Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The incidence of non-Hodgkin lymphoma (NHL) increased significantly in the United States and worldwide in the last two decades of the 20th century; most of this increase has been attributed to the HIV epidemic. The incidence of NHL appeared to reach a plateau around the turn of the 21st century, both in the human immunodeficiency virus (HIV)-infected and the non–HIV-infected population, and according to some reports may be decreasing in the HIV-infected population, presumably as a result of better antiviral therapy. Moreover, the 5-year cancer-specific survival rate for some major subtypes of NHL is improving, reflecting better cancer therapy, with current 5-year survival rates of 66% for diffuse large B-cell lymphoma (DLBCL), 84% for chronic lymphocytic leukemia/small lymphocytic lymphoma, and 82% for follicular lymphoma.
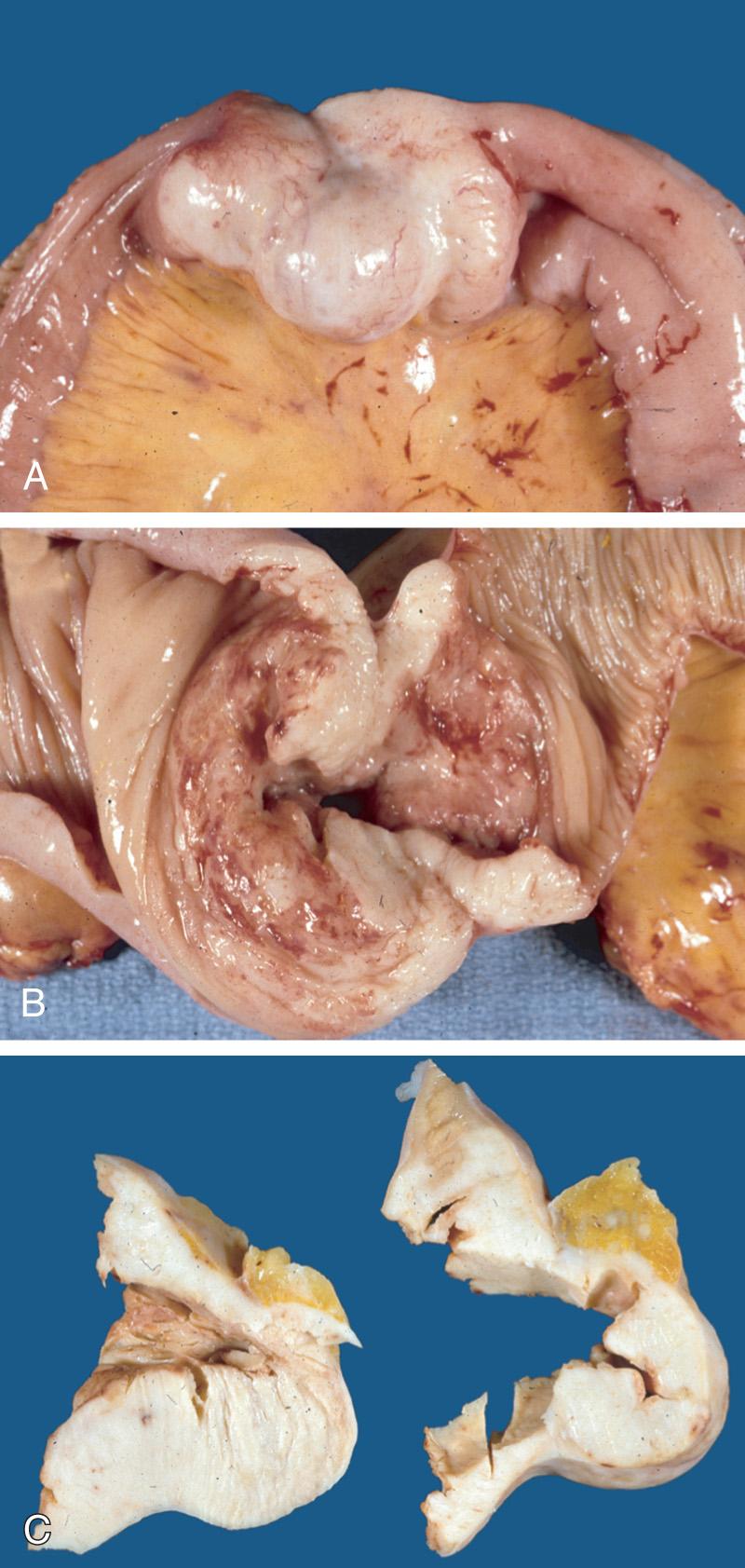
Approximately 30% of patients with NHL present as extranodal disease, and among these patients, the gastrointestinal (GI) tract is the most common site of presentation. Within the GI tract, the stomach is the most common site (65%), followed by the small intestine (18%), and colon and rectum (15%), while the liver, gallbladder, and pancreas collectively account for only 2%.
There has been debate over the years about the definition of primary GI tract NHL (PGINHL). Strict criteria were proposed by Dawson in 1961, who considered patients to have PGINHL only if no palpable superficial lymphadenopathy was found at first examination, chest radiograph showed no obvious enlargement of mediastinal lymph nodes, and the white blood cell count was within normal limits. Furthermore, upon laparotomy, the bowel lesion had to be shown to be dominant, and only involvement of lymph nodes in the immediate vicinity of the primary lesion was acceptable. Patients were excluded from analysis when distant abdominal lymph nodes, spleen, or liver were involved. A liberal definition of primary extranodal NHL has been proposed that includes all patients who present with NHL that apparently originated at an extranodal site, even in the presence of disseminated disease, as long as the extranodal component is clinically dominant. Use of strict criteria for PGINHL selects patients with earlier stage disease and, probably for this reason, for many years patients with GI tract NHL were thought to have a better prognosis than patients with nodal or disseminated NHL. However, the overall survival with treatment for extranodal NHL, including NHL of the GI tract, is similar to that for nodal NHL when patients with similar International Prognostic Index ( Table 82.1 ) and malignancy grade are compared.
| Risk factors | Ann Arbor stage III to IV (advanced disease) |
| >1 extranodal site | |
| Age >60 years | |
| High LDH | |
| Performance status ≥2 (ECOG) | |
| RISK ASSESSMENT | |
| Low risk | 0 to 1 risk factors |
| Low intermediate risk | 2 risk factors |
| High intermediate risk | 3 risk factors |
| High risk | 4 to 5 risk factors |
NHLs of the GI tract are a heterogeneous group of entities; there are multiple subtypes with different cells of origin, at different stages of maturation, and with different natural histories, biologic behavior, and prognosis. Ninety percent of PGINHL is of B-cell lineage; the two most common subtypes are DLBCL, which comprises approximately 60% of PGINHL, and mucosa-associated lymphoid tissue (MALT) lymphoma, primarily occurring in the stomach, which accounts for approximately 30% of all PGINHL. These two entities are the main focus of this chapter. Several other uncommon B-cell PGINHLs occur, including follicular lymphoma presenting typically in the duodenum, mantle cell lymphoma presenting typically in the terminal ileum, and Burkitt lymphoma, associated with Epstein-Barr virus infection, and typically presenting in the terminal ileum in children and young adults. These will not be discussed in detail in this chapter.
The only T-cell PGINHL of significance is the enteropathy-associated T-cell lymphoma (ETL), which accounts for approximately 5% of PGINHL and occurs most often in the jejunum but may affect multiple segments of small intestine. ETL can arise in the setting of refractory celiac disease (RCD), defined as celiac disease that does not respond histologically to at least 12 months of strict gluten-free diet, or in patients without a known history of celiac disease (primary ETL). In the setting of RCD, it often presents as an exacerbation with abdominal pain, diarrhea, and weight loss; as a de novo entity, it often presents acutely as GI perforation, obstruction, or hemorrhage. RCD can be classified based on intraepithelial lymphocyte (IEL) populations into two distinct subtypes; RCD type II, which involves clonal expansion of abnormal IELs lacking surface cluster of differentiation (CD)3, CD8, and T-cell receptor markers, but expresses intracellular CD3, is the subtype which evolves into ETL in 60% to 80% of patients within 5 years. Patients with ETL have a poor prognosis with less than 30% of patients alive at 2-year follow-up, and ETL that occurs in the setting of RCD has a worse prognosis.
Risk factors for the development of gastrointestinal lymphoma include Helicobacter pylori infection, immunosuppression after solid organ transplantation, celiac disease, Epstein-Barr viral infection, inflammatory bowel disease, and HIV infection.
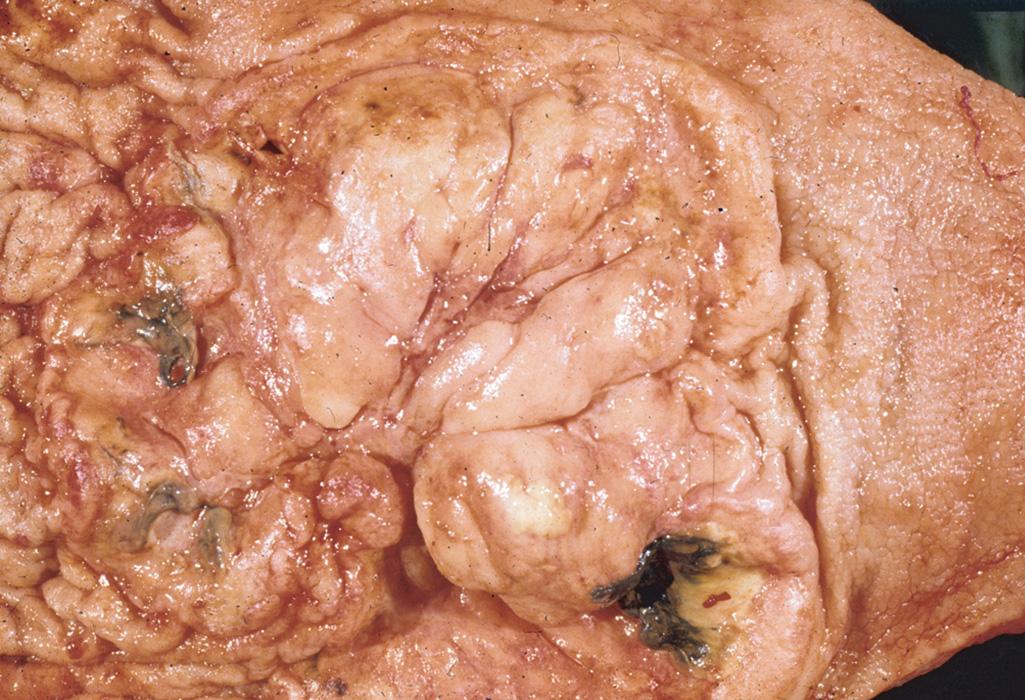
The most common histologic subtype of PGINHL, DLBCL accounts for slightly less than 50% of primary gastric lymphomas and the great majority of PGINHL occurring in the small bowel, colon, and rectum. DLBCLs of the GI tract are morphologically similar to DLBCLs at other sites. Histologically, tumors consist of diffuse sheets of large, blastic lymphoid cells, 2 to 4 times larger than normal lymphocytes, often infiltrating and destroying the gastric glandular architecture. Vesicular nuclei, prominent nucleoli, a basophilic cytoplasm, and a moderate to high proliferation fraction may also be noted ( Figs. 82.1 and 82.2 ). A variety of cell types may be observed but the most common cell appears as a large noncleaved cell, an immunoblast, or a mixture of these two types. Approximately 30% to 50% of primary gastric DLBCLs have components of MALT lymphoma, but the extent of this low-grade component varies from only small residual foci to dominant MALT lymphoma with only a small proportion of solid or sheet-like transformed blasts. In gastric DLBCLs with a component of MALT lymphoma, the tumor should be diagnosed as a DLBCL, noting the presence of accompanying MALT lymphoma, and the term high-grade MALT lymphoma should not be used for this entity.
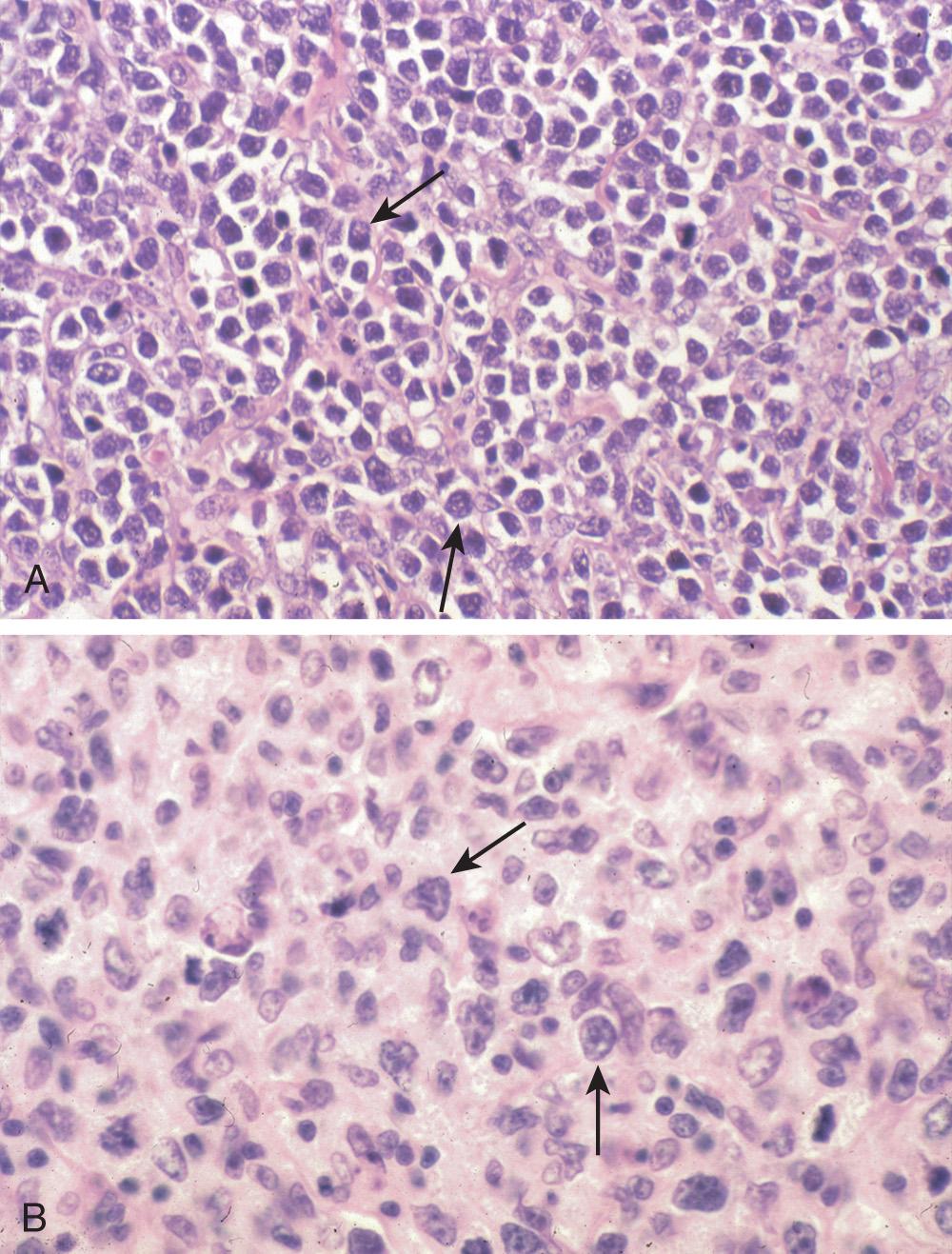
DLBCL has recently been found to be a heterogeneous disease, with different prognoses for different subtypes. It may be subdivided into germ-center B-cell and activated B-cell subtypes. Germ-center subtypes typically exhibit a translocation in the antiapoptotic BCL2 gene (t[14;18]) and amplification of REL (chromosome 2p). Although germ-center variants will exhibit downregulation of factors influencing cellular growth, such as nuclear factor-κB (NF-κB), activated B-cell subtypes exhibit upregulation. DLBCL is frequently the result of a mutation in BCL6 (involved in T-cell–dependent antigen responses), which may be associated with longer disease-free survival. Overall survival prognostication may require more integrative modeling of multiple factors and may need to account for multiple underlying genetic mutations.
The neoplastic cells are of B-cell phenotype, expressing pan B-cell antigens (CD19, CD20, CD22, and CD79a) but may lack one or more of these. The typical immunophenotype is CD20 + , CD45 + , and CD3 − . Adequate immunophenotyping to establish the diagnosis and to differentiate germinal center B-cell–like (GCB) vs. non-GCB origin requires an immunohistochemical (IHC) panel including CD20, CD3, CD5, CD10, CD45, B-cell lymphoma 2 (BCL2), BCL6, Ki-67, interferon regulatory factor 4 (IRF4) /melanoma associated antigen (mutated) 1 (MUM1), v-myc avian myelocccytomatosis viral oncogene homolog (MYC), with or without cell surface marker analysis by flow cytometry to include kappa/lambda, CD45, CD3, CD5, CD19, CD10, CD20. Also useful under certain circumstances are additional studies to establish lymphoma subtype, including IHC staining for cyclin D1, kappa/lambda, CD30, CD138, Epstein-Barr virus in situ hybridization, anaplastic lymphoma receptor tyrosine kinase (ALK), human herpesvirus-8 (HHV8), SRY-box 11 (SOX11), and karyotype or fluorescence in situ hybridization (FISH) analysis for MYC, BCL2, and BCL6 rearrangements.
MALT lymphomas comprise about 50% of all primary lymphomas of the stomach. Interestingly, the stomach is normally devoid of lymphoid tissue, but it is the most common site for MALT lymphoma. Lymphoid follicles develop in the presence of chronic inflammation and gastritis associated with H. pylori infection, as evidenced by a rate of H. pylori infection of greater than 90% in patients with MALT lymphoma. These lymphoid follicles resemble lymph node tissue and are composed of reactive T cells, activated plasma cells, and B cells. It is the B cells that undergo clonal expansion and subsequently develop into a MALT lymphoma. The neutrophilic response to the bacterial infestation promotes neutrophil migration and the release of reactive oxygen free radicals that may induce genetic mutations secondary to the oxidative stress.
Histologically, the characteristic marginal zone B cells are of intermediate size with pale cytoplasm and a slightly irregular nucleus. The most significant finding is the presence of a variable number of lymphoepithelial lesions defined by evident invasion and partial destruction of mucosal glands by the tumor cells ( Figs. 82.3 and 82.4 ). MALT lymphoma shows the immunophenotype of B cells in the normal marginal zone of spleen, Peyer patches, and lymph nodes. The tumor B cells can express the surface immunoglobulin and pan-B antigens (CD19, CD 20, and CD79a), the marginal zone–associated antigens (CD35 and CD21, and lack CD5, CD10, CD23), and cyclin D1. MALT lymphoma can be divided into H. pylori positive or negative based on the presence of H. pylori . H. pylori– negative MALT lymphomas have a higher positive rate for t(11;18)(q21;q21) translocation than H. pylori– positive MALT lymphoma.
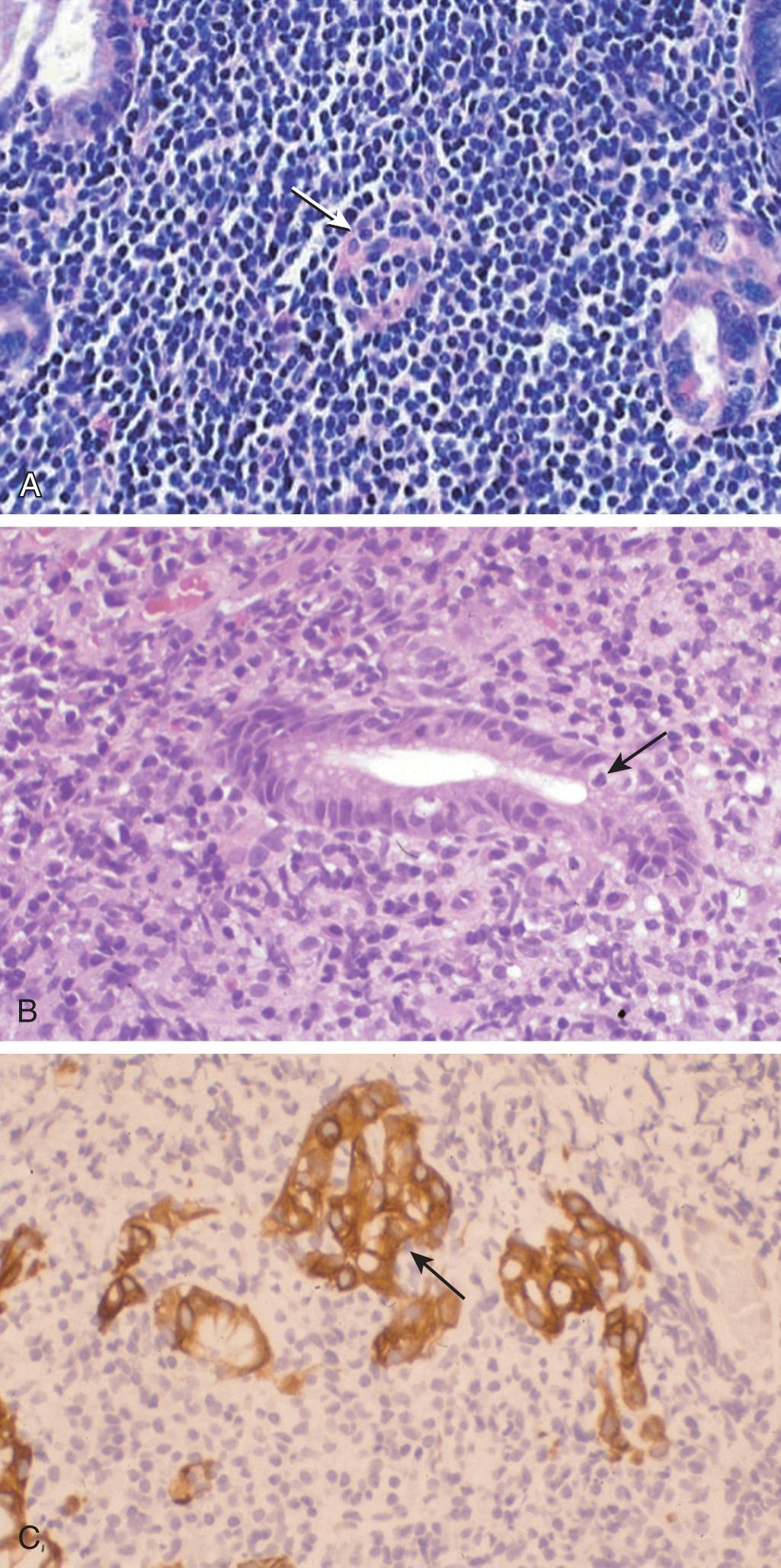
Adequate immunophenotyping to establish the diagnosis of MALT lymphoma includes an IHC panel consisting of CD20, CD3, CD5, CD10, BCL2, kappa/lambda, CD21 or CD23, cyclin D1, and BCL6, with or without cell surface marker analysis by flow cytometry including kappa/lambda, CD19, CD20, CD5, CD23, CD10. Also, H. pylori staining (gastric) of biopsy tissue should be obtained; if positive, then PCR or FISH for t(11 : 18). If H. pylori staining is negative by histopathology, then use noninvasive H. pylori testing (stool antigen, urea breath, or blood antibody test).
Several genetic alterations may promote the pathogenesis of MALT lymphoma. One translocation (t[11 : 18]) joins the inhibitor of apoptosis gene-2 (IAP2) to the MALT-1 gene (MLT). The resultant fusion protein not only has proapoptotic and transforming functions via the Bcl-10 protein, but also activates the NF-κB pathway, further promoting growth. Approximately 21% to 60% of MALT lymphoma may be attributed to this genetic alteration, and this particular translocation may portend a more aggressive underlying tumor biology. A second translocation (t[1;14]) occurs in less than 5% of MALT lymphomas and results in the constitutive activation of the bcl-10 gene as a result of the transfer of the immunoglobulin heavy chain promoter. Although differing in the locus of translocation, this genetic mutation shares the final common pathway of antiapoptosis, activation of NF-κB, and progression of disease. Because the possibilities of these genetic alterations exist and, when present, affect the biologic behavior and treatment responsiveness of gastric MALT lymphomas, cytogenetics or FISH analysis for t(1 : 14), t(11 : 14), t(11 : 18), and t(3 : 4) may be useful in selected cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here