurovirulence viral genes and enhanced for immunogenicity by the deletion of the viral ICP47 gene. Immunogenicity is further supported by the addition of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, which helps prime T-cell responses.
T-VEC demonstrated significant improvement in durable response rate, objective response rate, and progression-free survival in a randomized phase III clinical trial for patients with advanced melanoma.
Oncolytic viruses have an excellent safety profile and can be genetically manipulated to prevent toxicity, enhance immune responses, and induce tumor rejection.
Oncolytic viruses can be combined with other forms of immunotherapy, chemotherapy, targeted therapy, and radiation therapy to improve the efficacy of both therapeutic approaches.
The origin of vaccinia virus (VV), the virus used for vaccination against smallpox, is not known, but it was probably derived from cowpox virus, variola virus, or a hybrid of the two. Percutaneous VV vaccine administration results in protective cellular and humoral immune responses in more than 95% of primary vaccines. Recombinant VV vectors are highly attenuated, host-restricted, and nonreplicating or poorly replicating poxvirus strains (including the modified vaccinia Ankra [MVA] and canarypox or avipox vector [Alvac]) and thus do not create productive infections. MVA is avirulent in normal and immunosuppressed animals and safe in humans. Recent studies using transgenic mice provided a comparison of VV immunogenicity, including MVA and Western Reserve (WR). These studies demonstrated that MVA vaccines elicited CD8+ T-cell responses that are comparable to those induced by the replication-competent WR strain. Furthermore, MVA vaccination was shown to be protective against a lethal respiratory challenge with the virulent WR strain. The most frequent adverse complication of VV vaccination is inadvertent inoculation (usually autoinoculation) at other sites. Serious complications, which are more common among primary vaccinees and infants than among revaccinees and adults, include the following:
Generalized vaccinia in otherwise healthy individuals, which is generally self-limiting
Eczema vaccinatum, which consists of disseminated cutaneous lesions in highly susceptible patients with eczema or other chronic skin diseases, which can be severe or even fatal
Progressive vaccinia (vaccinia necrosum), which is a severe, potentially fatal illness seen in patients with immunodeficiency, whether congenital, acquired (e.g., via leukemia or lymphoma), iatrogenic (e.g., via chemotherapy or glucocorticoid treatment), or HIV induced
Postinfectious encephalitis, which is rare (three cases per million primary vaccines) but can be fatal in 15% to 25% of patients and can leave 25% of patients with permanent neurologic sequelae
Similar to Adv vectors, VV vectors are used for immune manipulation and as a vector for vaccines. VV vectors have been used worldwide to eradicate smallpox and, as discussed previously, provide a relatively safe live vaccine. Vaccinia vectors do not integrate into the genome of the host cell; however, they can accommodate large transgenes and are extremely immunogenic. VV vectors are used to immunize patients against tumor antigens (Ags) by cloning Ags and/or genes encoding proteins with adjuvant activity (e.g., cytokine or costimulating factor genes) into the viral genome. Most transgenes are expressed at high levels in vivo, eliciting an Ag-specific response. Vector-induced immunity, however, can limit the ability of the vaccinia transgenes to boost an immune response, which is an observation similar to that seen with Adv vectors. The current emphasis is on VV infection of dendritic cells (DCs) with use of a vector with an antigenic transgene.
In association with the immunogenicity of VV vectors and their ability to deliver an antigenic transgene, they have been used clinically as a melanoma vaccine. In clinical studies by Wallack and colleagues, during a phase III trial, a vaccinia melanoma oncolysate delivered as an active specific immunotherapy was found to increase the disease-free survival or OS of patients with stage III melanoma in a surgical adjuvant setting. Other studies have used VV mutants that are conditionally replicative and can lyse cancer cells after viral replication. These vectors have been used in a strategy whereby insertional inactivation of the VV thymidine kinase (tk) gene was used to limit viral replication in cells with large intracellular nucleotide pools, such as tumor cells. In a similar approach, Mastrangelo and coworkers inserted the gene for GM-CSF into the VV tk gene locus as a strategy to generate an oncolytic virus that induced antitumor immunity after infection of malignant melanoma. This vector is currently in a clinical trial of intralesional administration to patients with refractory recurrent melanoma. In the first seven patients studied, two patients had a complete response and three other patients had partial responses. Other oncolytic VV vectors have been engineered with cDNAs for cytokines such as interleukin-2 (IL-2) or with prodrug-activating enzymes such as cytosine deaminase to augment antineoplastic efficacy.
The role of VV vectors as vaccines has focused predominantly on carcinoembryonic antigen (CEA) as the vaccine Ag. CEA is a glycoprotein self-Ag found on breast, lung, gastric, colon, and ovarian tumors. One such vector is a recombinant VV containing the CEA gene (rV-CEA). In a phase I clinical trial, the safety of rV-CEA was demonstrated; however, no significant antineoplastic effects were observed. Possible reasons for the lack of clinical efficacy in these trials include:
Prior exposure to the VV, leading to the development of antivaccinia immune responses after repeated vaccinations
Advanced state of the patients' tumors
Potentially compromised immune status of the patients
Another phase I rV-CEA vaccine study demonstrated that CEA-specific T-cell responses could be generated in humans after vaccination. A second recombinant anti-CEA vaccine, Alvac-CEA, has been developed. Similar to rV-CEA, Alvac-CEA contains the CEA gene; however, unlike rV-CEA, it cannot replicate in mammalian cells. The safety of Alvac-CEA has been documented in phase I trials in patients with advanced carcinomas. A moderate but statistically significant increase in the number of CEA-specific CTL precursors was observed in seven of nine HLA-A2–positive patients treated with Alvac-CEA, although objective anticancer effects were not observed. Preclinical studies have suggested that the combination of rV-CEA and Alvac-CEA in a prime-boost protocol can induce a more vigorous T-cell response than either vaccine alone. In a clinical prime-boost study, 18 patients with advanced tumors expressing CEA were randomized to receive either rV-CEA followed by three Alvac-CEA vaccinations, or Alvac-CEA (three times) followed by one rV-CEA vaccination. In this study, vaccination with rV-CEA followed by Alvac-CEA resulted in an increased frequency of Ag-specific interferon-γ (IFN-γ)–positive cells by enzyme-linked immunospot assay (ELISPOT) relative to the reverse order of vaccination.
Another method to enhance the responses to a vaccine is to incorporate a costimulatory signal. In the absence of a costimulatory signal, presentation of an Ag to T cells can result in anergy. B7.1, which binds to CD28 on T cells, is one such costimulatory signal that results in the production of IL-2 and IFN-γ by T cells. In a vaccine study using VV vectors, 39 patients were treated with Alvac-CEA B7.1. In one study using the Alvac-CEA-B7.1 vaccine, patients with metastatic CEA-expressing adenocarcinomas received vaccine intradermally every 2 weeks for a total of four injections. In this phase I trial, 27% of the patients had disease stabilization after four vaccinations. Six of 31 patients with elevated serum CEA levels had a temporary decline in CEA. In addition, HLA-A2–positive patients demonstrated increased CEA-specific T-cell frequencies after three vaccinations. Based on these studies and additional phase II data, a phase III trial was initiated in 255 patients with advanced pancreatic cancer at approximately 60 medical centers. The protocol was powered to detect a 2-month improvement over control chemotherapy based on a median OS of 6 months. Unfortunately, this study did not meet its primary end point of improving OS compared with palliative chemotherapy or best supportive care. However, this outcome is not unexpected, given that patients with advanced pancreatic cancer have a rapid disease progression and are poorly responsive to intervention in general ( Box 29.3 ).
Vaccinia virus (VV) vectors have a profile of activity analogous to that of adenovirus (Adv) vectors—that is, they can easily transduce a wide range of cells, resulting in transient expression, and have as a negative attribute a brief transgene expression as a result of the innate antigenicity of the vector. In contrast to Adv vectors, VV vectors almost inevitably lyse the transduced cell, rendering it potentially less attractive as a vector (especially for dendritic cell [DC] transduction) owing to the shorter half-life of the transduced cell. Significant experience with the administration of both VV and Adv vectors as vaccines has provided a strong safety profile for both. In theory, the concomitant use of VV and Adv vectors makes possible cycles of vaccine delivery via transduced DCs, allowing a prime-boost immunization with DCs, which have a high frequency of transduction and levels of transgene expression, while reducing the concerns associated with the innate antigenicity of the viral vectors.
High-titer alphavirus vectors can provide efficient gene delivery both in vitro and in vivo. In addition, efficient CNS infections via intranasal and vascular injections with virulent and avirulent replication-competent Semliki Forest virus (SFV) strains have been shown in animal models. Replication-deficient alphavirus particles have a high local and transient transgene expression in rodent brains. Furthermore, repeated SFV injections are possible in the absence of an immunogenic response against SFV, which is in contrast to Adv and VV vectors. Modifications to the envelope structure of Sindbis virus are possible with resultant changes in host range and targeting. The favorable characteristics of alphavirus vectors include:
Rapid production of high-titer virus
Broad host range
High RNA replication rate in the cytoplasm
High transgene expression levels
Negative attributes include:
Short-term expression
Strong cytotoxic effects on host T cells
Nonetheless, both these properties are advantageous for certain indicators, particularly vaccine production.
Nonessential genes can be removed from viral vectors to allow room for transgene(s) to reduce inflammatory responses and to increase safety. This process involves simplifying the virus, sometimes to an extreme. After undergoing such a process, a virus vector can be an artificial “vector shell,” allowing the gene of interest to be expressed at high levels, in a highly regulated manner, and for a controlled period of time. Another approach to achieve the same result is to produce a vector that can introduce genetic material to the nucleus of cells. This strategy has resulted in the development of several nonviral vector systems; however, the efficiency of “naked DNA” as a therapeutic is suboptimal without some form of carrier or formulation ( Box 29.4 ).
The transduction efficiency of plasmid vectors is low, even with the use of formulations to improve transfection efficiency and increase transgene expression. Furthermore, this approach appears to work better in vitro than in vivo. In contrast to viral vectors, plasmid vectors offer little innate antigenicity, although there have been reports of immune responses to bacterial genes. Positive attributes of plasmid vectors include the low level of innate immunogenicity and the potential for genomic integration. The use of hydrodynamic delivery in rodents has provided a powerful preclinical tool. However, clinical translation is problematic with the potential for use in an isolated limb. In contrast, retroviral and lentiviral vectors provide the same characteristics with higher levels of transgene expression and improved transduction efficiency relative to plasmids. However, the improved transgene expression and transduction levels of retroviral and lentiviral vectors remain significantly lower than those of adenoviral and vaccinia vectors.
One form of nonviral gene delivery is the use of purified DNA plasmids. The transgene expression is low after intramuscular or intratumoral injection; however, high levels are observed if hydrodynamic injection is used. Naked DNA injection is typically performed as an intramuscular or intratumoral injection. Despite the simplicity of this approach, transfection efficiency is low and results in limited expression. Various formulations, including lipid or pluronic formulations, and incorporation into nanoparticles or liposomes, have been used to improve transduction efficacy and gene expression.
Nonviral liposomal delivery systems can be intravenously injected with limited vector-associated toxicity, but with transgene expression, especially in the lungs. Tumor targeting using tumor specific promoters, ligandation of receptors to the liposome surface, and pegylation of liposomes have all been studied. Although some degree of tumor targeting has been observed with these delivery systems, the level of transgene expression is generally low. Studies have revealed that liposome/DNA complexes can also elicit an inflammatory response when injected systemically, resulting in suppression of transgene expression. Furthermore, failure to achieve increased or sustained gene expression after repeated injections has been a major obstacle in the development of liposomes. It has been shown that cationic liposome (DOTAP:cholesterol or DOTAP:Chol)/DNA complexes can achieve effective levels of transgene expression in tumor-bearing lungs and, when injected intravenously, can achieve levels sufficient to cure immunocompetent mice with disseminated experimental metastases. Furthermore, repeated daily injections can result in a dose-dependent increase in transgene expression in tumor-bearing lungs.
Hydrodynamic tail vein plasmid delivery results in high levels of transgene expression in the livers of rodents. Lower levels of transgene expression (100- to 1000-fold) are found in the spleen, heart, kidneys, and lungs. This simple nonviral gene transfer procedure entails the rapid delivery of naked plasmid DNA in a relatively large volume of physiologic saline. In a typical mouse, weighing 20 g, the plasmid is delivered in a total volume of 2.0 mL over a period of 5 to 7 seconds.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here