Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A textbook of human embryology could begin at any of several points in the human life cycle. This textbook starts with a discussion of the origin of specialized cells called primordial germ cells (PGCs) . PGCs can be first identified within the wall of the yolk sac , one of the extraembryonic membranes, during the fourth to sixth weeks of gestation. These PGCs will give rise to the germ line , a series of cells that form the sex cells, or gametes (i.e., the egg and sperm ). However, these gametes will not function to form the next generation for several decades (i.e., after the onset of puberty ). Yet, remarkably, one of the first things that happen in the developing embryo is that the germ line is set aside for the next generation. Similarly, the germ lines that gave rise to the developing embryo were established a generation earlier, when the embryo’s father and mother were developing in utero (i.e., when the embryo’s paternal and maternal grandmothers were pregnant with the embryo’s father and mother).
From the wall of the yolk sac, PGCs actively migrate between the 6th and 12th weeks of gestation to the dorsal body wall of the embryo, where they populate the developing gonads and differentiate into the gamete precursor cells called spermatogonia in the male and oogonia in the female. Like the normal somatic cells of the body, the spermatogonia and oogonia are diploid , that is, they each contain 23 pairs of chromosomes (for a total of 46 chromosomes each). When these cells eventually produce gametes by the process of gametogenesis (called spermatogenesis in the male and oogenesis in the female), they undergo meiosis , a sequence of two specialized cell divisions by which the number of chromosomes in the gametes is halved. The gametes thus contain 23 chromosomes (one of each pair); therefore, they are haploid . The developing gametes also undergo cytoplasmic differentiation, resulting in the production of mature spermatozoa in the male and definitive oocytes in the female.
In the male, spermatogenesis takes place in the seminiferous tubules of the testes and does not occur until puberty . By contrast, in the female, oogenesis is initiated during fetal life. Specifically, between the third and fifth months of fetal life, oogonia initiate the first meiotic division, thereby becoming primary oocytes. However, the primary oocytes then quickly enter a state of meiotic arrest that persists until after puberty. After puberty, a few oocytes and their enclosing follicles resume development each month in response to the production of pituitary gonadotropic hormones. Usually, only one of these follicles matures fully and undergoes ovulation to release the enclosed oocyte, and the oocyte completes meiosis only if a spermatozoon fertilizes it. Fertilization , the uniting of egg and sperm, takes place in the oviduct. After the oocyte finishes meiosis, the paternal and maternal chromosomes come together, resulting in the formation of a zygote containing maternal and paternal chromosomes aligned on the metaphase plate. Embryonic development is considered to begin at this point.
The newly formed embryo undergoes a series of cell divisions, called cleavage , as it travels down the oviduct toward the uterus. Cleavage subdivides the zygote first into two cells, then into four, then into eight, and so on. These daughter cells do not grow between divisions, so the entire embryo remains the same size. Starting at the 8- to 16-cell stage, the cleaving embryo, or morula , differentiates into two groups of cells: a peripheral outer cell layer and a central inner cell mass . The outer cell layer, called the trophoblast , forms the fetal component of the placenta and associated extraembryonic membranes, whereas the inner cell mass, also called the embryoblast , gives rise to the embryo proper and associated extraembryonic membranes. By the 30-cell stage, the embryo begins to form a fluid-filled central cavity, the blastocyst cavity . By the fifth to sixth day of development, the embryo is a hollow ball of about 100 cells, called a blastocyst . At this point, it enters the uterine cavity and begins to implant into the endometrial lining of the uterine wall.
A couple, both in their late thirties, is having difficulty conceiving a child. Early in their marriage, about 10 years ago, they used birth control pills and condoms thereafter, but they stopped using all forms of birth control more than 2 years ago. Despite this and despite having intercourse three or four times a week, a pregnancy has not resulted. On routine physical examination, both the man and the woman seem to be in excellent health. The woman is an avid runner and competes in occasional marathons, and she has had regular periods since her menarche at age 13. The man had a varicocele, which was corrected when he was 19; the urologist who performed the surgery assured him that there would be no subsequent adverse effects on his fertility.
Because no obvious cause of their fertility problem is noted, the couple is referred to a local fertility clinic for specialized treatment. At the clinic, the man has a semen analysis. This reveals that his sperm count (60 million sperm per ejaculate), sperm mobility (vigorous motility and forward progression [i.e., straight swimming movement]), sperm morphology (70% with an oval head and a tail 7 to 15 times longer than the head), and semen volume (3.5 mL with a normal fructose level) are within normal ranges. Semen viscosity and sperm agglutination are also normal. As a next step, a postcoital test is planned. Using the woman’s recent menstrual history to estimate the time of her midcycle, and daily basal body temperature measurements and urine luteinizing hormone (LH) tests to predict ovulation, intercourse is timed for the evening of the day on which ovulation is expected to occur. The next morning, the woman undergoes a cervical examination. It is noted that the cervical mucus contains clumped and immotile sperm, suggesting sperm-cervical mucus incompatibility.
Based on the results of the postcoital test, the couple decides to undergo artificial insemination . After five attempts in which the man’s sperm are collected, washed, and injected into the uterus through a sterile catheter passed through the cervix, a pregnancy still has not resulted. The couple is discouraged and decides to take some time off to consider their options.
After considering many options, including adoption, gestational surrogacy, and remaining childless, the couple returns 3 months later and requests in vitro fertilization (IVF) . On the second of two very regimented attempts, the couple is delighted to learn that a pregnancy has resulted. A few weeks later, Doppler ultrasound examination detects two fetal heartbeats. This is confirmed two months later by ultrasonography. Early in the ninth month of gestation, two healthy babies are delivered—a 6-pound 2-ounce girl and a 5-pound 14-ounce boy.
Cells that give rise to gametes in both males and females can be identified during the fourth week of gestation within an extraembryonic membrane called the yolk sac ( Fig. 1.1A ). Based on studies in animal models, it is believed that these cells arise earlier in gestation, during the phase of gastrulation (covered in Chapter 3 ). These cells are called primordial germ cells (PGCs) , and their lineage constitutes the germ line . PGCs can be recognized, in histologic sections, within the yolk sac and during their subsequent migration (see next paragraph) because of their distinctive pale cytoplasm and rounded shape (see Fig. 1.1B,C ), and because they can be specifically labeled with molecular markers.
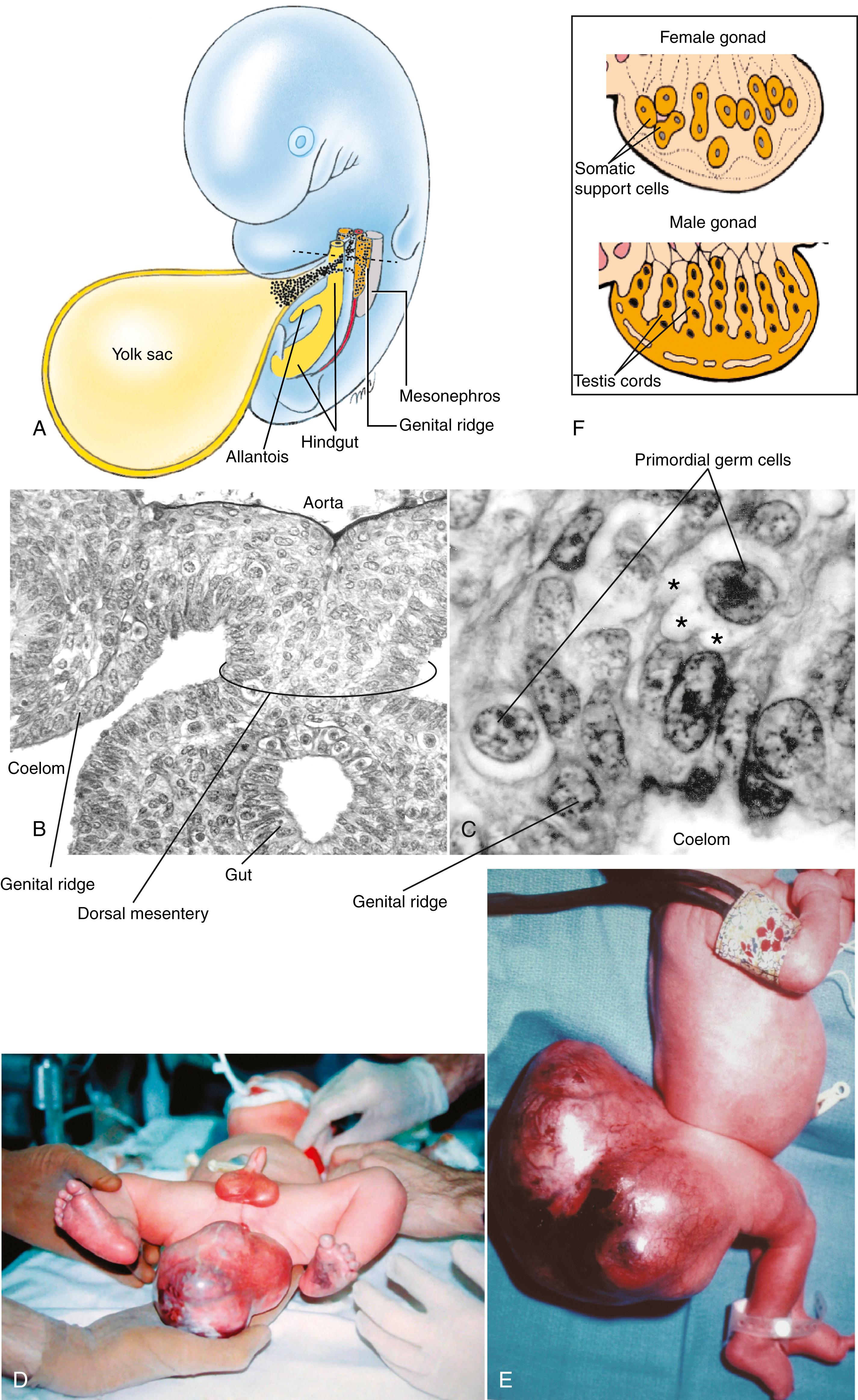
Between 4 and 6 weeks, PGCs migrate by ameboid movement from the yolk sac to the wall of the gut tube, and from the gut tube via the mesentery of the gut to the dorsal body wall (see Fig. 1.1A,B ). In the dorsal body wall, these cells come to rest on either side of the midline in the loose mesenchymal tissue just deep to the membranous (epithelial) lining of the coelomic cavity. Most PGCs populate the region of the body wall at the level that will form the gonads (covered in Chapter 16 ). PGCs continue to multiply by mitosis during their migration. Some PGCs may become stranded during their migration, coming to rest at extragonadal sites. Occasionally, stray germ cells of this type may give rise to a type of tumor called a teratoma ( Fig. 1.1D,E ).
Teratomas , tumors composed of tissues derived from all three germ layers, can be extragonadal or gonadal and are derived from PGCs. Sacrococcygeal teratomas, the most common tumors in newborns, occur in 1 in 20,000 to 70,000 births ( Fig. 1.1D,E ). They occur four times more frequently in female newborns than in male newborns, and they represent about 3% of all childhood malignancies. Gonadal tumors are usually diagnosed after the onset of puberty. Both ovarian and testicular teratomas can form. The pluripotency (ability to form many cell types, not to be confused with totipotency , the ability to form all cell types) of teratomas is exhibited by the fact that they can give rise to a variety of definitive anatomic structures, including hair, teeth, pituitary gland, and even a fully formed eye.
Differentiation of the gonads is described in detail in Chapter 16 . When PGCs arrive in the presumptive gonad region, they stimulate cells of the adjacent coelomic epithelium to proliferate and form somatic support cells ( Fig. 1.1F ; see also Figs. 16.1D and 16.5 ). Proliferation of the somatic support cells creates a swelling just medial to each mesonephros (embryonic kidney) on both right and left sides of the gut mesentery. These swellings, the genital ridges , represent the primitive gonads. Somatic support cells invest PGCs and give rise to tissues that will nourish and regulate development of maturing sex cells— ovarian follicles in the female and Sertoli cells of the germinal epithelium (seminiferous epithelium) of the seminiferous tubules in the male. Somatic support cells are essential for germ cell development within the gonad: if germ cells are not invested by somatic support cells, they degenerate. Conversely, if PGCs fail to arrive in the presumptive gonadal region, gonadal development is disrupted. Somatic support cells in the male quickly assemble into epithelial cords called testis cords .
Although the exact time and place of origin of PGCs in humans are unknown, cell tracing and other experiments in the mouse demonstrate that PGCs arise from the epiblast (one of the layers of the bilaminar and trilaminar blastoderm stages; covered in Chapters 2 and 3 ). During gastrulation, these cells move through the caudal part of the primitive streak and into the extraembryonic area. From there, they migrate to the gut wall and through the gut mesentery to the gonadal ridges, as in humans. In some species of monkeys, PGCs are also observed in the amnion (the extraembryonic membrane that contains the amniotic fluid surrounding the embryo; covered in Chapter 3, Chapter 4, Chapter 6 ).
Migration of PGCs to the developing gonads involves processes shared by migrating neural crest cells (see Chapter 4 ), neuronal processes (see Chapter 10, Chapter 9 ), and developing blood and lymphatic vessels (see Chapter 13 ). These include intrinsic motility programs involving cytoskeletal dynamics (note pseudopods on one of the PGCs shown in Fig. 1.1C ), adhesive substrates (such as tenascin C, β2 integrin, and laminin, all of which seem to be required for PGC migration), and extracellular attractive and repulsive cues. As covered in Chapter 10 , chemokines (a type of cytokine ) and their receptors direct the migration of sympathetic precursor cells. Similarly, chemokines play important roles in PGC migration by acting as chemotropic signals (i.e., attractive signals produced by the developing gonads) to regulate PGC honing. Such chemokines include the ligand stromal cell–derived factor-1 (Sdf1, also known as Cxcl12) and its receptor Cxcr4. PGC migration toward the gonad is disrupted in mouse or zebrafish embryos lacking the ligand or its receptor. In addition, Sdf1 acts as a PGC survival factor. Moreover, factors involved in the migration of melanocytes (covered in Chapter 4 ) also are involved in PGC migration. These include steel factor (also known as stem cell factor), the c-kit ligand, and its receptor, c-kit.
Development of the germ line involves the sequential activation of genes that direct the initial induction, proliferation, survival, migration, and differentiation of PGCs. Animal models have been very useful for understanding these events and have been used to show that the functions of many genes controlling PGC development are conserved across diverse organisms. However, mechanisms underlying the initial events of PGC formation in mammals seem to be very different from those of lower organisms.
In some model organisms, such as the fruit fly, worm, and frog, maternal effect genes (covered in Chapter 5 ) are required for initiation of germ cell formation. Activation of these maternal genes regulates the segregation of the germ plasm (cytoplasm containing determinants of the germ line) to a specific region of the zygote, so that it becomes incorporated during cleavage into a unique group of cells that will form the germ cell precursors.
The Drosophila gene vasa is segregated to germ cells in this fashion. Vasa transcripts are expressed ubiquitously in the oocyte cytoplasm, but vasa protein becomes specifically localized in the germ plasm. Vasa is an RNA-binding protein of the dead box family, and its possible role is to bind mRNAs involved in germ line determination, such as oskar and nanos, and to control the onset of their translation. Vertebrate orthologs of vasa exist, and in some vertebrates, vasa protein is expressed in germ cell precursors as they are forming (however, in mice, vasa is expressed in germ cells only much later, after they have differentiated and are about to colonize the gonads).
In contrast to lower organisms, in which germ cells are usually specified by the inheritance of maternal gene products, in the mouse, and probably also in humans, the germ line is induced. All cells of the mammalian morula are seemingly capable of forming pluripotent germ cells, but their capacity to do so becomes rapidly restricted first to the inner cell mass and then to the epiblast. Therefore, in mammals, the initiation of germ line development requires activation of genes that maintain pluripotency within the precursors that will form the germ line. One such gene encodes a pou domain transcription factor (Oct4, also called Pou5f1; transcription factors are covered in Chapter 5 ). Its activity is present initially in all cells of the morula, but then only in the inner cell mass. It is then restricted to the epiblast, and finally it is expressed only in the presumptive germ cells themselves.
Further development of the germ line requires inductive signals from the trophoblast (induction is covered in Chapter 5 ). In mouse embryos, two signaling pathways are involved: the Wingless pathway (in particular, Wnt3) and the bone morphogenetic protein pathway (in particular, Bmp4). These two proteins in turn result in the expression of key transcription factors (i.e., Ap2γ, Blimp1, and Prdm14) in a subset of cells in the caudal epiblast, which then repress somatic (non-germ) genes and upregulate germline-specific genes. A study using human induced pluripotent stem cells (iPSCs; covered in Chapter 5 ) showed that the transcription factor Sox 17 (a gene involved in endoderm differentiation; covered in Chapter 2 ), and downstream of Blimp1 signaling, plays a critical role in specifying PGC fate in humans.
Proliferation and survival of PGCs are ensured by the expression of trophic factors (factors that promote cell growth and survival) within the PGCs or within associated cells. A trophic factor expressed by PGCs and required for their early survival and proliferation is the RNA-binding protein tiar. Another is a mouse ortholog of the Drosophila gene nanos (nanos3). Many other trophic factors seem to be required for the survival and proliferation of PGCs along their migratory pathway from the yolk sac to the gut and dorsal mesentery and then to the dorsal body wall. These include several factors expressed by tissues along the pathway, including the c-kit ligand (stem cell factor or steel factor) and members of the interleukin/Lif cytokine family (a cytokine is a regulatory protein released by cells of the immune system that acts as an intercellular mediator in the generation of an immune response). Study of c-kit and steel mutants has revealed that this signaling pathway suppresses PGC apoptosis (cell death) during migration. This finding provides an explanation for why PGCs that stray from their normal migratory path and come to rest in extragonadal sites usually (but not always; see previous discussion of extragonadal teratomas) degenerate.
Once PGCs arrive within the presumptive gonad, numerous genes must be expressed to regulate the final differentiation of cells of the germ line . Three new germ cell–specific genes are expressed shortly after PGCs enter the genital ridge (after which they are usually called gonocytes ): murine vasa homolog (mVh; the vasa gene was covered earlier), germ cell nuclear antigen 1 (Gcna1), and germ cell–less (Gcl1). The last is expressed in the Drosophila germ line shortly after it is established and is named after the mutation in which the gene is inactivated and the germ line is lost.
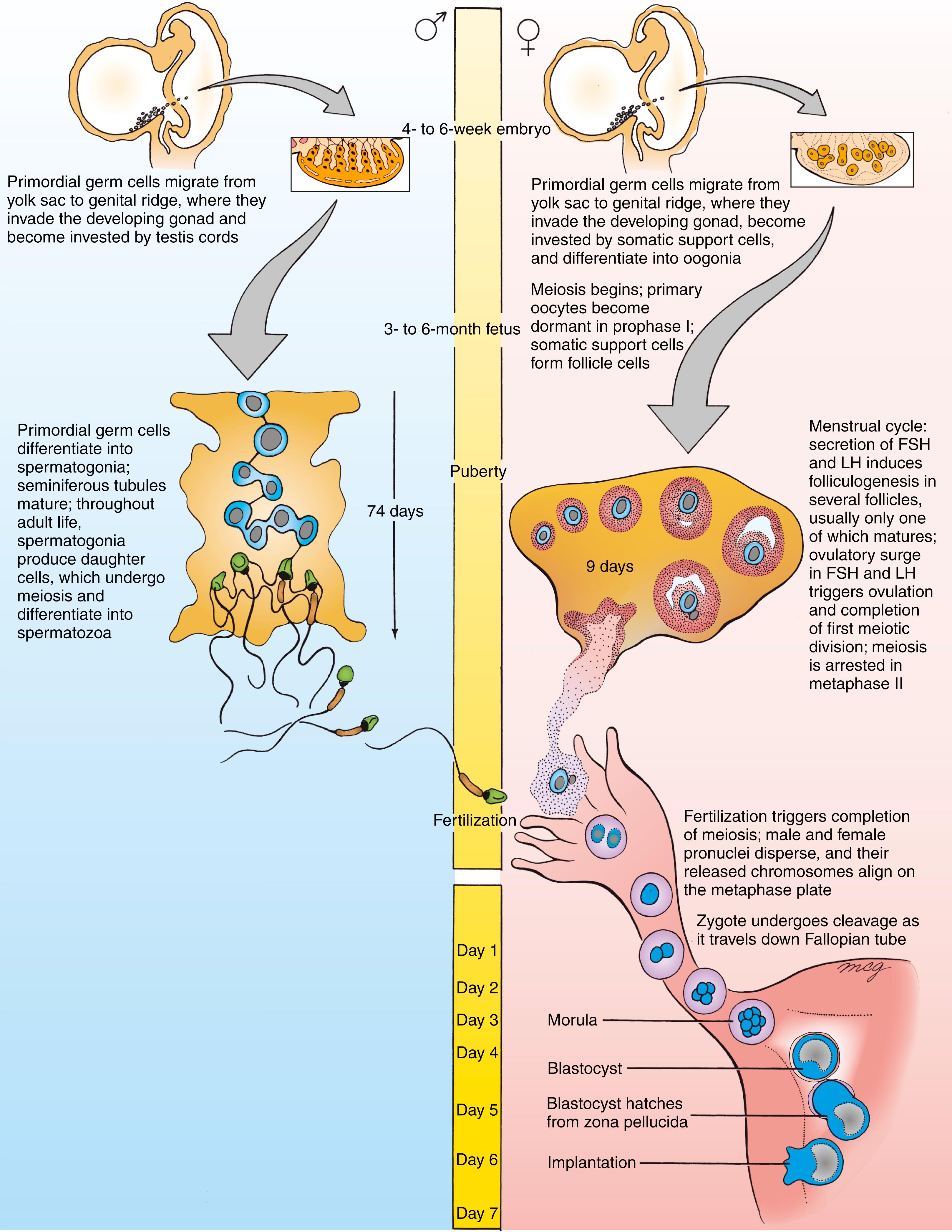
In both males and females, PGCs undergo further mitotic divisions within the gonads and then commence gametogenesis , the process that converts them into mature male and female gametes ( spermatozoa and definitive oocytes , respectively). However, the timing of these processes differs in the two sexes (see Timeline for this chapter). In males, PGCs (usually now called gonocytes ) remain dormant from the sixth week of embryonic development until puberty. At puberty, seminiferous tubules mature and PGCs differentiate into spermatogonia . Successive waves of spermatogonia undergo meiosis (the process by which the number of chromosomes in the sex cells is halved; see following section) and mature into spermatozoa. Spermatozoa are produced continuously from puberty until death.
In contrast, in females, PGCs (again, usually now called gonocytes ) undergo a few more mitotic divisions after they become invested by the somatic support cells. They then differentiate into oogonia . By the fifth month of fetal development, all oogonia begin meiosis, after which they are called primary oocytes . However, during an early phase of meiosis, all sex cells enter a state of dormancy, and they remain in meiotic arrest as primary oocytes until sexual maturity. Starting at puberty, each month a few ovarian follicles resume development in response to the monthly surge of pituitary gonadotropic hormones, but usually only one primary oocyte matures into a secondary oocyte and is ovulated. This oocyte enters a second phase of meiotic arrest and does not actually complete meiosis unless it is fertilized. These monthly cycles continue until the onset of menopause at approximately 50 years of age. The process of gametogenesis in the male and female (called spermatogenesis and oogenesis , respectively) is covered in detail later in this chapter.
Experiments in mouse embryos provide insight into why the timing of gametogenesis differs in males and females. Shortly after PGCs enter the genital ridge, they stop their migration and undergo two or three further rounds of mitosis and then enter a premeiotic stage, during which they upregulate meiotic genes. In the male genital ridge, germ cells then reverse this process and arrest, but in the female genital ridge, they enter the meiotic prophase as primary oocytes and progress through meiosis until the diplotene stage, at which time they arrest. If male (XY) PGCs are transplanted into female (XX) embryos, the male PGCs follow the course just described for normal female PGCs in females. Moreover, PGCs in female or male embryos that fail to reach the gonad also progress through meiosis as oocytes, regardless of their genotype. These two results suggest that all germ cells, regardless of their chromosome constitution, are programmed to develop as oocytes and that the timing of meiotic entry seems to be a cell-autonomous property rather than being induced. In support of this, Tet1, a member of the Tet family of proteins, is required for the activation of meiosis in female mice. Although it is unclear how Tet1 functions, Tet proteins play a role in erasing epigenetic marks in DNA—a critical event in the development of PGCs, as covered in Chapter 2 .
In males, the genital ridge prevents prenatal entry into meiosis, and experiments suggest that there is a male meiosis inhibitor and that this inhibitor is a diffusible signaling factor produced by Sertoli cells. Possible candidates for this factor include the protein prostaglandin D2 and the protein encoded by the Tdl gene (a gene showing sequence homology to antimicrobial proteins called beta-defensins; prostaglandins are synthesized from fatty acids and modulate several physiological functions, such as blood pressure, smooth muscle contraction, and inflammation).
Although the timing of meiosis is very different between males and females, the basic chromosomal events of the process are the same in the two sexes. Like all normal somatic (non-germ) cells, PGCs contain 23 pairs of chromosomes, or a total of 46 chromosomes. One chromosome of each pair is obtained from the maternal gamete and the other from the paternal gamete. These chromosomes contain deoxyribonucleic acid (DNA) , which encodes information required for development and functioning of the organism. Of the total complement of 46 chromosomes, 22 pairs consist of matching, homologous chromosomes called autosomes . The remaining two chromosomes are called sex chromosomes because they determine the sex of the individual. There are two kinds of sex chromosomes, X and Y. Individuals with one X chromosome and one Y chromosome (XY) are genetically male; individuals with two X chromosomes (XX) are genetically female. Nonetheless, one of the X chromosomes in the female genome is randomly inactivated, leaving only one active X chromosome in each cell (X-inactivation is covered in Chapter 2 ; mechanisms underlying sex determination are covered in detail in Chapter 16 ).
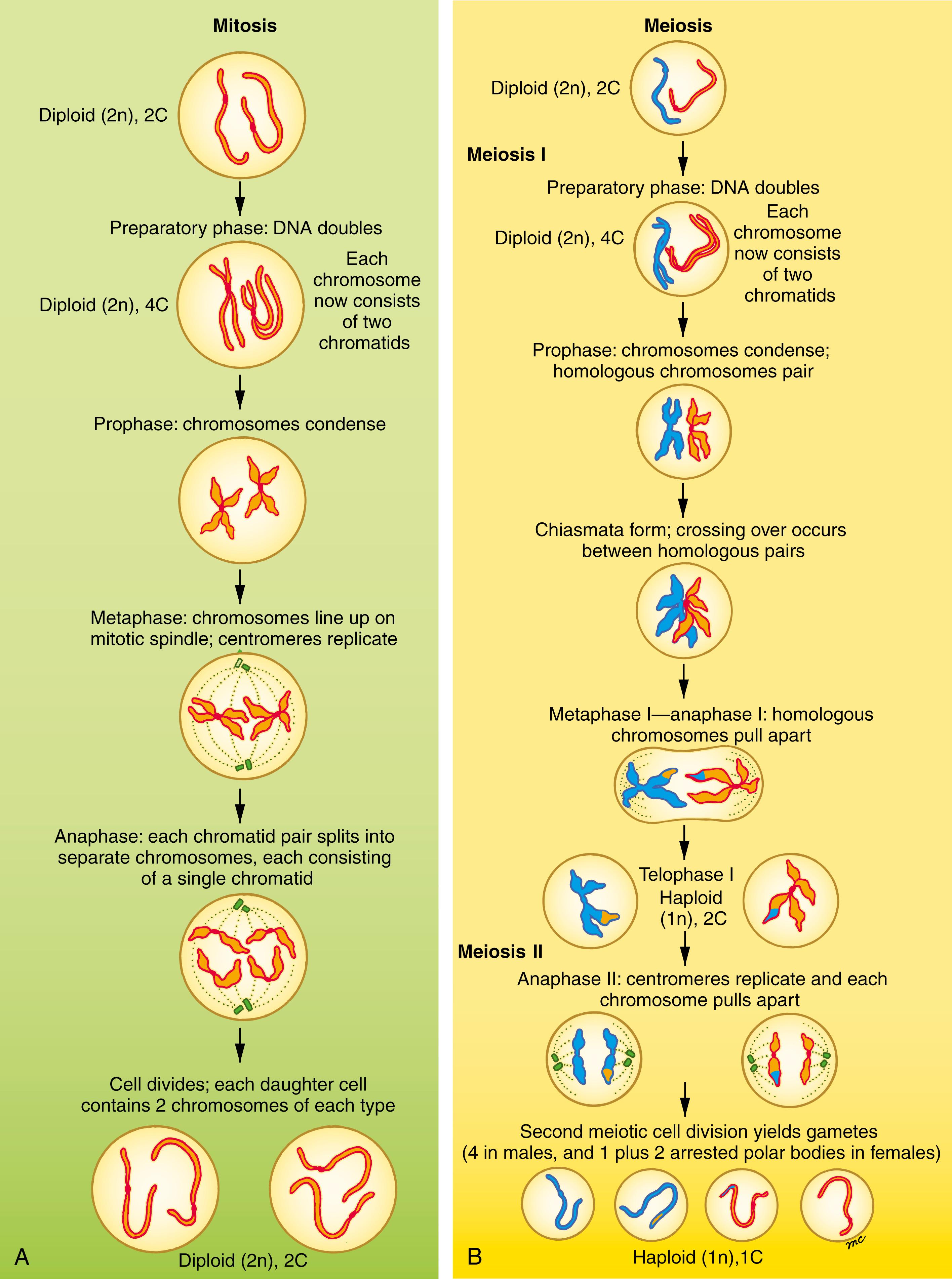
Two designations that are often confused are the ploidy of a cell (typically designated as its n value ) and its C value . Ploidy refers to the number of copies of each type of chromosome present in a cell nucleus, whereas the C value refers to the number of copies of each unique double-stranded DNA molecule in the nucleus. Each chromosome contains one or two double-stranded DNA molecules depending on the stage of the cell cycle, so the ploidy/n value and the C value of a cell do not always coincide. At stages when each chromosome contains two double-stranded DNA molecules it is organized into two chromatids, with each chromatid containing one of the double-stranded DNA molecules. Somatic cells and PGCs have two copies of each kind of chromosome; hence, they are diploid (2n) . In contrast, mature gametes have just one copy of each kind of chromosome and are haploid . Haploid gametes with only one double-stranded DNA molecule per chromosome are said to be 1C . In some stages of the cell cycle, diploid cells also have only one double-stranded DNA molecule per chromosome, and so are 2C (because they contain two of each type of chromosome). However, during earlier phases of meiosis or mitosis, each chromosome of a diploid cell has two double-stranded DNA molecules (with each having two chromatids), and so the cell is 4C .
Mitosis (normal cell division without reduction in DNA content) is common throughout most tissues of the body, especially during development. Meiosis is a specialized process of cell division that occurs only in the germ line. Fig. 1.2 compares mitosis (A) and meiosis (B). In mitosis , a diploid (2n), 2C cell replicates its DNA (becoming diploid, 4C) and undergoes a single division to yield two diploid, 2C daughter cells. In meiosis , a diploid germ cell replicates its DNA (becoming diploid, 4C) and undergoes two successive, qualitatively different nuclear and cell divisions to yield potentially four haploid (1n) 1C offspring. In males, the cell divisions of meiosis are equal and yield four identical spermatozoa ( Fig. 1.3 ). However in females, the meiotic cell divisions are dramatically unequal and yield a single, massive, haploid definitive oocyte and typically one polar body (the first polar body arrests; see Fig. 1.3 ).
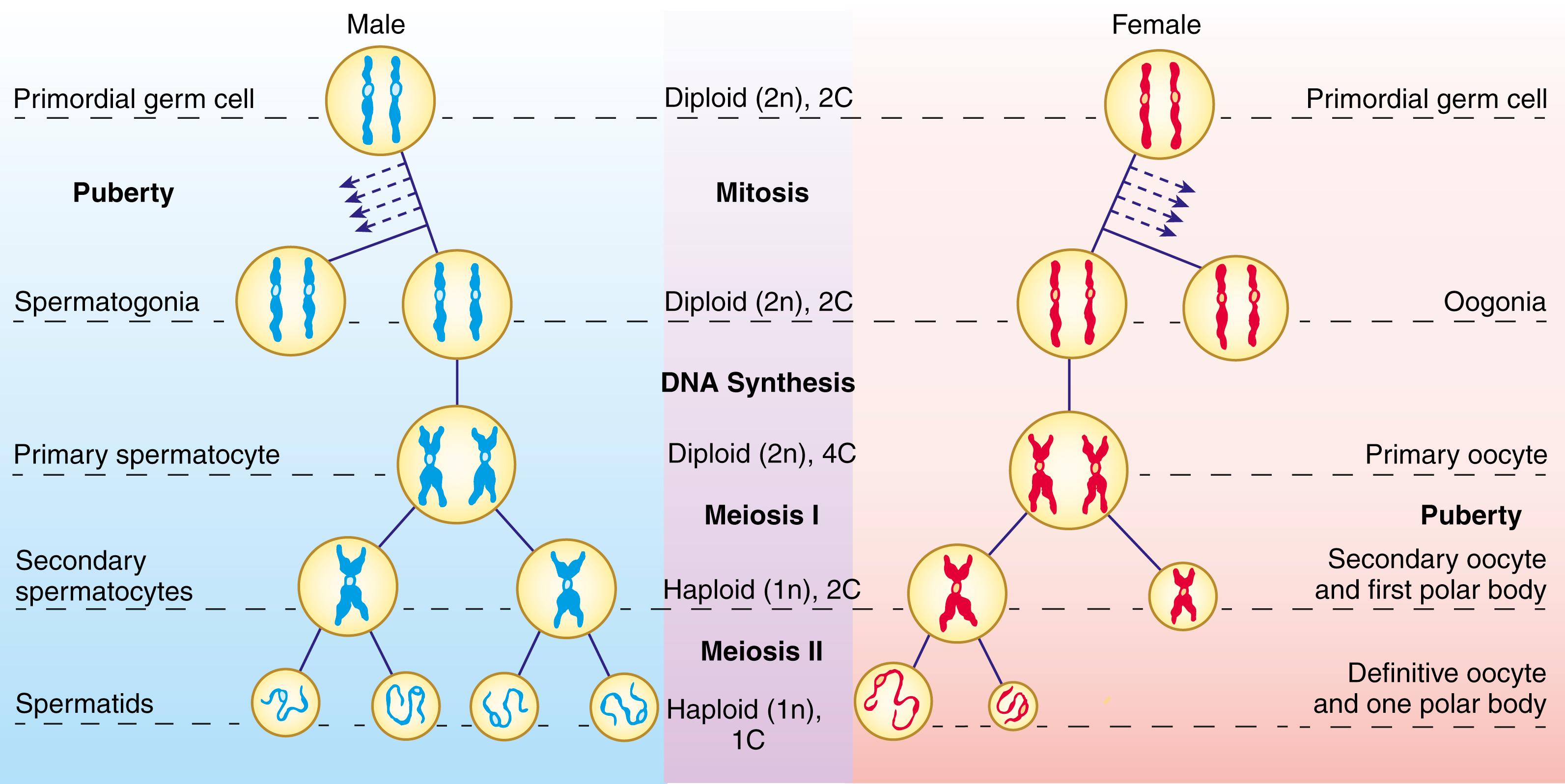
The steps of meiosis are illustrated in Fig. 1.2B and are summarized in Table 1.1 . Meiosis involves two sequential cell divisions; thus, these two divisions are called the first and second meiotic divisions or meiosis I and II. The preliminary step in meiosis I, as in mitosis, is the replication of each chromosomal DNA molecule; thus, the diploid cell is converted from 2C to 4C. This event marks the beginning of gametogenesis. In the female, the oogonium is now called a primary oocyte , and in the male, the spermatogonium is now called a primary spermatocyte (see Fig. 1.3 ). Once the DNA replicates, each chromosome consists of two parallel components, called chromatids, joined together at a structure called the centromere . In addition, sister chromatids are held together by a protein called Cohesion. Each chromatid contains a single double-stranded DNA molecule.
| Stage | Events | Name of Cell | Condition of Genome |
|---|---|---|---|
| “Resting” interval between mitotic cell divisions | Normal cellular metabolism occurs | ♀ Oogonium ♂ Spermatogonium |
Diploid (2n), 2C |
| Mitosis | |||
| Preparatory (S) phase | DNA replication results in each chromosome forming two chromatids | ♀ Oogonium ♂ Spermatogonium |
Diploid (2n), 4C |
| Prophase | Chromosomes condense | ||
| Metaphase | Chromosomes align along the equator; centromeres replicate | ||
| Anaphase and telophase | Each chromatid pair splits into two separate chromosomes (each consisting of a single chromatid), one of which is distributed to each daughter nucleus | ||
| Cytokinesis | Cell divides | ♀ Oogonium ♂ Spermatogonium |
Diploid (2n), 2C |
| Meiosis I | |||
| Preparatory phase | DNA replication results in each chromosome forming two chromatids | ♀ Primary oocyte ♂ Primary spermatocyte |
Diploid (2n), 4C |
| Prophase | Chromosomes condense; two chromosomes of each homologous pair align at the centromeres to form a four-limbed chiasma; recombination by crossing over occurs | ||
| Metaphase | Chromosomes align along the equator; centromeres do not replicate | ||
| Anaphase and telophase | One chromosome (consisting of two chromatids) of each homologous pair is distributed to each daughter cell | ||
| Cytokinesis | Cell divides | ♀ One secondary oocyte and the first polar body ♂ Two secondary spermatocytes |
Haploid (1n), 2C |
| Meiosis II | |||
| Prophase | No DNA replication takes place during the second meiotic division ; chromosomes condense | ||
| Metaphase | Chromosomes align along the equator; centromeres replicate | ||
| Anaphase and telophase | Each chromosome splits into two separate chromosomes (each consisting of a single chromatid), one of which is distributed to each daughter nucleus | ||
| Cytokinesis | Cell divides | ♀ One definitive oocyte and three polar bodies ♂ Four spermatids |
Haploid (1n), 1C |
In the next step, called prophase , the chromosomes condense into compact structures (i.e., two chromatids joined by one centromere). During the late stages of prophase, the chromosomes of each homologous pair match up, centromere to centromere, to form a joint structure called a chiasma (composed of two chromosomes, consisting of four chromatids, and two centromeres). Chiasma formation makes it possible for the two homologous chromosomes to exchange large segments of DNA by a process called crossing over . The resulting recombination of the genetic material on homologous maternal and paternal chromosomes is largely random; therefore, it increases the genetic variability of future gametes. As mentioned earlier, the primary oocyte enters a phase of meiotic arrest during the first meiotic prophase.
During metaphase , the four-stranded chiasma structures are organized on the equator of a spindle apparatus similar to the one that forms during mitosis, and during anaphase , each homologous pair is distributed to one of the two daughter nuclei. During the first meiotic division, the centromeres of the chromosomes do not replicate; therefore the two chromatids of each chromosome remain together during telophase . The resulting daughter nuclei thus are haploid (1n) but 2C: they contain the same amount of DNA as the parent germ cell but half as many chromosomes. As daughter nuclei form, the cell itself divides (undergoes cytokinesis ). The first meiotic cell division produces two secondary spermatocytes in the male and a secondary oocyte and a first polar body in the female (see Fig. 1.3 ).
No DNA replication occurs during the second meiotic division. The 23 chromosomes (each consisting of two chromatids) condense during the second meiotic prophase and line up during the second meiotic metaphase. The chromosomal centromeres then replicate, and during anaphase, the two chromatids of each chromosome pull apart into two chromosomes (each consisting of a single chromatid), one of which is distributed to each of the daughter nuclei. In males, the second meiotic cell division produces two definitive spermatocytes , more commonly called spermatids (i.e., a total of four from each germ cell entering meiosis). In the female, the second meiotic cell division, like the first, is radically unequal, producing a large definitive oocyte and another diminutive polar body, which is also arrested (see Fig. 1.3 ).
In the female, the oocyte enters a second phase of meiotic arrest during the second meiotic metaphase before replication of the centromeres. Meiosis does not resume unless the cell is fertilized.
Now that meiosis has been described, it is possible to describe and compare the specific processes of spermatogenesis and oogenesis. At puberty, the testes begin to secrete greatly increased amounts of the steroid hormone testosterone . This hormone has a multitude of effects. In addition to stimulating development of many secondary sex characteristics, it triggers growth of the testes, maturation of seminiferous tubules, and commencement of spermatogenesis.
Under the influence of testosterone, Sertoli cells differentiate into a system of seminiferous tubules. The dormant PGCs resume development, divide several times by mitosis, and then differentiate into spermatogonia. These spermatogonia are located immediately under the basement membrane surrounding the seminiferous tubules, where they occupy pockets between Sertoli cells ( Fig. 1.4A ). Adjacent Sertoli cells are interconnected between the pockets by tight junctions , which help establish a blood-testis barrier . Thus, developing spermatogonia reside within an immune-privileged site during their development in the testes.
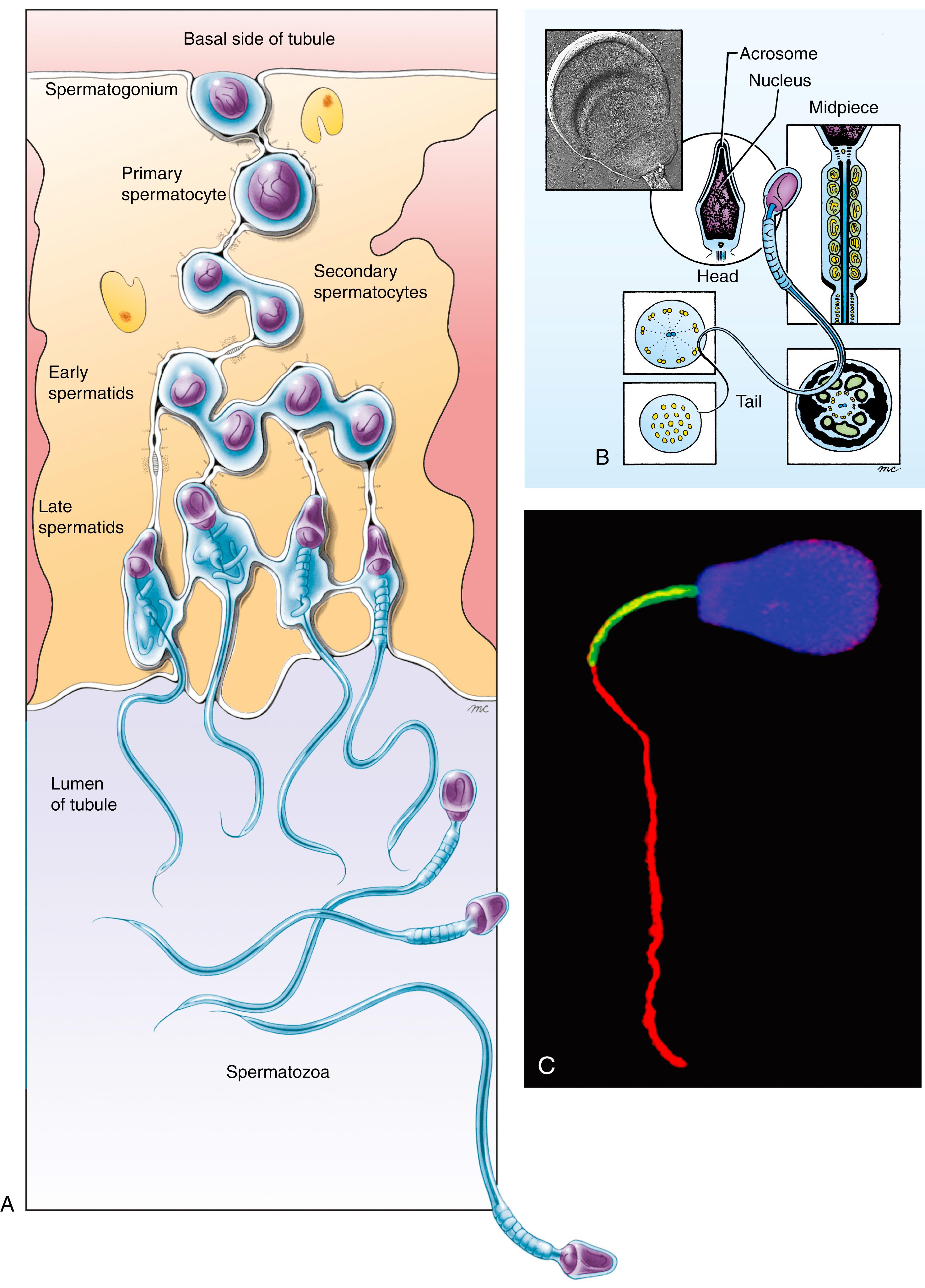
Cells that will undergo spermatogenesis arise by mitosis from the spermatogonia. These cells are gradually translocated between the Sertoli cells from the basal to the luminal side of the seminiferous epithelium while spermatogenesis takes place (see Fig. 1.4A ). During this migratory phase, primary spermatocytes pass without interruption through both meiotic divisions, producing first two secondary spermatocytes and then four spermatids. The spermatids undergo dramatic changes that convert them into mature sperm as they complete their migration to the lumen. This process of sperm cell differentiation is called spermiogenesis .
Sertoli cells participate intimately in differentiation of the gametes. Maturing spermatocytes and spermatids are connected to surrounding Sertoli cells by intercellular junctions, typical of those found on epithelial cells, and unique cytoplasmic processes called tubulobulbar complexes that extend into the Sertoli cells. The cytoplasm of developing gametes shrinks dramatically during spermiogenesis; the tubulobulbar complexes are thought to provide a mechanism by which excess cytoplasm is transferred to Sertoli cells. As cytoplasm is removed, spermatids undergo dramatic changes in shape and internal organization that transform them into spermatozoa. Finally, the last connections with Sertoli cells break, releasing the spermatozoa into the tubule lumen. This final step is called spermiation .
As shown in Fig. 1.4B,C , a spermatozoon consists of a head , a midpiece , and a tail . The head contains the condensed nucleus and is capped by an apical vesicle filled with hydrolytic enzymes (e.g., acrosin, hyaluronidase, and neuraminidase). This vesicle, the acrosome , plays an essential role in fertilization. The midpiece contains large, helical mitochondria and generates energy for swimming. The long tail contains microtubules that form part of the propulsion system of the spermatozoon.
Errors in spermatogenesis or spermiogenesis are common. Examination of a sperm sample will reveal spermatozoa with abnormalities such as small, narrow, or piriform (pear-shaped) heads, double or triple heads, acrosomal defects, and double tails. If at least 50% of the spermatozoa in an ejaculate have a normal morphology, fertility is not expected to be impaired. Having a larger number of abnormal spermatozoa (called teratospermia if excessive) can be associated with infertility.
Spermatogenesis takes place continuously from puberty to death. Gametes are produced in synchronous waves in each local area of the germinal epithelium, although the process is not synchronized throughout the seminiferous tubules. In many different mammals, the clone of spermatogonia, derived from each spermatogonial stem cell, populates a local area of the seminiferous tubules and displays synchronous spermatogenesis. This may be the case in humans as well. About four waves of synchronously differentiating cells can be observed in a given region of the human tubule epithelium at any time. Ultrastructural studies provide evidence that these waves of differentiating cells remain synchronized because of incomplete cytokinesis throughout the series of mitotic and meiotic divisions between division of a spermatogonium and formation of spermatids. Instead of fully separating, daughter cells produced by these divisions remain connected by slender cytoplasmic bridges (see Fig. 1.4A ) that could allow passage of small signaling molecules or metabolites. In the human male, each cycle of spermatogenesis takes about 74 days.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here