Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mature and differentiated mammalian kidney assumes a number of diverse, complex, and related functions in the postnatal environment. These functions include glomerular filtration, salt and water reabsorption, acid-base homeostasis, regulation of blood pressure, tubular secretion, and endocrine functions including vitamin D, calcium and phosphate metabolism, erythropoiesis, immune regulation, and circulating drug and toxin metabolism, among others. Most of these important postnatal physiologic functions that regulate normal body homeostasis have their origins in fetal life. Their ontogeny is directed by the development of kidney structure, which itself is under the tight control of precisely orchestrated spatial and temporal gene and protein expression.
The important events in fetal life that precede the acquisition of kidney function include the induction of embryonic kidney progenitor cell populations to differentiate into kidney-specific cells, the development and differentiation of glomeruli and associated vascularization, segment-specific differentiation of the kidney tubules, and terminal differentiation of the various cellular compartments of the kidney.
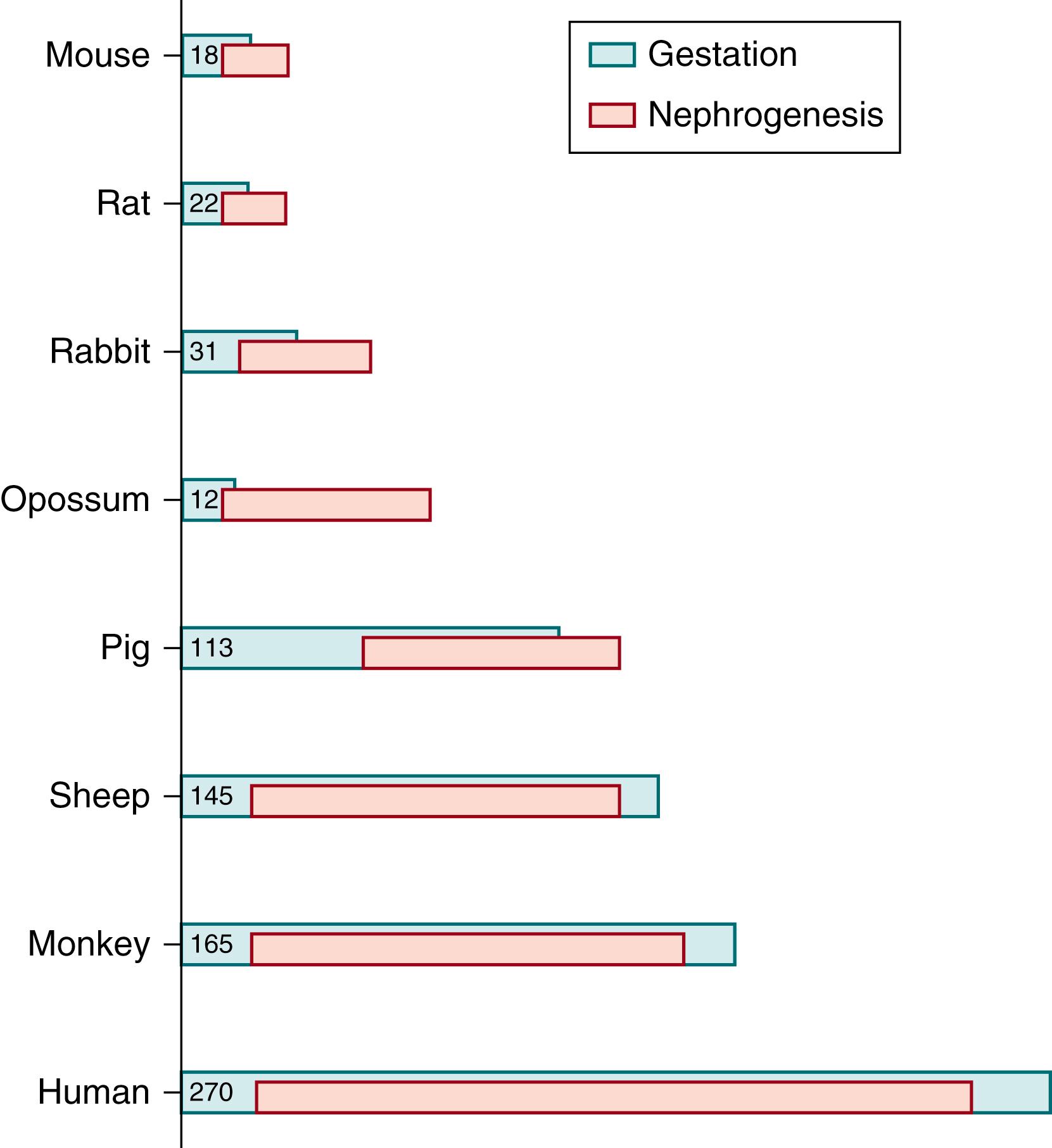
While much of our knowledge to date regarding fetal kidney physiology is based on large mammal experimental observation, and while there are a number of phylogenetic similarities in kidney embryogenesis (e.g., pro- and metanephric development of glomeruli and tubules), it should be noted that substantial variation exists in the periods of nephrogenesis that occur during fetal life and postnatally.
For example, in humans, nephrogenesis, as defined by the period of time during which new nephrons are formed, begins at approximately 8 weeks gestation and is complete by 36 weeks gestation. In other species (and in human infants born prematurely) nephron induction continues postnatally and is influenced by ex utero factors. The requirements for mature kidney function in the human fetus are supplanted by the interposition of the maternal placenta. Therefore, our knowledge of normal fetal kidney physiology is inferred from either observational data in preterm infants or from experimental data from animal models, whose period of gestational nephrogenesis may or may not correspond to the length of human gestation ( Fig. 94.1 ).
Why then is an understanding of normal fetal renal physiology important, particularly if growth and maturation of the fetus is dependent on the placenta and maternal circulation?
The development of normal kidney function originates in utero as soon as nephrogenesis begins and continues into the neonatal period. Fetal kidney function impacts normal fetal development and is linked to the normal development of other fetal organ systems such as the lung and genitourinary systems.
Alteration of normal kidney development and, therefore, normal kidney structure and kidney function by factors such as placental insufficiency, protein restriction, preterm birth, in utero exposure to toxins such as inhibitors of prostaglandin synthesis, corticosteroids, and inhibitors of the renin-angiotensin-aldosterone axis, impacts not only fetal development but also postnatal kidney function and long-term kidney health. Abnormal fetal kidney development and fetal kidney function therefore portend long-term physiologic consequences.
In a more practical sense, in the developed world there is almost universal access to antenatal screening to evaluate fetal health. The most common abnormalities discovered during early second trimester ultrasound screening (16 to 20 weeks gestation) are anomalies associated with urinary tract and kidney structure. Information gained from early gestation fetal ultrasounds, particularly in the cases in which kidney abnormalities are found, is used to predict fetal outcome, to influence families’ decisions regarding the future of their pregnancies, and to inform counseling around future pregnancies.
A number of tests are used to estimate kidney health and to extrapolate postnatal kidney function during antenatal assessments of pregnancies complicated by fetal kidney anomalies. These include imaging studies of fetal kidney morphology, structure and differentiation, fetal blood and urine sample estimates of fetal glomerular filtration rate (GFR), amniotic fluid (AF) concentrations of analytes, AF volumes, fetal urine flow rates, and proteomic and metabolomic analysis of fetal urine and AF.
Antenatal imaging using second trimester screening ultrasound identifies most cases of severe developmental kidney anomalies including congenital urinary tract obstruction. Typically, the normal fetal bladder can be visualized from the onset of urine production at approximately 10 weeks gestation and in all pregnancies by 18 to 20 weeks. Increased echogenicity of the kidneys, a poorly defined cortico-medullary border, and parenchymal cystic changes are suggestive of abnormal kidney development. When associated with severe oligohydramnios in early pregnancy, these changes carry a poor prognosis and are associated with a less than 90% perinatal mortality.
Studies analyzing the value of screening antenatal ultrasounds in predicting postnatal kidney outcomes are conflicting. The predictive value of echogenic and/or cystic changes of fetal kidneys to detect either dysplasia histologically or chronic kidney disease on postnatal follow-up was only 59%. In contrast, the predictive value of normal parenchyma on prenatal ultrasound to detect no dysplasia or normal renal function was 56%. Detailed ultrasound examination of the urinary tract, particularly when performed by an experienced operator, yields a higher accuracy of diagnosis of congenital kidney anomalies. Fetal magnetic resonance imaging (MRI) can provide more detailed assessment of the fetal urinary tract, and future applications of functional MRI, such as diffusion weighted imaging, may provide information regarding fetal GFR. Predictive values of MRI for congenital urinary tract obstruction, however, are difficult to ascertain due to its selective use and small numbers of published cases.
Given the inherent drawbacks of antenatal imaging in affected fetuses with kidney disorders, antenatal evaluation strategies have included measures of fetal renal function. Measurement of fetal GFR would be an ideal determinant of renal function and likely a good surrogate for the severity of dysplasia; however, there are no normative data in uncomplicated pregnancies, and GFR is somewhat challenging to determine accurately during prenatal life. β 2 -microglobulin, the light chain of the class I major histocompatibility antigens, has been used as an indirect measure of fetal GFR. It is an attractive candidate for GFR estimation because of a constant production rate by the fetus, inability to cross the placenta, and free filtration at the level of the glomerulus. Thus, fetal blood levels reflect fetal GFR in the same manner that blood levels of creatinine reflect kidney function postnatally. Several groups of investigators have measured the levels of β 2 -microglobulin in fetal blood obtained by cordocentesis at different stages of pregnancy. Fetuses with congenital kidney anomalies affecting normal kidney function, such as urinary tract obstruction, had higher serum levels than those without obstruction, and β 2 -microglobulin was shown to be a better predictor of postnatal kidney function in fetal uropathy than either α1-microglobulin or cystatin C ( Fig. 94.2 ).
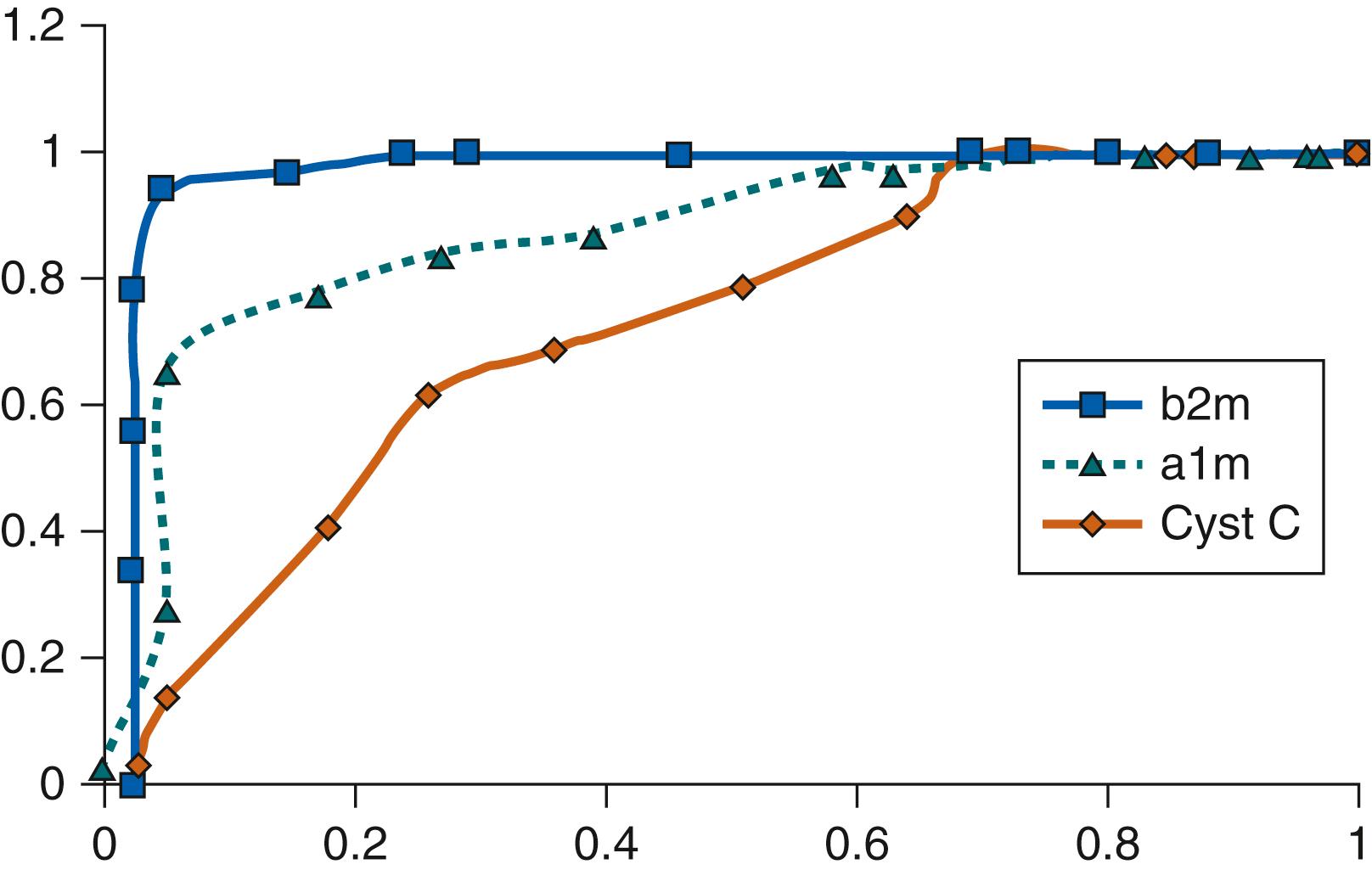
A fetal serum β 2 -microglobulin cutoff of 5.6 mg/L had a sensitivity of 80%, a specificity of 98.6%, a positive predictive value of 88.9%, and a negative predictive value of 97.1% for postnatal renal failure. Filtered β 2 -microglobulin normally undergoes 99.9% degradation by proximal tubular cells. Urinary levels lower than 6 mg/L are considered “normal” in fetuses with congenital urinary tract obstruction, while levels greater than 13 mg/L, which reflect tubular damage, were invariably associated with perinatal death. The usefulness of serum β 2 -microglobulin is, however, limited due to the lack of normative data based on the small numbers of patients, measurements at different stages of gestation, and variable measures of outcome. In addition, fetal blood sampling carries the risks of bleeding, AF leak, infection, and fetal death.
Fetal urine analyte analysis has also been used in the antenatal evaluation of kidney function. Similar to GFR, tubular function undergoes significant changes during fetal maturation. During early development, the urinary ultrafiltrate is minimally modified by passage through the nephron. With maturation, the tubules and collecting ducts become more efficient in water and electrolyte reabsorption. Based on these physiologic observations, normal fetal urinary thresholds have been correlated with autopsy and biopsy findings and clinical outcome in fetuses with bladder outlet obstruction. Fetal urine sodium less than 100 mEq/L, chloride less than 90 mEq/L, and urine osmolality greater than 210 mEq/L at a mean gestational age of 23.8 weeks predicted a “good outcome,” based on the presence of non-dysplastic kidneys at autopsy or biopsy, or normal renal and pulmonary function at birth. Values above or below these thresholds reflected poor tubular function of dysplastic kidneys with presumed altered reabsorption of filtered electrolytes and water. Since this report, multiple studies of the best predictors of poor outcome based on the composition of fetal urine, including sequential fetal urine sampling, have added little information, correlating only modestly with postnatal renal function. A comprehensive systematic literature review on the accuracy of fetal urine analysis to predict postnatal renal function revealed that there is currently no individual analyte or threshold with significant clinical accuracy. The thresholds of the most widely investigated analytes (e.g., sodium, chloride, calcium, osmolality, and β 2 -microglobulin) varied widely among the studies, and not all studies correlated these thresholds with gestational age. The same analytes in fetal urine have also been used to select fetuses for prenatal intervention but, unfortunately, have not resulted in improved outcomes. More recently unbiased analyses of fetal urine peptidome and metabolome signatures have been used to more reliably predict postnatal kidney function outcomes in fetuses affected by bladder outlet obstruction.
Serum cystatin C has also been used and promoted as a reliable measure of postnatal GFR. It is a 13-kDa protein produced by all nucleated cells that is freely filtered in the glomerulus and almost completely reabsorbed in the proximal tubule. Serum cystatin C levels are higher in preterm infants than in newborn infants. The metabolism of cystatin C by fetal kidneys has not been described, and the value of fetal urine, fetal blood, or AF cystatin C levels in assessing fetal kidney function is unknown. During normal pregnancy AF cystatin C levels decrease with increasing gestational age but are increased in pregnancies associated with fetal uropathy.
AF volume can also be used as a surrogate marker of fetal GFR during the second half of pregnancy. In the early fetal period, most of the AF is produced by the amnion, placenta, and umbilical cord. AF volume increases from approximately 25 mL at 10 weeks gestation to approximately 400 mL at 20 weeks gestation when fetal kidneys become the main source, although the total volume of AF can vary substantially. By 28 weeks gestation, AF volume reaches a plateau of approximately 800 mL until term, with a slight decline post-term. Any impairment of fetal kidney function, including urinary tract obstruction, will manifest as oligo/anhydramnios from mid-trimester onward.
Since fetal GFR increases with fetal weight and urine output increases with advancing gestation, fetal urinary flow rates have been used as a surrogate marker of fetal GFR and can be calculated from changes in fetal bladder volumes on repeat ultrasound examinations over time. ,
Development of glomeruli, the glomerular vasculature, and the glomerular filtration barrier occurs in a centrifugal fashion continuously between 8 and 36 weeks gestation in humans. Therefore juxtamedullary glomeruli form first and outer cortical glomeruli form last.
Following induction by the ureteric bud, pluripotent cells of the metanephric mesenchyme condense to form aggregates adjacent to the ureteric bud. These aggregates subsequently undergo mesenchymal-epithelial transdifferentiation to form epithelialized structures called renal vesicles . Cell-to-cell adherence via the expression of adherens and tight junctional proteins allows cell polarization and the deposition of a provisional basement membrane and formation of a lumen. The immature epithelium of the renal vesicle will constitute all of the epithelial components of the nephron including the podocytes and parietal epithelium of the glomerulus. The differentiation of the simple renal vesicle to the complex structure of the final nephron proceeds through two recognizable intermediates: the comma shaped and S-shaped bodies. , The invasion of FLK1-positive angioblasts into the proximal cleft of the S-shaped body provides the precursors for the vascular bundles comprising the future glomerular capillary network. Mesangial precursors provide structural support for the growing capillary loops and serve an important role in the patterning and final development of the glomerular tuft.
The most proximal end of the S-shaped body, together with its vascularized cleft, differentiates into the glomerulus. The epithelium in this region specializes into precursors of the podocyte and parietal epithelium. Adjacent podocytes produce modified junctions that form the slit diaphragm, a crucial feature of the glomerular filtration barrier. The remaining epithelium of the proximal arm of the S-shaped body subsequently forms the parietal epithelial layer of the Bowman capsule and encloses the glomerular urinary space, or Bowman space. The glomerular filtration barrier then consists of the fenestrated glomerular capillary endothelium with its associated charged glycocalyx, the glomerular basement membrane whose components derive from both the developing endothelium and epithelium, and the developing podocyte or glomerular epithelial cell layer. Normal podocyte development is instrumental in determining normal glomerular filtration. Podocyte development and maintenance of glomerular integrity is under the control of numerous genes and the proteins they encode, and monogenic mutations have been defined in various childhood disorders associated with urinary protein losses and progressive decline in GFR. ,
Fetal renal function is neither sufficient to balance all the metabolic requirements of the growing fetus, nor is it necessary, given the interposition of the placenta and maternal-fetal membranes. Nevertheless, the contribution of fetal kidneys to fluid and electrolyte homeostasis gradually increases as gestation progresses, eventually replacing the placenta at the time of birth.
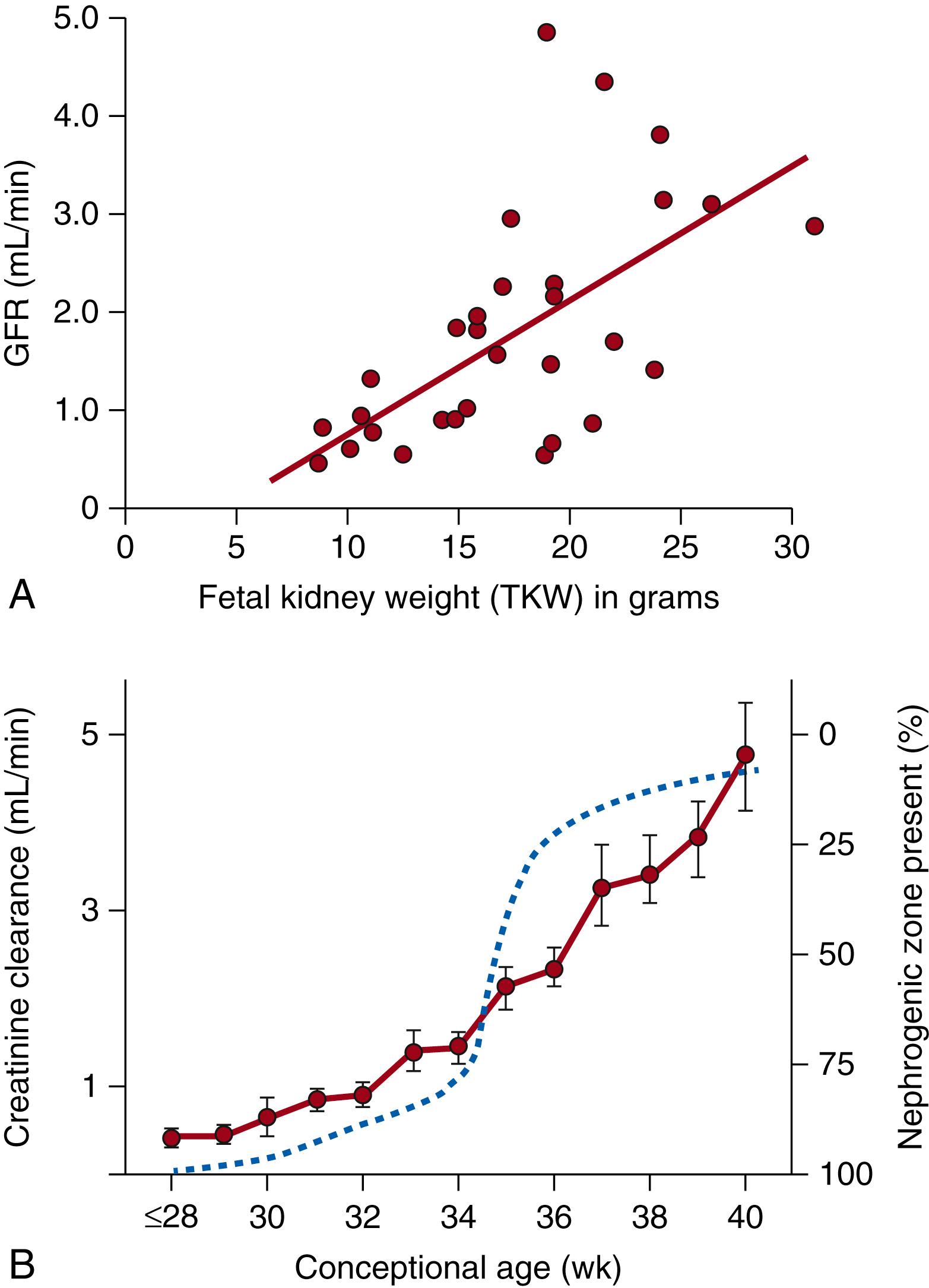
In humans, glomerular filtration begins shortly after 8 weeks gestation, when the first glomeruli appear, shows a slow linear increase reflecting new nephron development before 34 weeks gestation, and increases rapidly thereafter with completion of nephrogenesis, renal blood redistribution, and engagement of younger cortical nephrons ( Fig. 94.3 ).
With the increase in cardiac output and growth of the renal vascular bed during gestation, renal blood flow also increases. The kidneys of the human fetus at 10 to 20 weeks gestation receive about 5% of the cardiac output, compared to 9% in 1-week-old term infants and 25% in adults. The intrarenal distribution of blood flow also changes during gestation as a result of a centrifugal pattern of new glomerular development. The hemodynamic evolution of the fetal kidney is thus characterized by a shift from a low-flow, high-resistance organ, with most of the blood supply to the inner cortex to a high-flow, low-resistance organ, with most of the blood flow supplying the outer cortex.
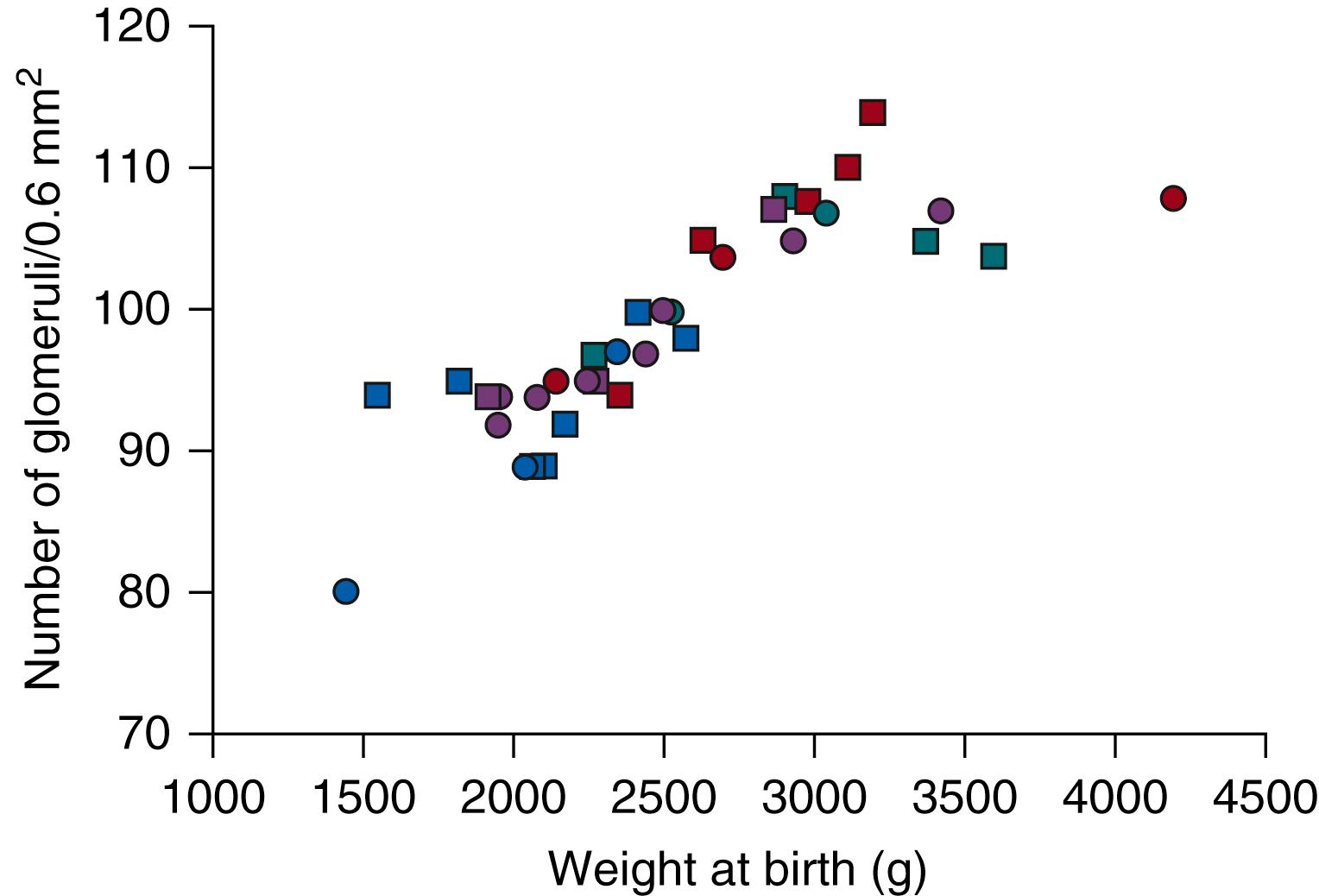
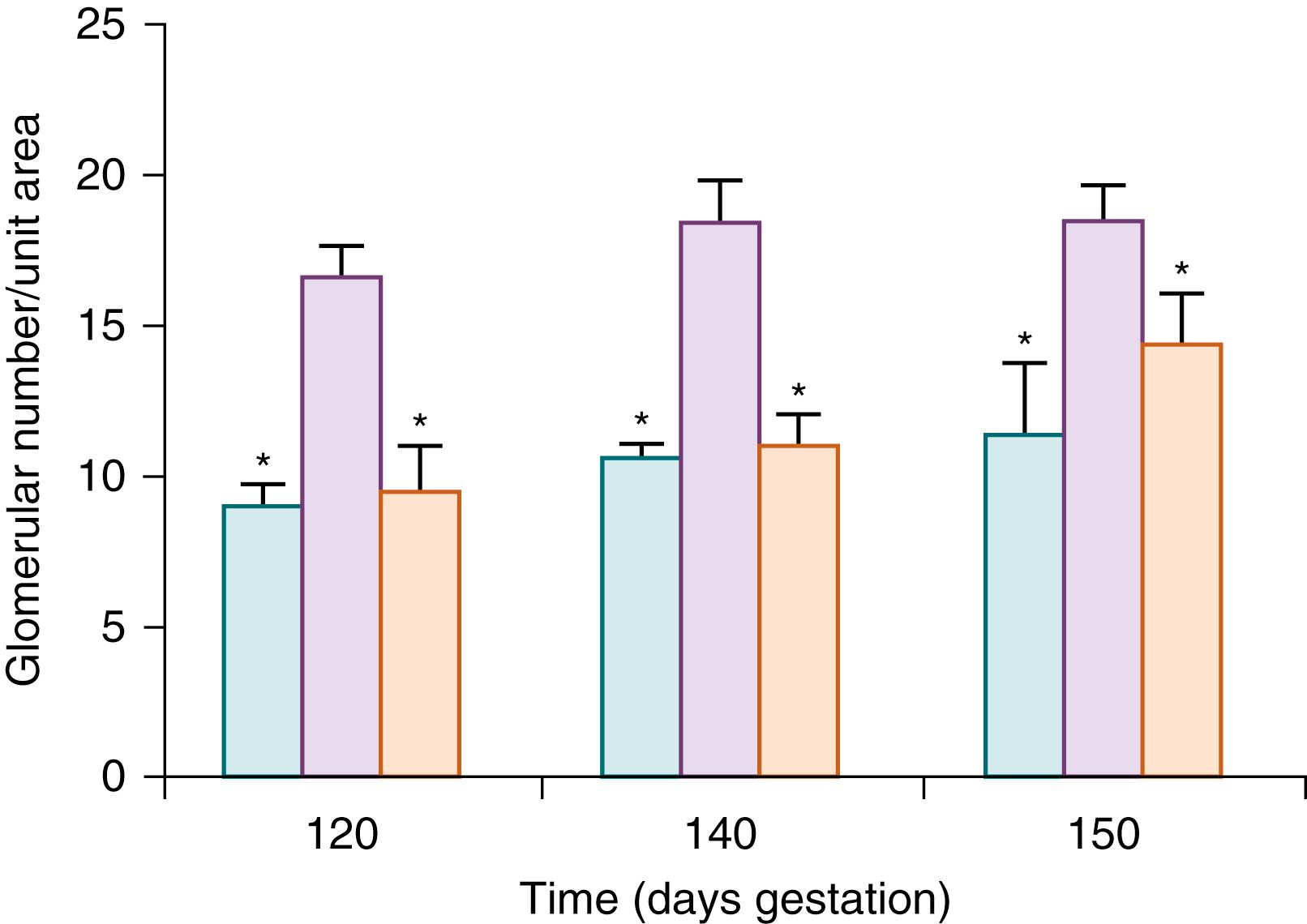
Glomerular numbers are also proportional to fetal weight. The increase in GFR and fetal urine flow correlate closely with the increase in kidney mass, and thus it is reasonable to assume that a main contributing factor is the addition of new nephrons ( Fig. 94.4 ).
The attainment of the final nephron number and, therefore, the establishment of GFR are influenced by genetic and environmental factors. Conditional gene targeting in mice has implicated a number of individual genes and their protein products in determining nephron number. These mutations include genes responsible for induction, specification, and maturation of glomerular cells. Similarly, in utero exposure to environmental factors, or developmental kidney injury, such as placental insufficiency, protein restriction, or urinary tract obstruction can impact normal nephrogenesis and result in a deficit of nephron endowment and associated decrease in renal function ( Fig. 94.5 ). ,
As in the postnatal kidney, glomerular filtration in the fetal kidney is the cumulative effect of single nephron GFR (SNGFR). The determinants of SNGFR in the fetal kidney, like the adult kidney, include the development of renal blood flow, the difference in trans capillary hydrostatic pressure across the filtration barrier, the difference in oncotic pressure between the capillary and urinary spaces, and the hydraulic permeability and surface area of the filtration membrane. The gestational changes in fetal GFR result from maturational changes in these variables, from changes in regulatory mechanisms with fetal maturation, and, ultimately, from an increase in number and size of glomeruli over the period of in utero nephrogenesis.
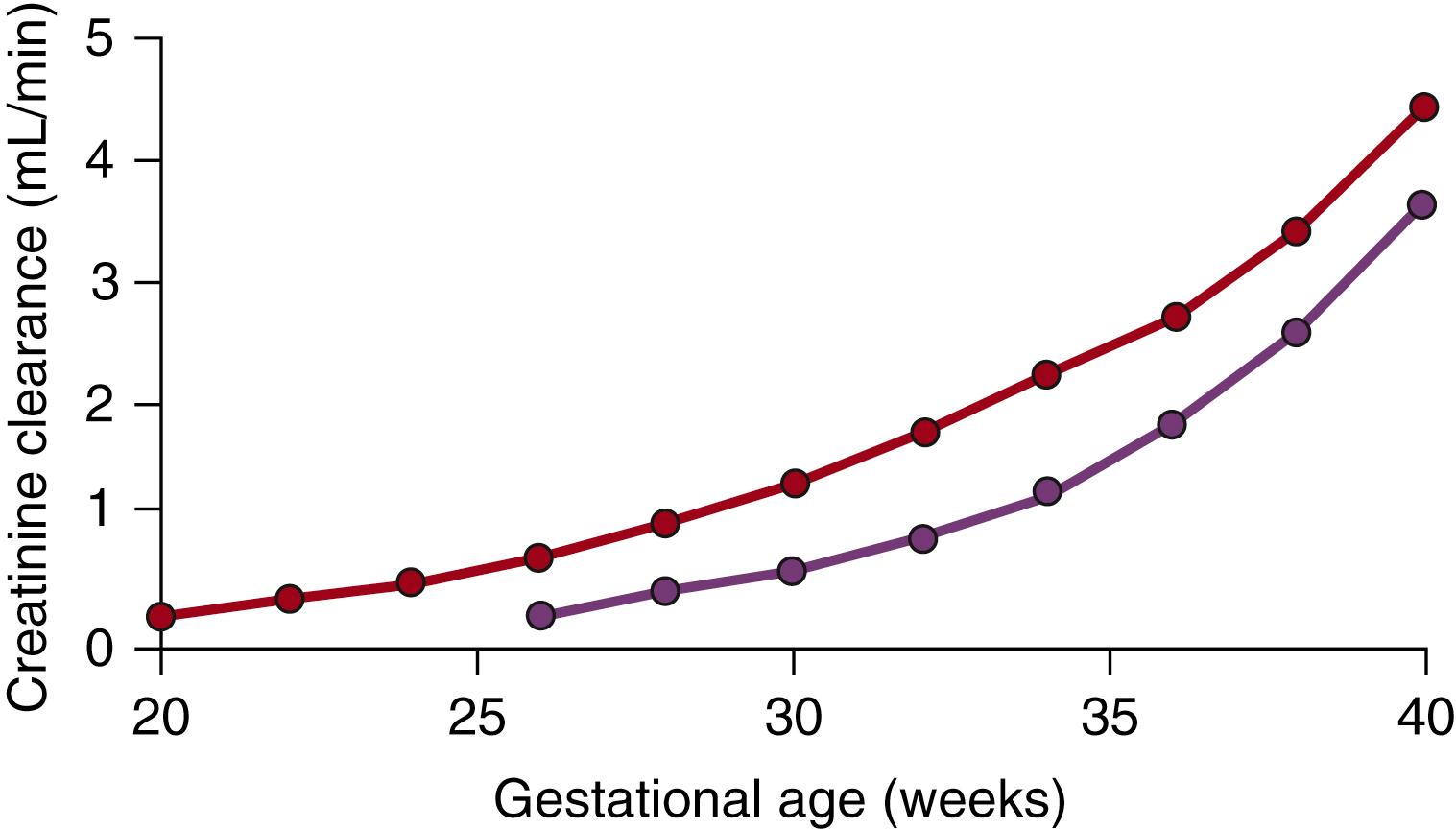
Using a combination of studies of fetal blood and urine sampling fetal GFR, as measured by creatinine clearance, has been estimated as early as 20 weeks gestation in humans. , , While the absolute creatinine clearance for a fetus at 20 weeks gestation is estimated to be less than 1 mL/min, over the subsequent period of nephrogenesis and at term, the GFR increases approximately fivefold to 4 to 5 mL/min, with a further doubling of the GFR in the 2 weeks after birth ( Fig. 94.6 ).
Hydraulic permeability and filtration membrane surface area constitute the filtration coefficient of the glomerular filtration membrane. Little is known about the changes that occur in hydraulic permeability in the developing fetal glomerulus; however, both indirect experimental evidence in late gestation fetal lambs and direct measurement using antiglomerular basement membrane antibodies in newborn and growing rats have demonstrated a significant increase in the glomerular filtration membrane surface area. Therefore, while an increase in the fetal filtration coefficient up to the end of nephrogenesis is accounted for by both the increase in the number of glomeruli and in the filtration surface area after nephrogenesis is complete, in later gestation this increase is due primarily to an increase in surface area.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here