Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Free tissue transfer involves auto-transplantation of skin, soft tissue, muscle, or bone isolated on a supporting vascular supply. Although more than 40 donor sites for free tissue transfer have been described, forearm, fibula, scapula, and anterolateral thigh flaps account for the majority of all the free flaps used for head and neck reconstruction.
A multidisciplinary team approach, especially for patients with head and neck cancer, is necessary to maximize both the oncologic and reconstructive efforts.
Some situations in which free tissue transfer has become the standard of care include treatment of oromandibular defects, especially those that involve the anterior mandible; circumferential pharyngo-esophageal defects; complex craniofacial defects; and salvage surgical resections of recurrent/persistent head and neck carcinomas following organ preservation chemotherapy or radiotherapy.
Aspirin, heparin, and dextran have all been extensively used in the management of patients undergoing microvascular surgery. Many microvascular surgeons use some form of postoperative anti-thrombotic therapy on a routine basis despite a paucity of prospective clinical trial data to guide such a practice.
Most ischemic complications in free flap anastomoses occur within the first 48 to 72 hours. After approximately 12 hours of ischemia, even with reestablishment of anastomotic flow, the free flap is usually not salvageable.
Systemic medical complications are common in patients with head and neck cancer and should be recognized and treated aggressively. Local complications—such as hematoma, salivary fistula, and infection—are especially common in previously irradiated patients and may lead to late flap compromise.
In addition to surgical expertise, free flap reconstruction requires significant health care resources that include time, equipment, and personnel. However, the actual costs of microvascular reconstruction are equivalent to or even lower than the costs of pedicled flap reconstruction, owing to the shorter length of hospitalization for patients who receive free flaps.
Since the early 1970s, the expansion of knowledge, expertise, and training in the techniques of free tissue transfer has redefined the standard of care in head and neck reconstructive surgery. Free tissue transfer refers to the isolation of tissues—fascia, skin, fat, muscle, nerve, and bone, either individually or in combination—on a supporting vascular supply with transfer to a new location in the body using microvascular surgical techniques to revascularize the tissues in a permanent fashion. This auto-transplantation of tissues relies entirely on the anastomosis of the feeding artery and draining vein to blood vessels in the head and neck for tissue survival. Upon revascularization, the transferred tissues can then be utilized to reconstruct various complex defects of the head and neck from any cause. Since the first reports of free tissue transfer for reconstruction in the head and neck in the early 1970s, the technique has gained widespread acceptance and has become more available. Advances in operative microscopes, instrumentation, and microsuture manufacturing technology; understanding of surgical anatomy; and formalized training opportunities, in addition to unparalleled success rates, have been largely responsible for this rapid growth. Because of the additional resources and training required (e.g., microvascular reconstructive surgery fellowships), the majority of these complex procedures have been completed in larger, academic medical centers.
A multidisciplinary team approach, especially for patients with neoplastic disease, is commonly used to maximize both the oncologic and reconstructive efforts. Postoperative management is often labor intensive and requires additional team members to achieve maximal rehabilitative potential. Although significant additional training and resources are required to perform free tissue transfer for head and neck reconstruction, the diversity of available tissues for harvest allows for the replacement of tissue with like tissue in most instances, with a focus on functional and cosmetic issues. Currently, more than 40 identified sites in the human body are candidates for the harvest of tissue for transfer, with new sites being added regularly. It becomes the reconstructive surgeon's mandate to determine the optimal flap for free tissue transfer and the anticipated defect for each patient. The advantages of free tissue transfer in the reconstruction of these complex defects include the following:
Immediate reconstruction at the time of tumor resection (avoiding multiple, staged procedures)
The transfer of well-vascularized tissue into a bed that often has salivary contamination and underlying tissue damage from earlier radiotherapy
Significantly improved wound healing
Immediate separation of critical compartments, including the aerodigestive tract and intracranial contents
Improved ability to manage massive defects that might otherwise preclude surgical resection of tumors
Better prospects for functional and cosmetic rehabilitation
Specific circumstances exist in which reconstruction using free tissue transfer has become the standard of care in most communities. These include oromandibular composite defects, particularly those that involve the anterior mandibular arch, subtotal or total pharyngoesophageal defects, and defects that result from resection of recurrent carcinoma failing organ-preservation nonsurgical therapy (combined chemotherapy and radiation therapy). These situations have traditionally been the most challenging in head and neck reconstructive surgery and have been associated with high complication and failure rates. The introduction of free tissue transfer for reconstruction of these defects has dramatically improved the overall course for these difficult cases.
This chapter reviews the indications, patient selection, and preparation for free tissue transfer as well as the dominant “free flaps” in use today for head and neck reconstruction. Perioperative issues, management of complications, and future directions are also discussed.
The first clinical application of free tissue transfer was in 1959 by Seidenberg and colleagues, who reported transfer of a free jejunal segment for cervical esophageal reconstruction. In 1961, Hiebert and Cummings transferred and revascularized a portion of the gastric antrum for pharyngoesophageal reconstruction. Other reports of the clinical application of free tissue transfer techniques began to appear in the literature in the early 1960s. The first reports of oral cavity reconstruction were made in the early 1970s by Kaplan and associates, who utilized a free groin flap, and Harii and coworkers, who described the microvascular distant transfer of a deltopectoral flap. Panje and associates at the University of Iowa are credited with being the first otolaryngologists to report the use of free tissue transfer for the reconstruction of the oral cavity, in 1976. Subsequent years saw rapid implementation of free tissue transfer in head and neck reconstruction by both plastic surgeons and otolaryngologists. Otolaryngologist pioneers such as William Panje, Mark Urken, Richard Hayden, and Michael Sullivan worked to establish head and neck microvascular reconstructive surgery as an important part of the specialty of otolaryngologic head and neck surgery.
With the recognition of the endless possibilities afforded by free tissue transfer came the progressive study and identification of vascular supply patterns and territories that could be potential donor sites. Vascular injection studies identified axial vessels and the presence of perforating vessels passing through muscles to supply the overlying subcutaneous tissues and skin. Identification of axial vessels that supply fasciocutaneous tissues without muscular perforators followed.
Ian G. Taylor and associates reported the first vascularized bone graft using a fibula for reconstruction of long bone injuries in 1975, and Hidalgo adapted the osteocutaneous fibula flap for mandibular reconstruction in 1989. Dye-injection studies proved a reliable vascular supply to the ilium from the deep circumflex iliac artery for vascularized bone transfer, and cadaver dissections identified the vascular branch of the circumflex scapular artery to the scapula, allowing vascularized scapula transfer. Throughout the 1980s, a progressive development and refinement of these and other vascularized bone flaps for oromandibular reconstruction have significantly expanded the availability of this technique.
Free tissue transfer is just one of many options available to the reconstructive surgeon. Well-established methods of primary closure, skin grafting, local flaps, and pedicled fasciocutaneous or musculocutaneous flaps all remain viable and necessary tools in the surgeon's armamentarium. The indications for free tissue transfer continue to evolve with experience and as additional donor site options have emerged. The etiologies of defects that may require free tissue transfer are listed in Box 78.1 . The advantages of free tissue transfer over less sophisticated techniques have become more apparent with greater experience; they are listed, along with the method's disadvantages, in Box 78.2 . The tremendous versatility offered by multiple potential donor sites can test the surgeon's imagination for new reconstructive methods. The result has been a progressive evolution of new ways to tackle old problems. Certainly, the driving force behind the expansion of free tissue transfer has been the potential for dramatically improved functional outcomes and a remarkable success rate in the head and neck of more than 90%. For some situations, such as reconstruction of the oral cavity, free tissue transfer offers a significant improvement from a functional and cosmetic standpoint over previously available techniques, particularly for composite defects. In other situations for which no reconstructive options had been available, such as large skull base and scalp defects, free tissue transfer offers excellent reconstructive possibilities. Clinical situations that commonly require free tissue transfer for reconstruction in the head and neck are listed in Box 78.3 .
Neoplasia
Trauma
Congenital defects
Infection, osteoradionecrosis
Secondary reconstructions
Versatility in tissue (skin, muscle, bone, and nerves)
Versatility in orientation (no limitations on flap “reach” as with pedicled flaps)
Restoration of shape, function, and sensation
Single operation for complex reconstructions
Multiple potential donor sites available
Simultaneous resection and flap harvest possible
Donor sites out of field of prior treatment
Extensive amounts of tissue available for large or massive defects
Postoperative irradiation tolerance
Independent blood supply for compromised tissue beds
Improved function and cosmesis
Dental rehabilitation possible
High success rates (>90%), including for bony reconstruction
Only available option for some patients
Increased technical difficulty (additional training required)
Two surgical teams required (surgeons and nurses)
Expensive instrumentation
Longer operation times
More intensive postoperative management
Donor site morbidity
Composite defects of the oral cavity
Three-layer (through-and-through) defects of the oral cavity
Total or near-total pharyngoesophageal defects
Extensive skull base defects
Extensive scalp defects
Massive defects not readily addressed with other techniques
Lack of other reconstructive options (failures or patient limitations)
Salvage surgery for chemoradiation failures
As with any new surgical procedure or technique, a significant learning curve exists. As a result, these procedures have been available primarily at major academic medical centers, and multiple fellowship training programs have been developed to safely convey the necessary knowledge and experience through advanced training. From a medical center perspective, a high level of investment in personnel and equipment is necessary. Anesthesia support is critical, and additional training and experience for dedicated microsurgical operating room nurses are also advised. The postoperative management of patients undergoing free tissue transfer is certainly more complex in comparison with that for most patients undergoing head and neck surgery, and therefore, the demands on personnel are more intensive. The overall cost of free tissue transfer has been examined and compared with the cost of more conventional (pedicled flap) techniques. The higher operation costs for free tissue transfer that result from longer operating room times and increased instrumentation are offset by the significantly shorter lengths of hospitalization. The resulting overall cost of free tissue transfer is either comparable to or less than the cost of pedicled flap reconstruction.
Although more than 40 donor sites for free tissue transfer have been described, only a handful have been found to be consistently useful in the routine reconstruction of head and neck defects. The dominant free tissue transfer flaps in use today are shown in Table 78.1 . In fact, we have found that the radial forearm fasciocutaneous, radial forearm osteocutaneous, and fibula osteocutaneous free flaps are utilized in more than 80% of cases of head and neck reconstruction. Disa and colleagues reported that forearm, fibula, rectus, and jejunum flaps accounted for 92% of free flaps they used for head and neck reconstruction. This distribution will certainly vary according to the makeup of the surgeon's practice and the surgeon's preferences and experiences. In recent years, anterolateral flap has gained popularity and is commonly used in reconstructions of large soft tissue defects previously fulfilled by rectus free flap. Varying degrees of vastus lateralis muscle may be included, according to perforator anatomy and configuration.
| Flap | Artery | Vein | Nerve | Reconstruction Uses |
|---|---|---|---|---|
| F asciocutaneous F laps | ||||
| Radial forearm | Radial | Venae comitantes or cephalic vein | Medial and lateral antebrachial cutaneous | Oral cavity, tongue, palate, nose, face, scalp, lip, pharynx, and larynx |
| Ulnar forearm | Ulnar | Venae comitantes or cephalic vein | Medial and lateral antebrachial cutaneous | Oral cavity, tongue, palate, nose, face, scalp, lip, pharynx, larynx, and cervical esophagus |
| Lateral arm | Posterior radial collateral | Posterior radial collateral | Posterior cutaneous nerve of the forearm | Oral cavity, tongue, palate, pharynx |
| Lateral thigh | Deep femoral | Venae comitantes | Lateral femoral cutaneous | Oral cavity, tongue, palate, and pharynx |
| Anterolateral thigh | Descending branch, lateral circumflex femoral | Venae comitantes | Lateral femoral cutaneous | Oral cavity, tongue, palate, pharynx, larynx, and cervical esophagus |
| Scapular-parascapular | Subscapular | Subscapular | None | Oral cavity, tongue, palate, pharynx, face, and lip |
| M uscle or M yocutaneous F laps | ||||
| Rectus abdominis | Deep inferior epigastric | Deep inferior epigastric | Intercostals (mixed motor and sensory) | Skull base and total glossectomy |
| Latissimus | Subscapular | Subscapular | Thoracodorsal | Skull base and scalp |
| O steocutaneous F laps | ||||
| Fibula | Peroneal | Peroneal | Lateral sural cutaneous | Mandible reconstruction |
| Radius | Radial | Venae comitantes or cephalic vein | Medial or lateral antebrachial cutaneous | Mandible and midface |
| Scapula | Subscapular | Subscapular | None | Mandible and midface |
| Iliac crest | Deep circumflex iliac | Deep circumflex iliac | None | Mandible and midface |
| O ther F laps | ||||
| Jejunum | Superior mesenteric branch | Superior mesenteric branch | None | Pharyngoesophageal reconstruction |
| Omentum | Gastroepiploic | Gastroepiploic | None | Scalp coverage |
| Temporal-parietal | Superficial temporal | Superficial temporal | None | Bone and cartilage coverage |
Nonetheless, it is important for the reconstructive surgeon to be familiar with a full array of flaps to manage the more difficult or unusual situations. Although several flap options are available for most defects, the surgeon must choose one on the basis of critical characteristics, such as those listed in Box 78.4 . Additionally, a thorough donor site evaluation and assessment of long-term donor site morbidity must be considered. A complete discussion of each of these flaps is beyond the scope of this chapter; however, some general comments are warranted.
Skin and soft tissue volume, bulk, and color
Pedicle length and vessel caliber
Innervation capacity (sensory motor)
Bone quality, quantity, and availability
Donor site location to allow concurrent resection and harvest
Donor site morbidity (dysfunction and cosmetic deformity)
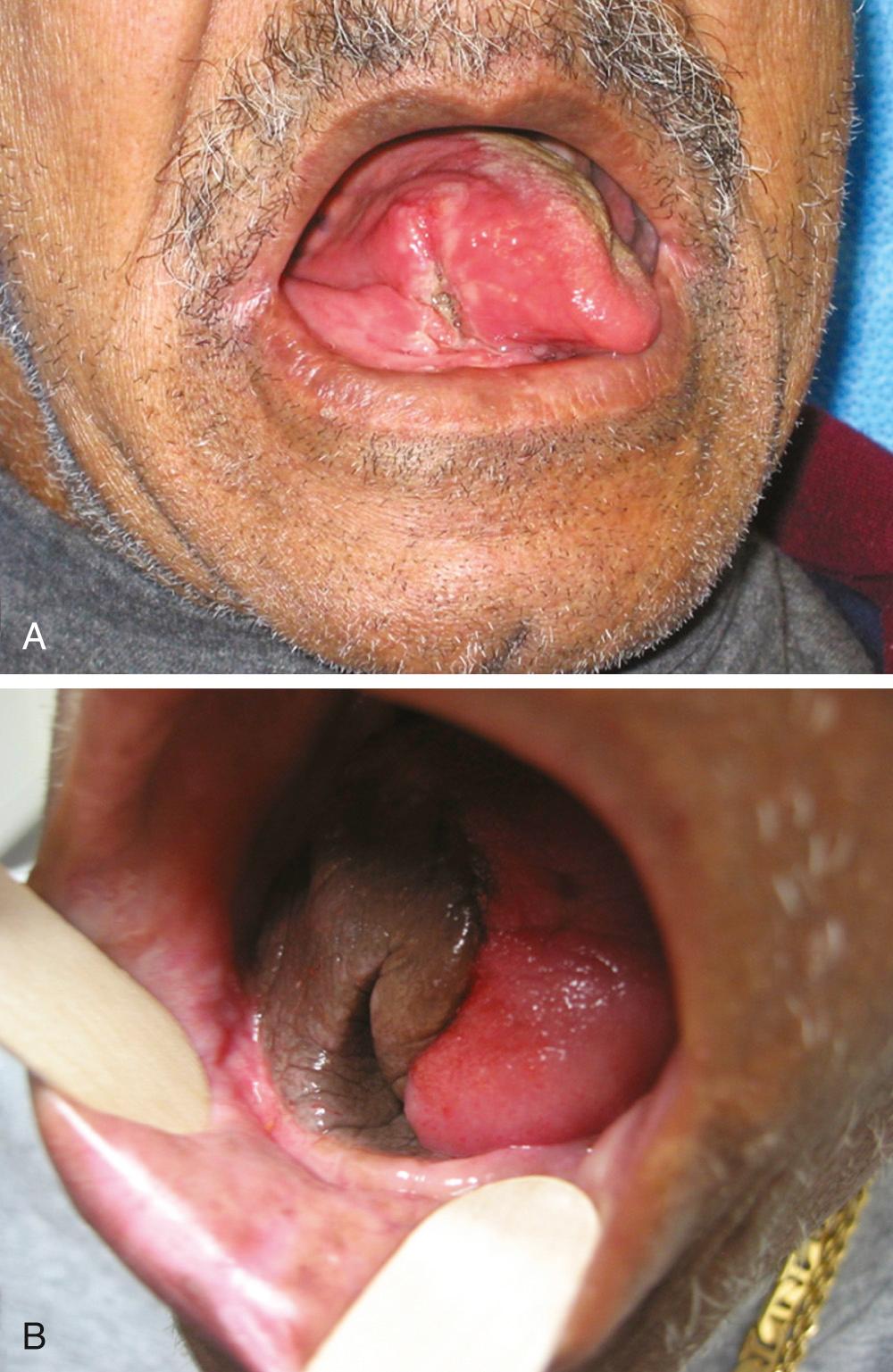
For many cutaneous, mucosal, and soft tissue defects in the head and neck, the radial forearm fasciocutaneous free flap has proved to be the most versatile and reliable. Forearm skin is available in large quantities, is thin and pliable, and has excellent sensory capability that is ideal for oral cavity reconstruction ( Fig. 78.1 ). The vascular pedicle is long, has a favorable vessel caliber, can easily be harvested concurrently, and exhibits acceptable functional morbidity at the donor site. However, up to 12% of people have an incomplete superficial palmar arch and poor communication between the deep and superficial arches, which precludes the safe harvest of the radial artery without causing hand ischemia.
The lateral arm flap is similarly thin and pliable, with sensory capability, but is limited in skin quantity, has a much shorter pedicle, and has vessels of small caliber. The scapular and parascapular fasciocutaneous flaps produce the largest volume of skin but are quite bulky, have no sensory capability, and have intermediate pedicle length with large vessel caliber. Although the donor site may be closed primarily, the donor site location (back) for these two flaps requires intraoperative patient repositioning, which precludes simultaneous resection and harvest.
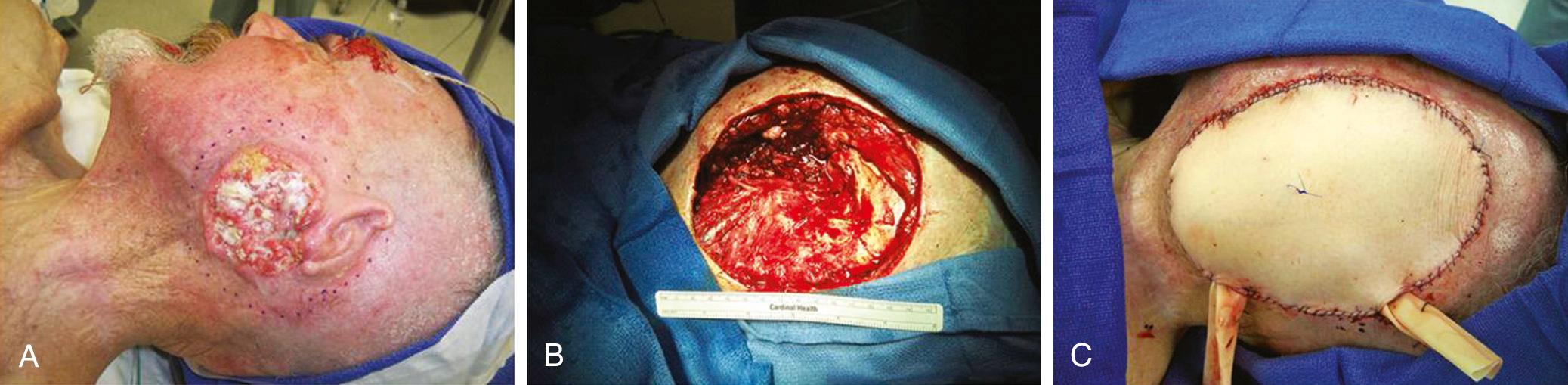
In order to maximize color match, eliminate tissue bulk, and streamline the process of simultaneous flap harvest, other donor site choices, such as the anterolateral thigh flap, have been popularized. Based on the perforators from the descending branch of the lateral circumflex femoral artery, the anterolateral thigh flap has been used extensively as a flap of choice for defects that require more soft tissue bulk than a radial forearm free flap (RFFF) can provide (see Table 78.1 ). Peripheral vascular disease is not a contraindication to the use of the anterolateral thigh flap, because the profundus femoris arterial system is not usually affected by atherosclerotic changes. A pedicle length up to 14 cm could be obtained by ligation of the transverse branch of the lateral circumflex femoral artery and the branch to the rectus femoris muscle; this may allow access to the neck vessels contralateral to the defect, if the ipsilateral neck has no suitable vessels. A skin paddle as wide as 12 cm may be harvested while still allowing primary closure of the donor site. Overall, this flap may allow harvest of a skin surface area up to 25 cm wide and up to 40 cm long as a single paddle or as multiple skin paddles ( Fig. 78.2 ). The two main reasons that the anterolateral thigh flap was initially slow in gaining popularity in the United States are the thickness of the subcutaneous tissue of the thigh in the Western population and the highly variable vascular anatomy of the flap. However, the negligible donor site morbidity, primary closure of the donor site, and significant experience gained by microvascular surgeons in harvesting and thinning the flap have led to popularization of the anterolateral thigh flap in many U.S. centers. Some writers describe using a handheld Doppler probe to identify perforators preoperatively before designing the skin paddle.
A lateral thigh fasciocutaneous flap based on the third and fourth perforators of the profunda femoris artery has been used with success in oral cavity and pharyngoesophageal reconstruction. It has the additional advantages of minimal donor site morbidity and ease of harvest in the supine, prone, or lateral decubitus position, which enables a two-team approach to resection and reconstruction.
Muscle and myocutaneous flaps have found a niche in the reconstruction of very large defects that require significant bulk. The most common of these defects are found at the base of the skull, where large portions of the facial skeleton, paranasal sinuses, facial skin, and palate may be missing. The rectus abdominis flap may be harvested as a muscle or myocutaneous flap with the patient in a supine position; a long vascularized pedicle of large-caliber vessels provides adequate reach to the defect site in most instances. The skin of the rectus myocutaneous flap is useful for lining the nasal and oral cavities of the defect, and the muscle provides the needed bulk to replace the missing facial skeleton. Donor site morbidity is minimal, although abdominal hernias may occur as a result of a weakening of the abdominal wall from the partial harvest of the anterior rectus sheath.
The latissimus flap may be harvested as a muscle or myocutaneous flap based on the subscapular vascular system. Originally described as a pedicled flap, the latissimus flap has been found to be much more versatile as a single-stage, free tissue transfer. The large amount of muscle available for harvest is useful for very large soft tissue defects, including those of the skull base and scalp. The subscapular system has additional versatility, with the option for vascularized bone (scapula bone or rib). The vascular pedicle has large vessel caliber and a length of 6 to 10 cm, and the donor site is repaired primarily without the need for a skin graft. A surmountable disadvantage of the latissimus flap is patient positioning for harvest. As with scapula flaps, the patient must be placed in the lateral decubitus position, making harvest concurrent with the head and neck procedure, depending on location, more difficult. The donor site morbidity from the loss of latissimus function is well tolerated in most people. The latissimus flap has been most useful in skull base defects and larger scalp defects that require cranial coverage.
One of the primary indications for free tissue transfer is the reconstruction of oromandibular defects. In this setting, no other reconstructive option offers a single-stage procedure with a greater than 90% success rate. The choices for vascularized bone flaps also dictate the need for careful evaluation and selection. Most bone reconstructions also require some soft tissue and/or mucosal reconstruction. The length of bone required, plans for future dental implantation, soft tissue and innervation requirements, and donor site suitability and morbidity must all be considered.
The osteocutaneous fibula free flap has become the mainstay for mandibular reconstruction at most institutions ( Fig. 78.3 ). The fibula is touted as “the most donatable bone in the body,” with up to 25 cm of bone available for harvest and adequate bone stock to support dental implantation. With such lengths of bone available, the entire mandible may be reconstructed with vascularized bone if required. Multiple osteotomies may be performed to shape the fibula to reconstruct the anterior arch, body, angle, or ramus of the mandible, as long as the fibular periosteum is not disrupted. The septocutaneous or musculocutaneous perforating vessels of the lower leg can be quite variable in location and quantity, which affects the placement and reliability of the skin paddle. The skin of the lower lateral leg is thin and pliable, and fairly large amounts of skin are available and may be transferred in a sensate fashion. With smaller skin paddles, the donor defect may be closed primarily. With larger defects, a split-thickness skin graft is utilized. The fibula flap harvest can easily be performed simultaneously with the head and neck procedure.
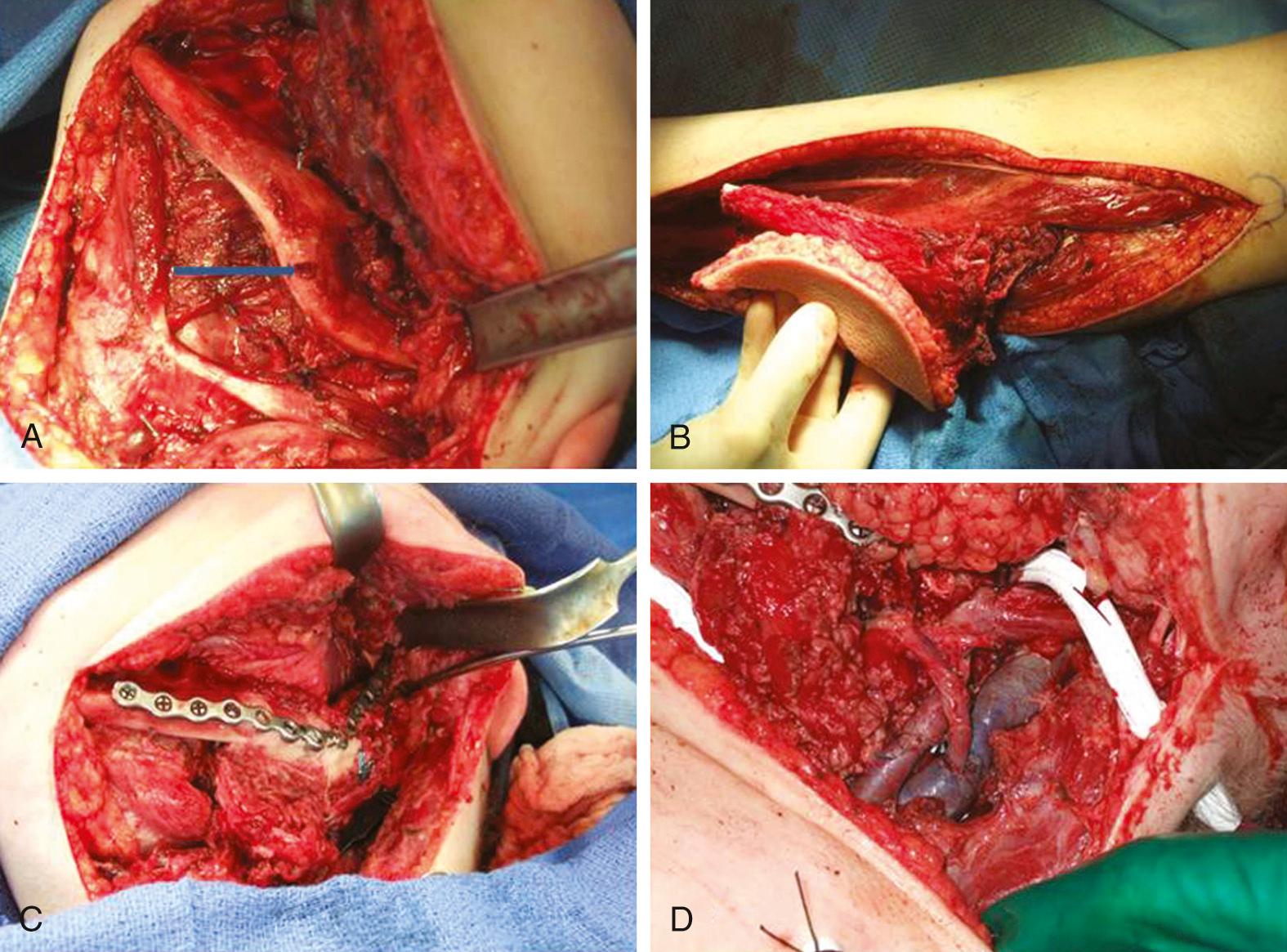
Confirmation of three-vessel flow to the distal lower extremity should be determined preoperatively to avoid vascular compromise of the foot after harvest of the peroneal artery. The primary disadvantage of the fibula flap is due to the limitations of the skin paddle: the skin is inadequate for larger soft tissue defects and most three-layer defects, which requires a second flap for soft tissue repair. The somewhat unreliable nature of the presence and location of the cutaneous perforator vascular supply can usually be minimized by inclusion of a cuff of soleus muscle with the flap, to include musculocutaneous perforators. If dental implants are not planned, use of the fibula bone results in a very broad and rounded neomandible, which is quite difficult to fit for a tissue-borne prosthesis. The donor site morbidity of the fibula flap involves prolonged pain on ambulation for some patients.
The RFFF has also been described as an osteocutaneous radial forearm free flap (OCRFFF), with harvest of a portion of the radius bone based on perforators in the intermuscular septum that pass to the periosteum. This addition significantly broadened the applicability of the already widely used forearm flap in reconstructive surgery. Although the OCRFFF is seemingly the best of options, with tremendous soft tissue characteristics and an option for bone harvest, its widespread acceptance has been limited by concerns about the bone quality and possible pathologic fracture of the radius bone postoperatively.
The length of radius bone that can be safely harvested without unacceptable forearm dysfunction is limited to 10 to 12 cm ( Fig. 78.4 ). To avoid pathologic fracture, most surgeons recommend that the thickness of the harvested bone be limited to 40% of the circumference of the radius. This thickness generally does not provide adequate bone stock to support endosseous dental implants. When a segment of bone is removed from the radius, the bone is significantly weakened, especially to torsional forces; the weakening previously resulted in a postoperative pathologic radius fracture rate of up to 66%, with an average of 23%. The prophylactic internal fixation of the radius bone after OCRFFF harvest has been shown to successfully eliminate this risk ( Fig. 78.5 ).
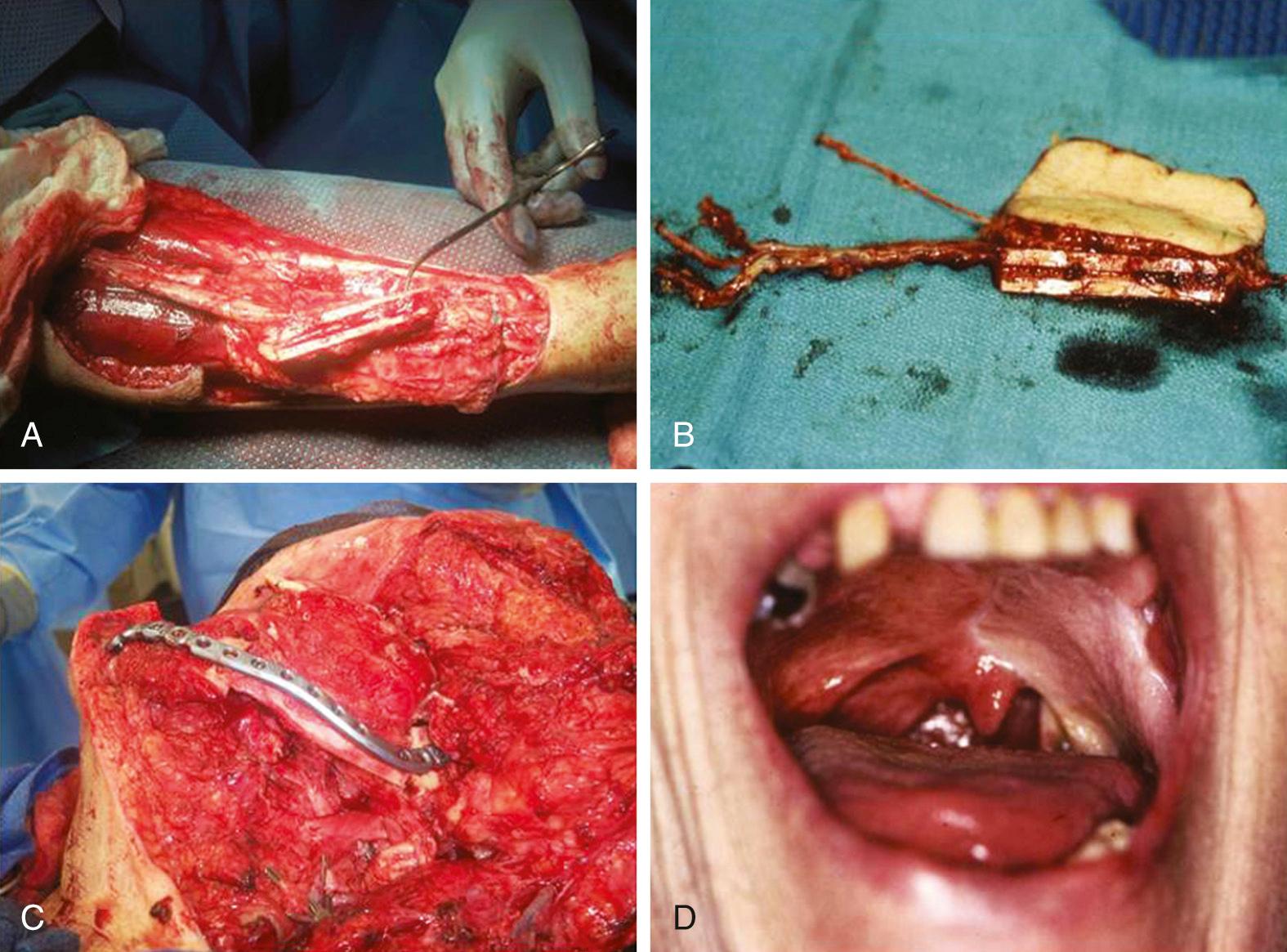
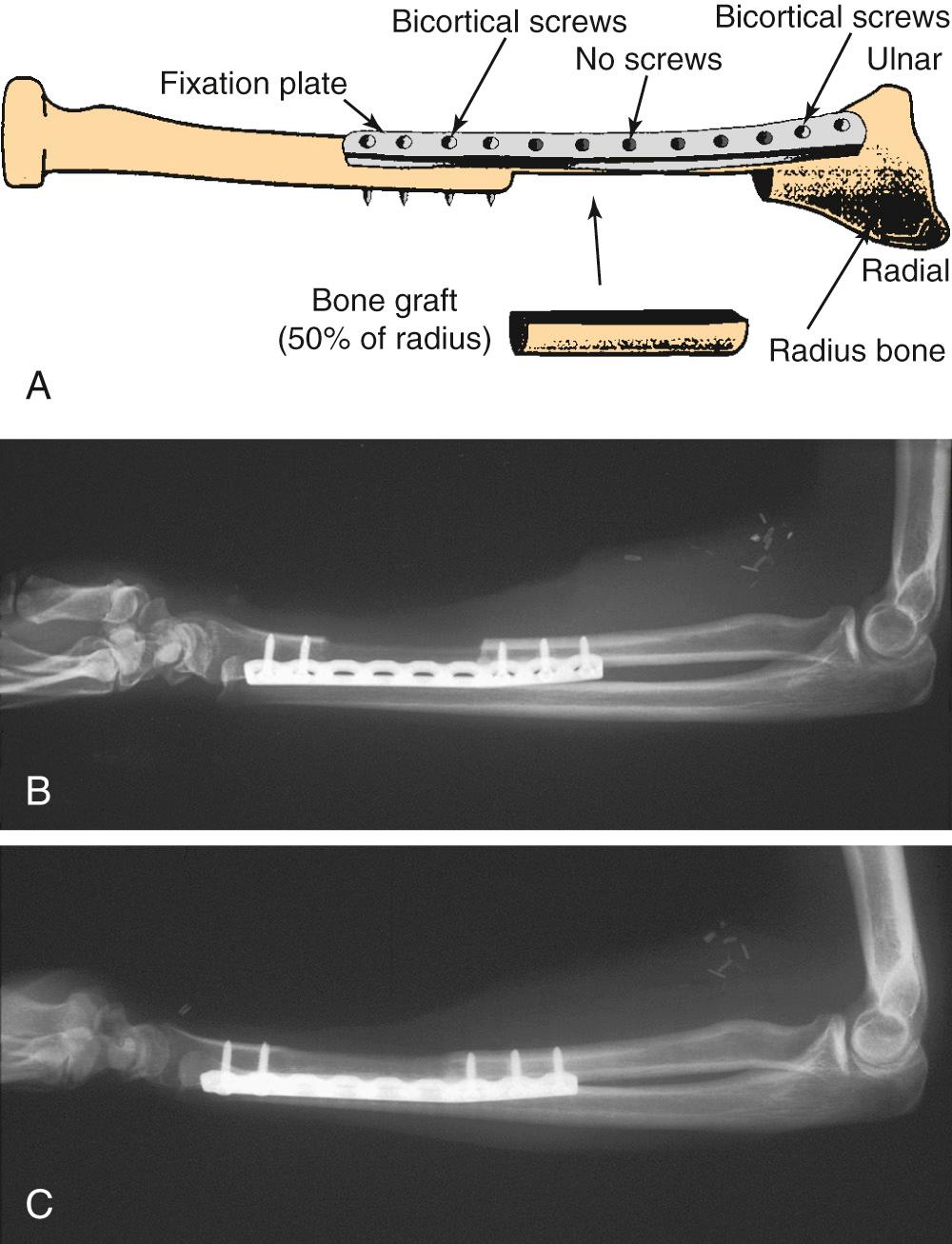
Despite the limitations of bone availability with the OCRFFF, it has been used successfully for oromandibular reconstruction with fewer complications than those associated with the fasciocutaneous RFFF with plate reconstruction. For limited mandibular defects, the radius bone is quite adequate and can easily bear a tissue-borne prosthesis (denture). This characteristic has proven beneficial, because many patients do not have the financial means for dental implantation, which is frequently not covered by many third-party payers. In this setting, the radius bone provides a better contour for the support of a tissue-borne prosthesis than either the fibula or scapula bone.
Reconstruction of maxillofacial defects is a challenging problem, owing to the complex three-dimensional, aesthetic, and functional roles of the midfacial structures. Cordeiro and Santamaria presented a classification of maxillofacial defects and suggested an algorithm for their reconstruction. A large skin paddle to line the palatal mucosal surface, small volume of the soft tissue, and moderate vascularized bone stock are the requirements fulfilled very well by a reconstruction with OCRFFF. The RFFF is ideal to solve one of the most problematic aspects of choosing the flap for a midfacial reconstruction: the need for a long (10- to 13-cm) pedicle to span the distance between the midface and cervical vessels. Using an OCRFFF eliminates the need for vein grafts and thus shortens operative time and improves the success of the reconstruction. Chepeha and colleagues expanded the use of the OCRFFF for maxillectomy defects with infraorbital rim involvement in 10 patients. They advocate the choice of OCRFFF for total maxillectomy defects that involve less than 40% of the orbital floor, with or without orbital exenteration. Rather than using the vascularized bone to reconstruct the malar alveolar ridge, the authors used osteotomized radial bone to recontour the infraorbital rim and to provide support for the orbital contents when preserved. Dental rehabilitation was achieved through the use of a palatal obturator and dentures.
The osteocutaneous scapula free flap remains one of the most versatile flaps available for harvest in the human body. The subscapular arterial system offers tremendous amounts of skin; the latissimus dorsi and serratus anterior muscles are available for harvest, as is scapular bone—all supplied by one major vascular pedicle that is favorable in both length and caliber. Each of these different tissue components has a separate vascular branching supply that allows for almost limitless degrees of orientation in relation to one another and to the recipient bed. The skin of the upper lateral back is usually quite thick and overlies considerable subcutaneous fat; this results in significant soft tissue bulk, which can be advantageous in some reconstructive situations. This bulk reliably remains on a long-term basis without atrophy. The skin can also be separated into scapular and parascapular skin paddles based on the transverse and descending branches, respectively, of the circumflex scapular artery. Inclusion of the dorsal cutaneous rami of the T1 or T2 spinal nerve into the flap has been described, which may allow for sensate transfer of scapular cutaneous paddles. A total of 10 to 14 cm of bone is available for harvest from the lateral border of the scapula, supplied by the periosteal branch of the circumflex scapular artery. The separation of the bony and fasciocutaneous components of the flap, which results from unique vascular supplies, can be as much as 4 cm; this allows for significant versatility in hard and soft tissue orientation during reconstruction. Osteotomies may be safely performed for mandibular contouring, as long as the periosteum is preserved. The harvested scapular bone has a thick border along its free edge but transitions quickly to thin (1- to 2-cm) bone medial to the edge. The resulting limited bone stock may not be adequate for the support of endosseous dental implants.
The scapular osteocutaneous flap remains an optimal choice for reconstruction of the oromandibular complex when the surgeon is faced with large complex defects, especially those that involve a large surface area, or with full thickness composite defects that involve both the oral cavity and external soft tissues ( Fig. 78.6 ).
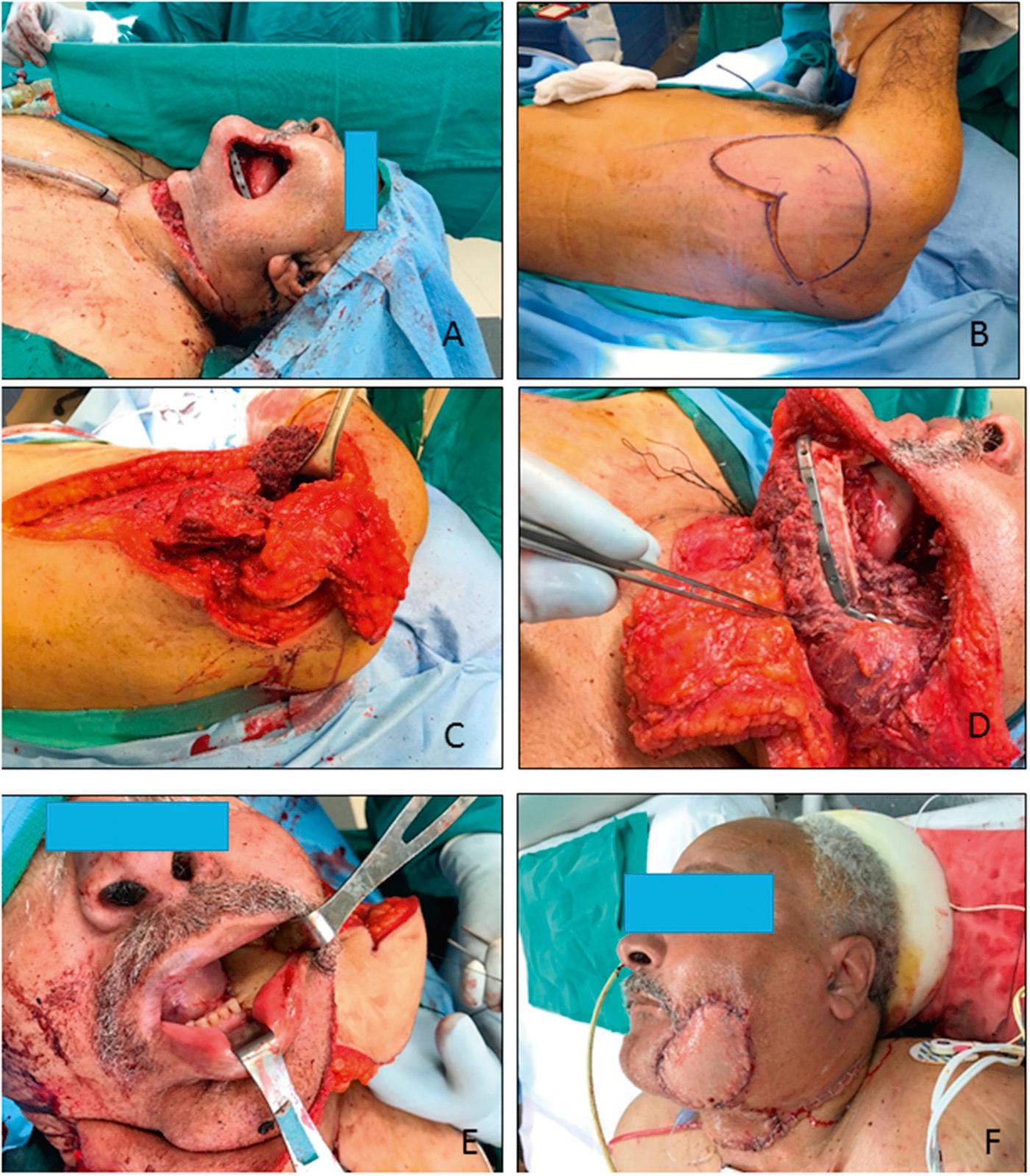
For extremely large defects, some surgeons have used the scapular “megaflap,” which includes not only the scapular bone and extensive skin, as described earlier, but also involves the latissimus dorsi muscle or the serratus anterior muscle for additional bulk and coverage. The muscles are based on the thoracodorsal artery and vein that branch off the subscapular vessels; therefore the megaflap can be harvested on the same vascular pedicle and requires only one arterial anastomosis and one venous anastomosis. The megaflap offers significant mobilization of the various tissue components relative to one another as a result of the branching vascular supply, which provides great reconstructive versatility for even the largest of defects.
The major disadvantages of the scapula flap include the bulky and insensate nature of the skin paddles and the necessity to reposition the patient several times during the operation, which adds significant time to the procedure. Long-term shoulder dysfunction can result from the harvest of the scapular osteocutaneous flap, with winging of the scapula, decreased range of motion, and chronic pain. These problems can usually be minimized with careful technique and aggressive physical therapy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here