Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The interventional radiologist can play a central role in the provision of enteral nutrition through the placement of gastrostomy and jejunostomy tubes. Traditionally, enteral feeding tubes have been placed by surgical or endoscopic techniques. The first successful placement of a percutaneous endoscopic gastrostomy (PEG) was described in 1979 by Gauderer and Ponsky. This was followed 2 years later by the first percutaneous radiologic gastrostomy (PRG), performed under fluoroscopic guidance by Preshaw. PRG is a well-tolerated procedure that provides nutritional support where required. The procedure is associated with low morbidity and mortality, with recent technical advances improving the long-term patency of enteral catheters. The advantage of PRG is the relative simplicity of the technique, facilitating enteral feeding in the hospital or home environment.
PRG is indicated in a wide range of patients who cannot maintain their nutrition orally. The most common patient subgroups are those with neurologic impairment resulting in absent gag reflex or disorders of swallowing, and esophageal or head and neck malignancy. Indeed, PRG is particularly advantageous in these patient subgroups because it can be performed with minimal sedation, thereby decreasing the risk of aspiration and avoidance of endoscopy, which is often precluded by upper gastrointestinal tract stenosis in the case of malignancy. Other patient groups who benefit from gastrostomy placement and enteral feeding include those with intestinal malabsorption secondary to small bowel pathology such as Crohn’s disease, radiation enteritis, and scleroderma; patients who require supplementary enteral support such as those with cystic fibrosis or significant burns; and patients with psychologic ailments such as eating disorders or profound depression.
Less commonly, gastrostomy is placed for decompression of gastrointestinal obstruction. Patients with diabetes-related gastroparesis can benefit from dual-lumen cannulation ; one lumen decompresses the stomach while a distal limb is placed beyond the ligament of Treitz for feeding. When not feeding during the day the two loops are attached, obviating the need for a drainage bag.
There are few contraindications to PRG placement. As with most interventional procedures the patient’s coagulation status should be evaluated, and any abnormality corrected before the procedure. An international normalized ratio ≤ 1.5 and a platelet count in excess of 50,000/mm 3 are considered acceptable.
Difficulties with percutaneous access to the stomach, such as interposition of the colon between the stomach and anterior abdominal wall, or a large or low-lying liver are considered relative contraindications. In the case of colonic interposition, an infracolic approach is possible. Previously it was believed that gastric fixation (gastropexy) should not be performed with this approach because of the increased potential for complications including colonic obstruction; however, more recently an infracolic approach with gastropexy has been described with no additional complications. In the presence of a low-lying or large liver the use of computed tomography (CT) and/or ultrasound can facilitate gastric cannulation. PRG placement can be challenging in patients with a high-lying stomach, as is often seen in patients with amyotrophic lateral sclerosis due to diaphragmatic weakness, and an intercostal approach may be required.
Previous surgery (e.g., Billroth II gastroenterostomy, partial gastrectomy, or gastric pull-through surgery) is considered a relative contraindication owing to anatomic distortion. Balloon distention of the stomach remnant and/or CT-guided placement of the gastrostomy tube may render the procedure possible.
In head and neck or esophageal malignancy significant stenosis of the upper gastrointestinal (GI) tract may preclude placement of the nasogastric tube required for gaseous distension of the stomach. In such cases a small-diameter catheter and hydrophilic guidewire may be used to cross the stenosis ; alternatively the stomach can be punctured under ultrasound or CT guidance.
The presence of ascites is no longer considered an absolute contraindication to PRG placement, and most interventional radiologists will perform the procedure in the presence of mild ascites. In the case of significant ascites preprocedural paracentesis and gastropexy is mandatory to reduce the risk of catheter looping in the peritoneal cavity, tube dislodgement, and peritubal leakage. It is also necessary to prevent reaccumulation of ascites after the procedure because the weight of fluid can result in separation of the stomach from the anterior abdominal wall despite gastropexy. Sonographic follow-up and repeated paracentesis should therefore be performed as required. Patients undergoing peritoneal dialysis require similar consideration.
The presence of gastric varices as in portal hypertension remains one of the only absolute contraindications to PRG due to the associated risk of significant hemorrhage.
The required equipment for PRG insertion is detailed in Table 91.1 . The procedure and potential complications are discussed with the patient and informed consent obtained. A coagulation study is performed and any abnormalities corrected. The preceding dose of low-molecular-weight heparin should be held. Although aspirin may be continued, clopidigrel should be discontinued 5 days in advance where safe to do so. The patient is required to fast from the evening before the procedure, and a nasogastric tube (NG) placed. In the past, 100–200 mL of dilute barium sulfate (50–100% w/v) was administered (orally or via NG) 24 hours before the procedure. This aids in identifying the colon so it can be avoided during PRG. Similar to other investigators, we have discontinued the routine use of barium in our unit because we find gas in the colon is usually a sufficient marker when lateral screening is used. If there is insufficient gas in the colon, a small amount of air can be insufflated per rectum. Although advocated by some, particularly in head and neck cancer patients, antibiotic prophylaxis does not appear to reduce the rate of infection in PRG and is therefore not recommended for routine use.
| Equipment | Comments |
|---|---|
| Nasogastric tube and air insufflation balloon | Placed before procedure |
| Dilute barium suspension | Optional; colonic air usually visible fluoroscopically |
| Noninvasive monitoring equipment | Monitor heart rate, blood pressure, oxygen saturation |
| Paralytic agents | Hyoscine- N -butylbromide or glucagon hydrochloride |
| Local anesthetic | 1% lidocaine |
| Medications for conscious sedation | Fentanyl citrate, midazolam |
| T-fasteners, slotted needle, and stylet | For gastropexy |
| 10-mL syringe with 5 mL of sterile saline | For gastropexy and gastric puncture |
| 18-gauge needle | For gastric puncture |
| 0.035-Inch Amplatz Super Stiff guidewire | |
| Serial fascial dilators or tapered dilator | |
| Measuring balloon catheter | Optional, for measuring length of tract |
| Gastrostomy catheter of choice | |
| Peel-away sheath | If balloon-retention–type catheter is used |
| Contrast medium | To confirm placement |
Noninvasive monitoring equipment is applied. The patient’s heart rate, blood pressure, and oxygen saturation are monitored throughout the procedure. When appropriate, intravenous midazolam and fentanyl citrate are used in combination for sedation and analgesia. We routinely start with 1 mg midazolam, and 50 μg fentanyl, giving further increments as required. The abdominal skin is cleansed with an appropriate solution, and sterile drapes placed.
One of the key factors to a successful outcome is maintaining adequate gastric distension. The stomach is insufflated with air via the indwelling NG tube and the degree of gastric distension monitored fluoroscopically. Hyoscine- N -butylbromide (Buscopan) or glucagon hydrochloride (Glucagen) is administered intravenously to facilitate gastroparesis and air retention within the stomach. This is of particular importance in cases where the stomach is suboptimally distended due to poor air retention.
The following is a description of technique for placing a standard gastrostomy tube with gastropexy under fluoroscopic guidance. Figs. 91.1 91.2, 91.3, 91.4, 91.5, 91.6, 91.7, 91.8, and 91.9 illustrate various aspects of the insertion technique. Certain tube types, such as low-profile gastrostomies, require some modification of technique and this is addressed later in the chapter with the discussion on tube types.
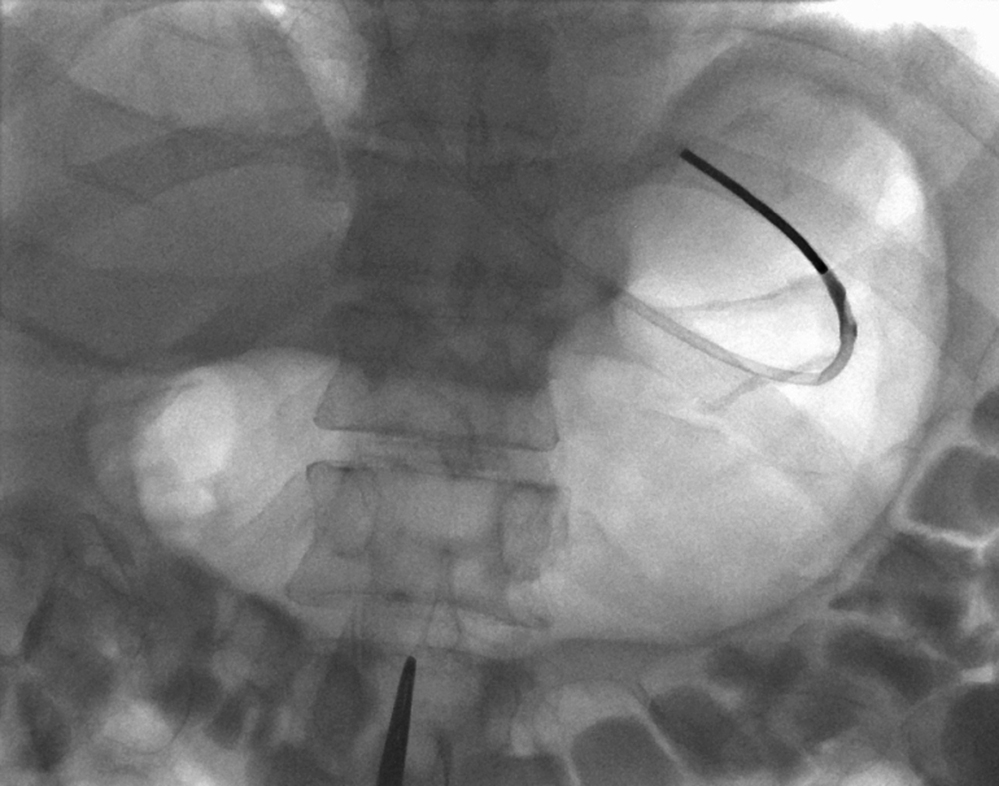
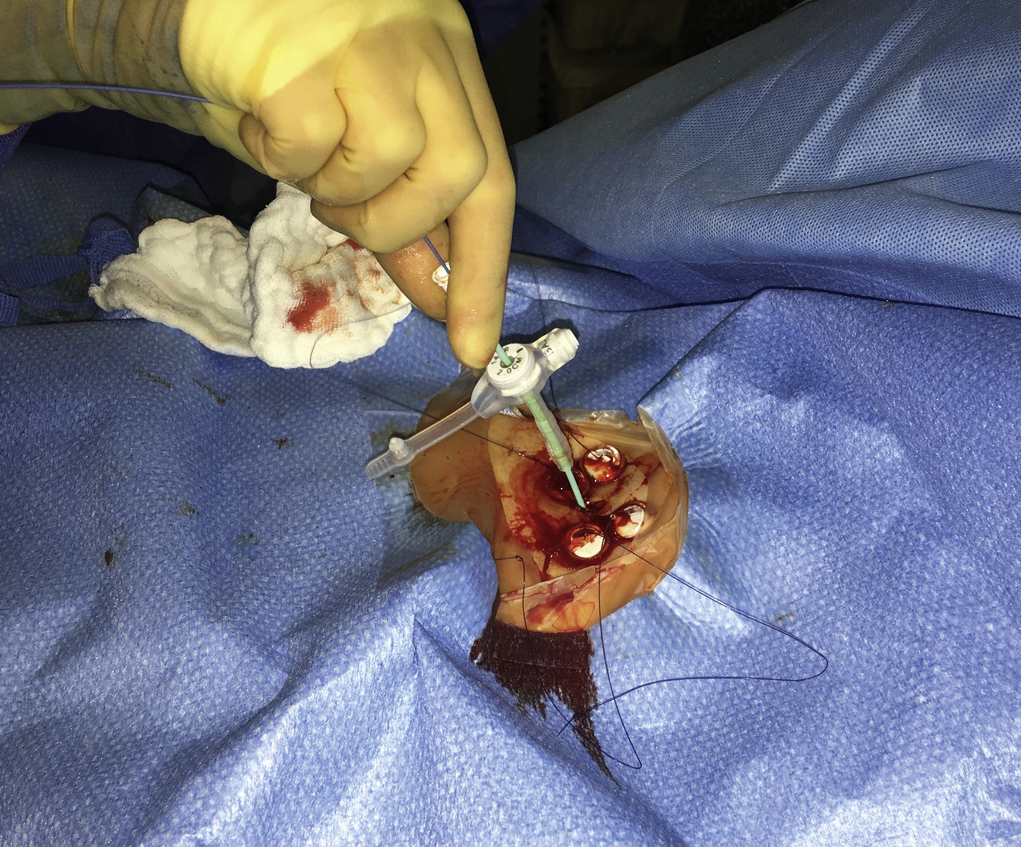
An appropriate puncture site for gastrostomy is chosen using fluoroscopy and anatomic landmarks; we do not routinely use ultrasound to delineate the liver edge. The optimum insertion site is usually to the left of midline, overlying the antrum or mid to distal body of the stomach, equidistant from the greater and lesser curves to avoid gastric and gastroepiploic vessels, and lateral to the rectus muscle to avoid the inferior epigastric vessels. A curved artery forceps is placed over the planned puncture site under fluoroscopy to confirm the proposed gastropexy position.
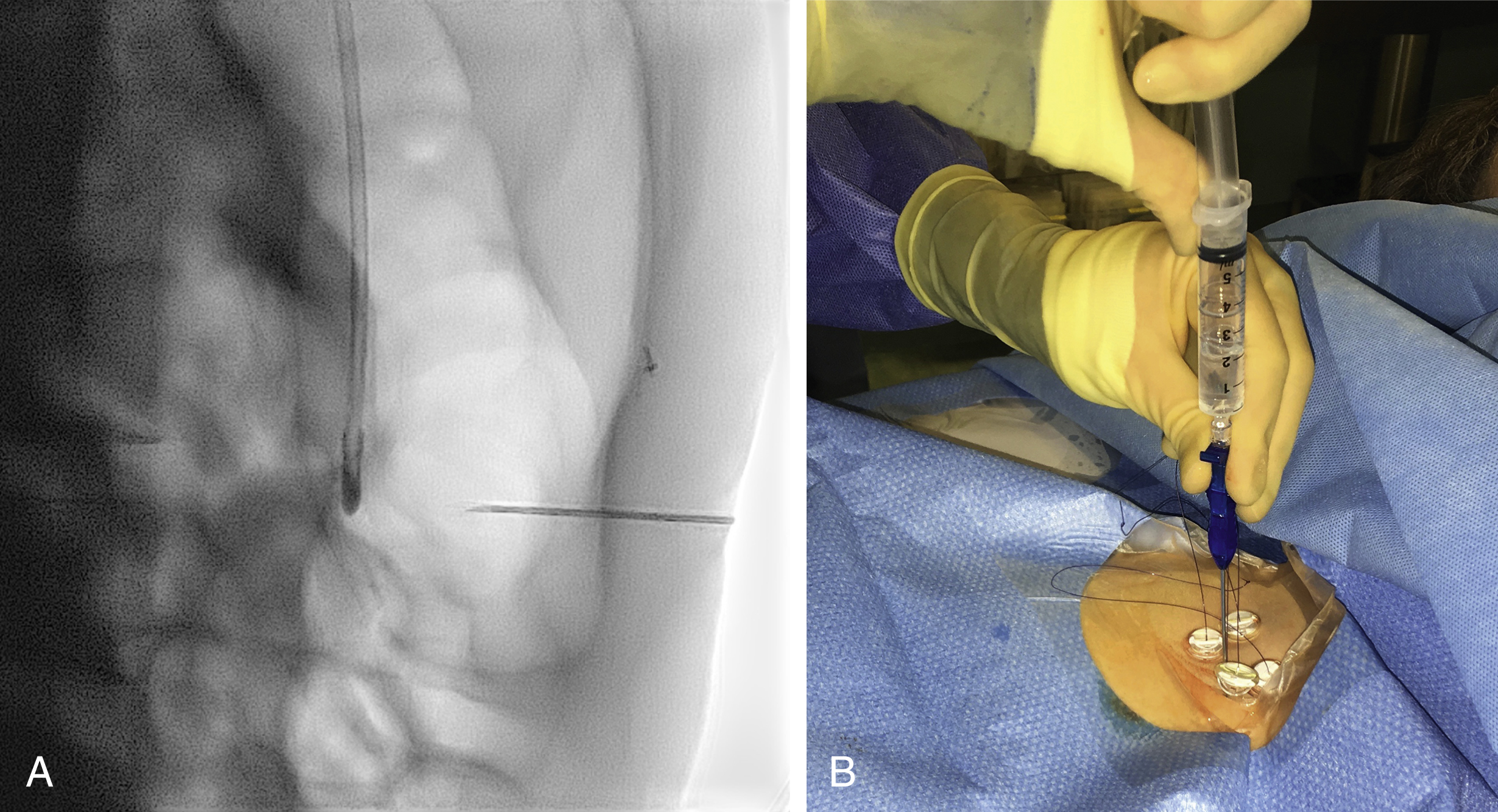
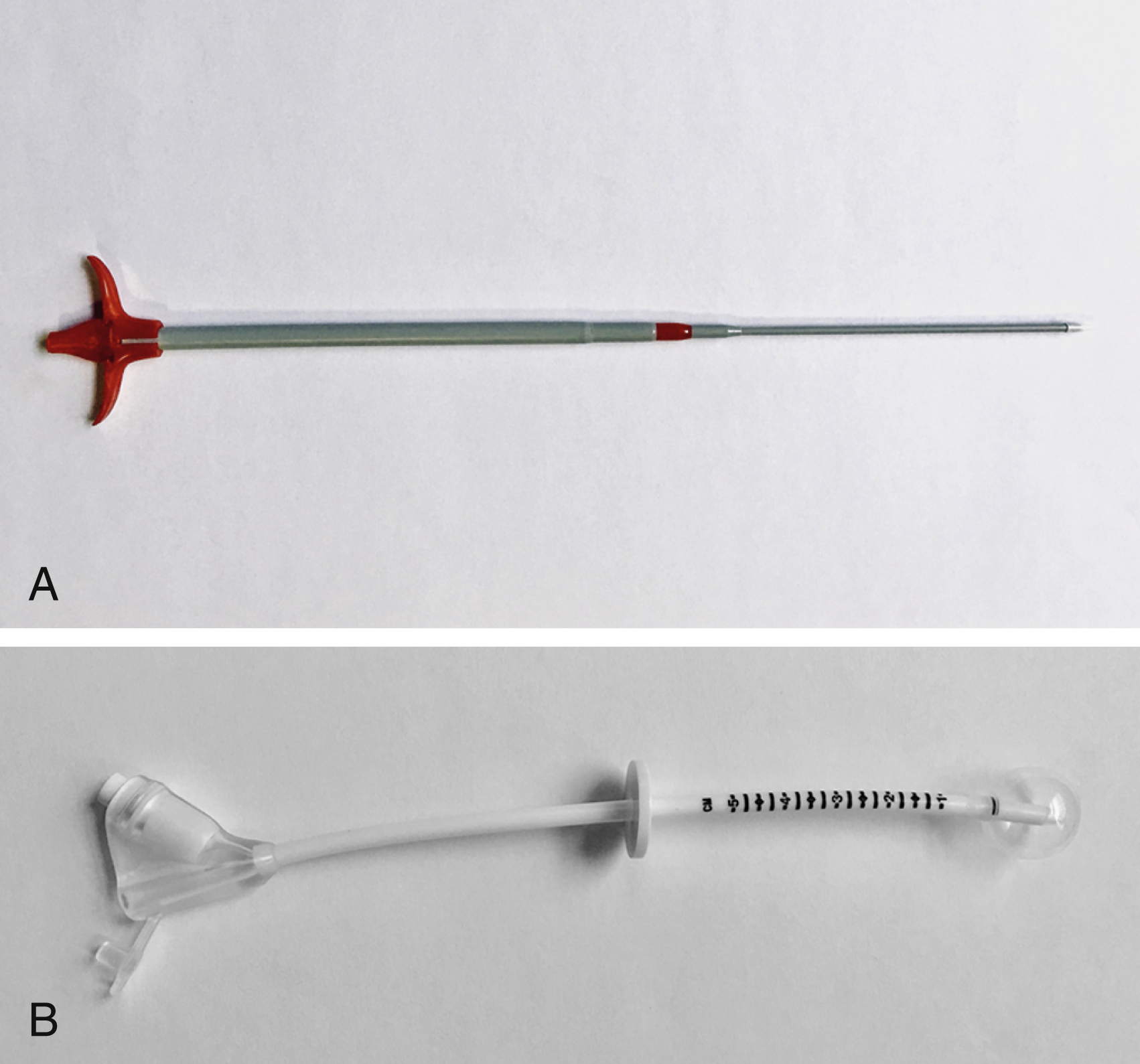
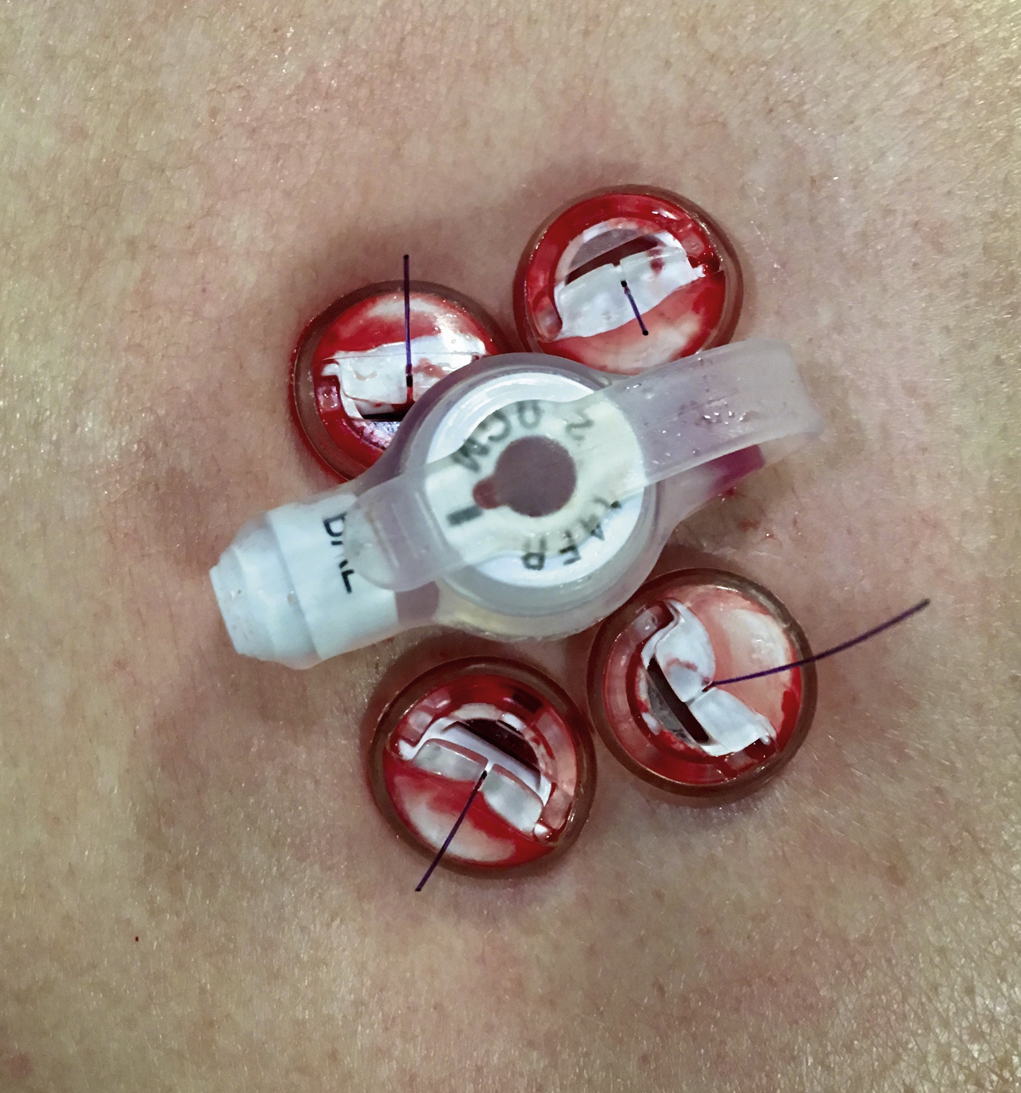
Local anesthetic (5–10 mL of 1% lidocaine) is infiltrated at the four corners of a 2-cm square surrounding the planned puncture site and the gastropexy is formed using four gastropexy sutures, one at each corner of the gastropexy square, ensuring deep infiltration to anesthetize the peritoneal lining. Traditional T-fasteners (Boston Scientific, Natick, MA) consist of a nylon suture with a metal T-bar, cotton wool pledget, two metal cylinders, and a plastic washer, and are inserted into the distended stomach using a slotted 18-gauge needle. Newer systems similarily deploy an intragastic metal T-bar on a suture, combined with lower-profile suture locking devices which can be tightened flush with the skin (SAF-T-PEXY T-fasteners Gastrointestinal Anchor Set, Halyard Health, Alpharetta, GA, and Entuit Secure Gastrointestinal Suture Set, Cook Medical, Bloomington, IN)
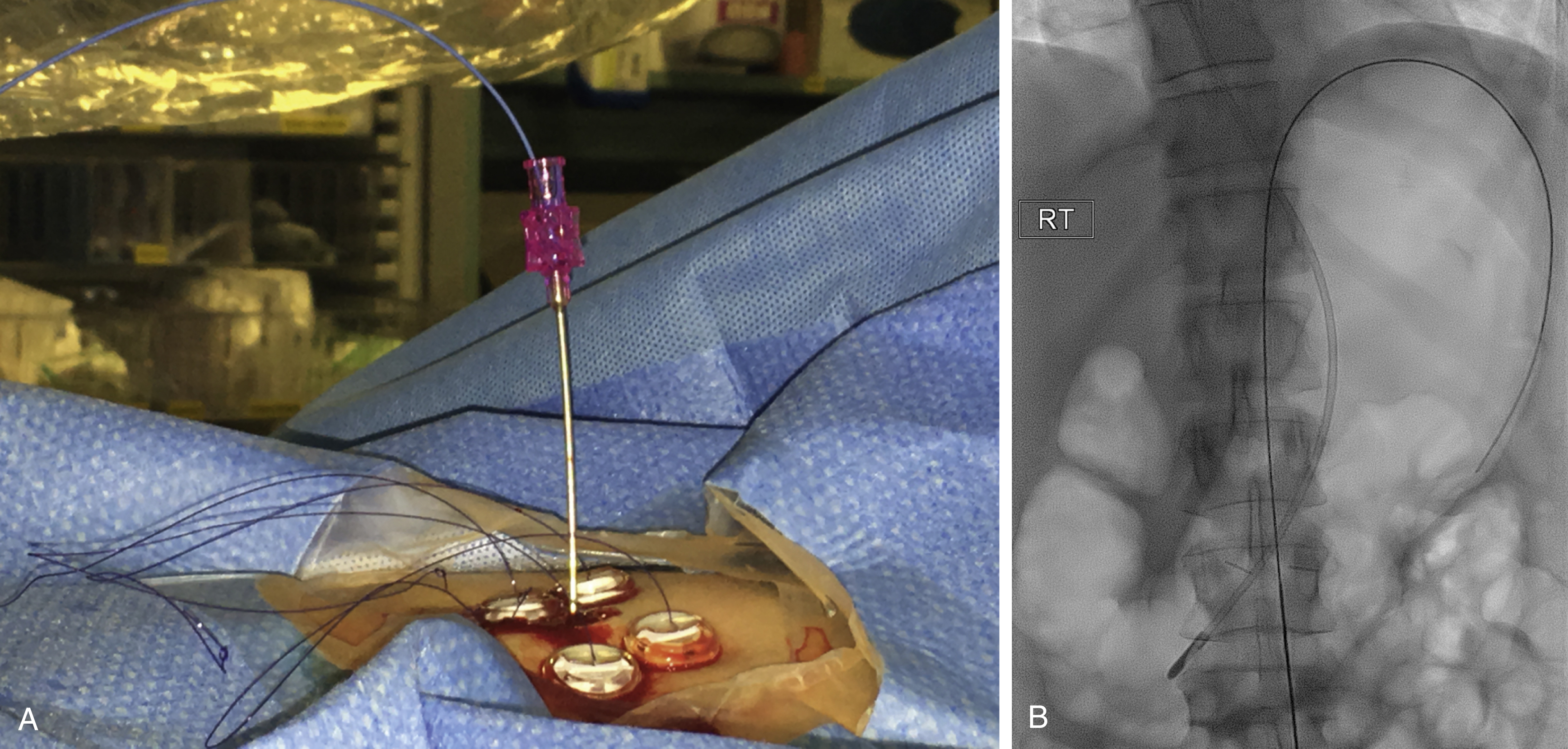
A syringe containing sterile saline is attached to the slotted needle, and an intragastric position confirmed by aspiration of air into the syringe. A stylet is then passed through the slotted needle to deploy the T-fastener. Stylet and needle are subsequently removed and the stomach is gently approximated to the anterior abdominal wall by traction on the T-fastener suture. T-fasteners are locked, in accordance with device instructions. Traditional T-fasteners required removal after the procedure; however, newer T-fasteners typically have an absorbable suture, which do not require removal after the procedure.
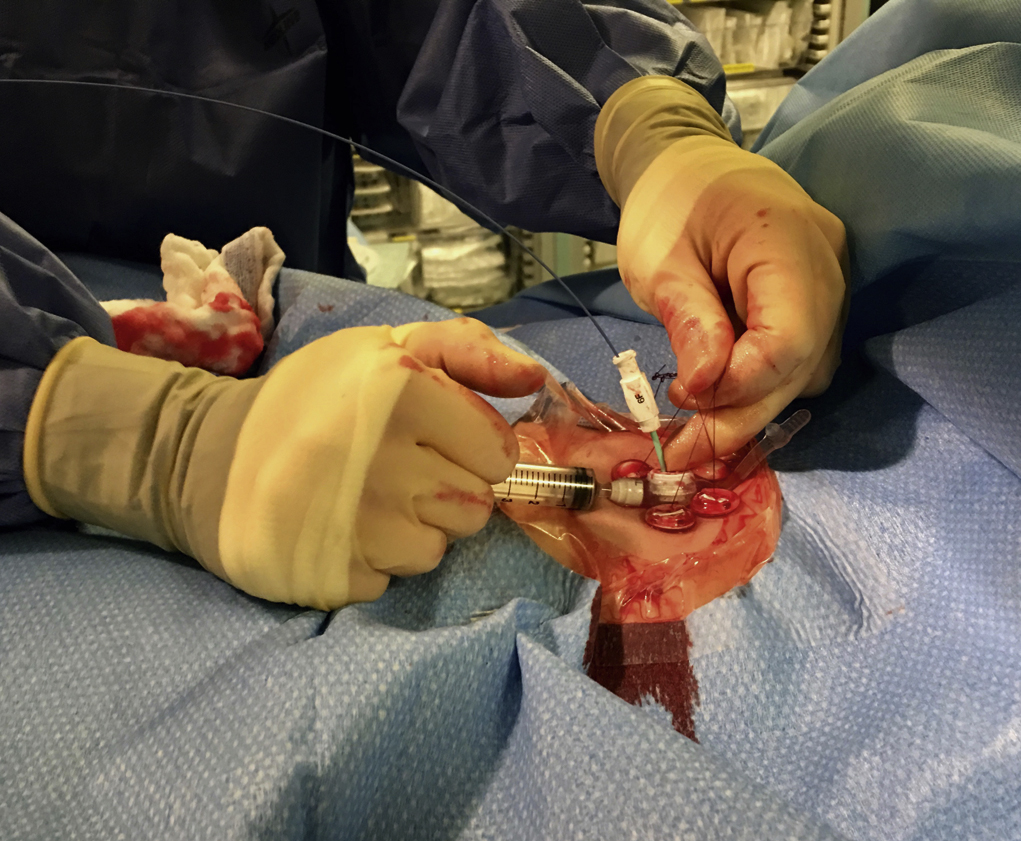
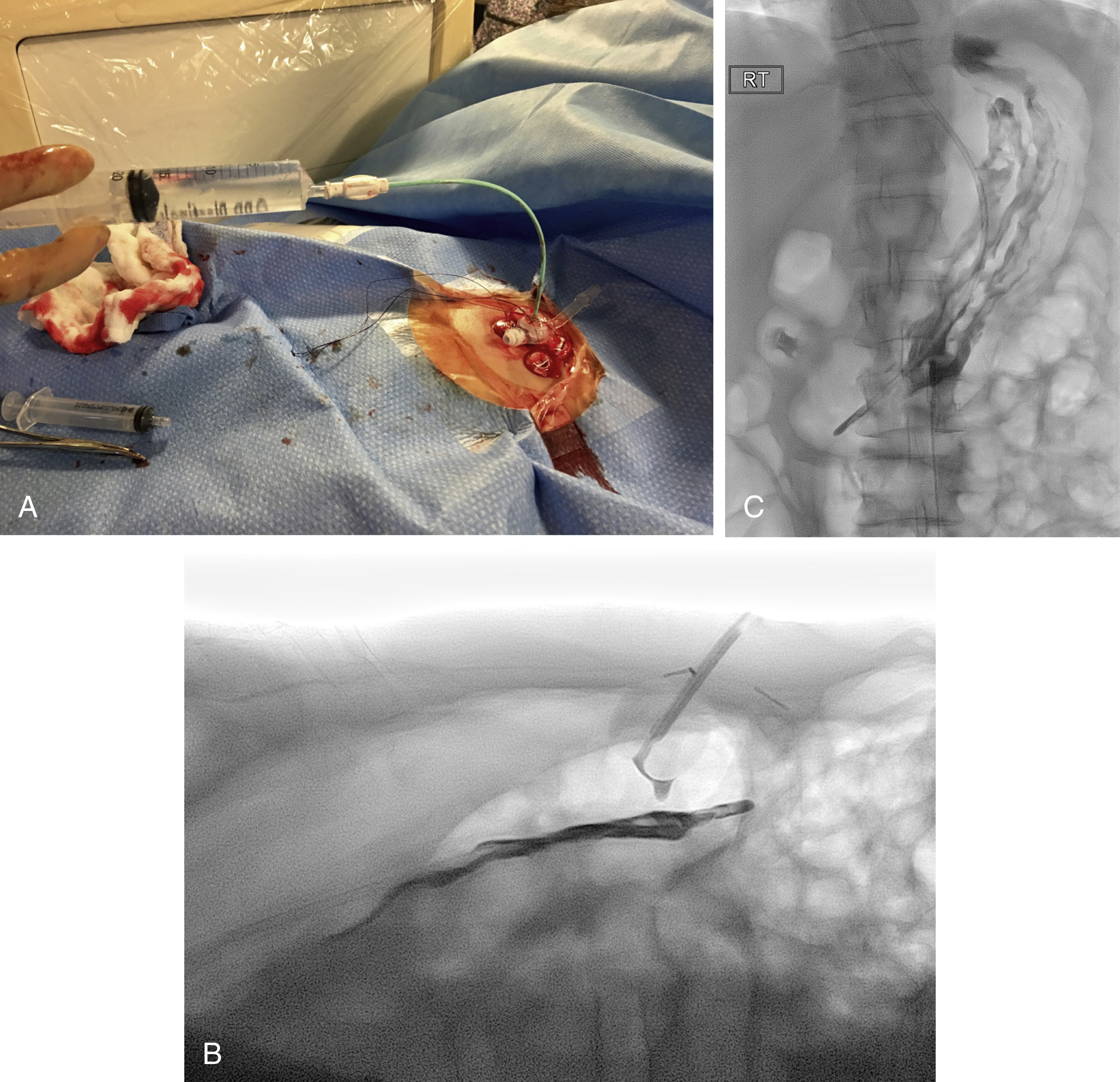
Following gastropexy, the center of the square is incised using a no. 11 blade, and the subcutaneous tissue dissected. An 18-gauge needle is inserted through the incision and into the stomach. Usually the puncture needle is directed vertically down or slightly toward the fundus; however, if conversion to a transgastric jejunostomy procedure is anticipated then the needle should be directed toward the pylorus. Again, aspiration of air confirms an intragastric position, and a 0.035-inch Amplatz Super Stiff guidewire (Boston Scientific) is introduced through the needle and coiled within the gastric lumen. The percutaneous tract is then dilated to a size 2F larger than the selected gastrostomy catheter, and each dilation is monitored fluoroscopically. Previously we used a series of fascial dilators; however, several current sets include a tapered telescopic dilator, combining serial dilators and a peel-away sheath in a single device. Alternatively an angioplasty balloon can be used to dilate the tract.
Finally, a gastrostomy catheter (typically 14–18F) is placed into the stomach, either through a peel-away sheath if a soft balloon retention catheter is used or directly over the guidewire if a pigtail-retention–type catheter is used. Depending on tube type, normal saline is then instilled into the balloon, or the pigtail formed. At the end of the procedure, contrast material is injected through the gastrostomy to confirm an intraluminal position.
For gastrostomy catheter exchange in mature tracts, the old tube can be removed over a wire and a new tube placed. In immature tracts, where the gastrostomy tube is no longer in place, a small hockey-stick catheter can be used to probe the tract and gain access to the stomach under fluoroscopic guidance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here