Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Over the past 50 years, advances in technology, endoscopic techniques, and team approaches have revolutionized the abilities of surgeons to successfully treat sinonasal and skull base malignancies as well as repair skull base and orbital defects. Advancements in reconstruction using pedicled flaps have allowed surgeons to resect lesions more successfully while minimizing postoperative morbidities. In many instances, however, these pedicled flaps are not always available because of prior surgeries or compromised donor sites, or they are not appropriate because of the size and extent of the defect. The gravitational pull or rotational arc of pedicled flaps also limit the ability of a reconstructive surgeon when it comes to large or complex skull bases or orbital defects. In these cases, microvascular free tissue transfer has become a safe, reliable, and versatile method of dealing with complicated defects.
Skull base and orbital defects can occur secondary to a number of causes, including ablation of locally advanced malignancies, osteoradionecrosis with chronic infection, and trauma. The complex anatomy and nature of reconstruction have led to a variety of classification systems and methods for describing defects. Urken et al. proposed a classification system in 1993 that was based on the integrity of dura, mucosa, skin, bone, cavities (maxillary, orbital, infratemporal), nerves, and the carotid artery. Although the classification system is quite extensive with a large number of associated variables, it has not been widely adopted. In 2000, Yamamoto et al. described a classification system based on their experiences with resection, but the system was primarily based around orbital defects and was therefore mainly used for anterior skull base defects.
In 1994, Irish et al. proposed a classification system that categorized skull base lesions into three different regions. Their classification system was primarily used to describe the location of primary disease, not the defect that remained after tumor ablation. In an attempt to assist surgeons when communicating and exchanging information regarding surgical results, complications, and the advantages of different reconstructive procedures, Yano et al. described their own classification system. The classification systems of both groups are outlined as follows.
Region 1 includes the anterior defects, orbital roof, superior nasal cavity, cribriform plate, and frontal bone.
Region 2 includes lateral defects involving the infratemporal pterygopalatine fossae and communicates with the middle fossa.
Region 3 involves posterior defects, which are within or around the temporal bone and extends to the middle or posterior fossa.
Anterior skull base defects (cribriform plate is defined as the center of the defect)
Defect is localized to the cribriform plate
Defect extends horizontally from the cribriform plate to the orbital roof
Defect extends vertically from the cribriform plate to the deep part of the sinonasal cavity
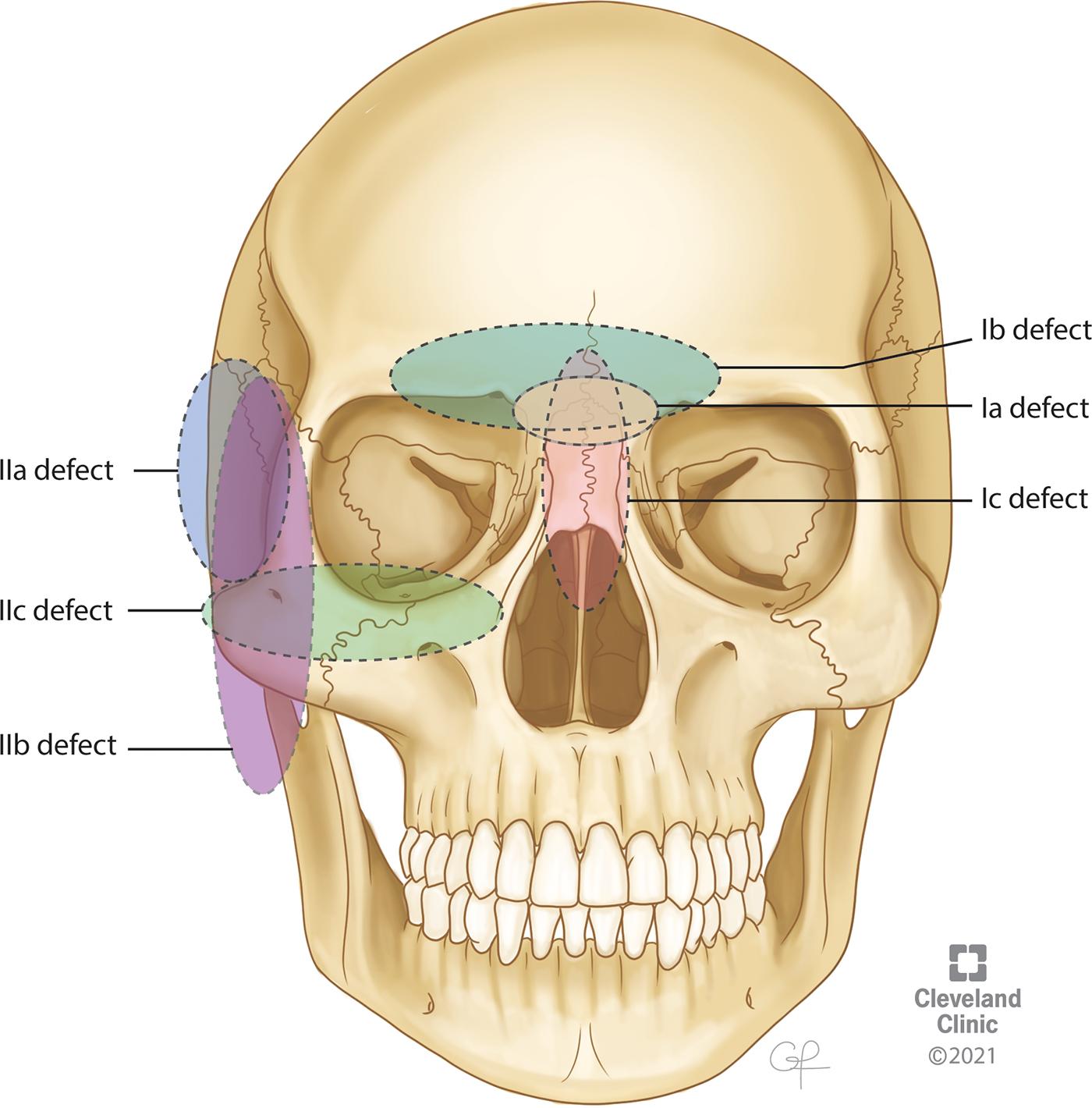
Middle skull base defects (infratemporal fossa is defined as the center of the defect)
Defect localized to the infratemporal fossa
Defect extends horizontally from the infratemporal fossa to the pterygoid muscle or mandible
Defect extends vertically from the infratemporal fossa to the maxillary sinus or epipharynx
Posterior skull base defects
Defects of the skin or orbital contents and combined defects (if a defect of the skin or orbital contents is also present, an “S” or “O” is added to each classification
The goals of a reconstructive surgeon after surgical extirpation of a skull base mass or repair of a skull base defect focus primarily on one goal: restoring the structure and function of the skull base. The skull base is one of the most complex anatomic regions of the body and is often described in relation to three distinct regions as alluded to earlier, each with its own anatomic boundaries and functions.
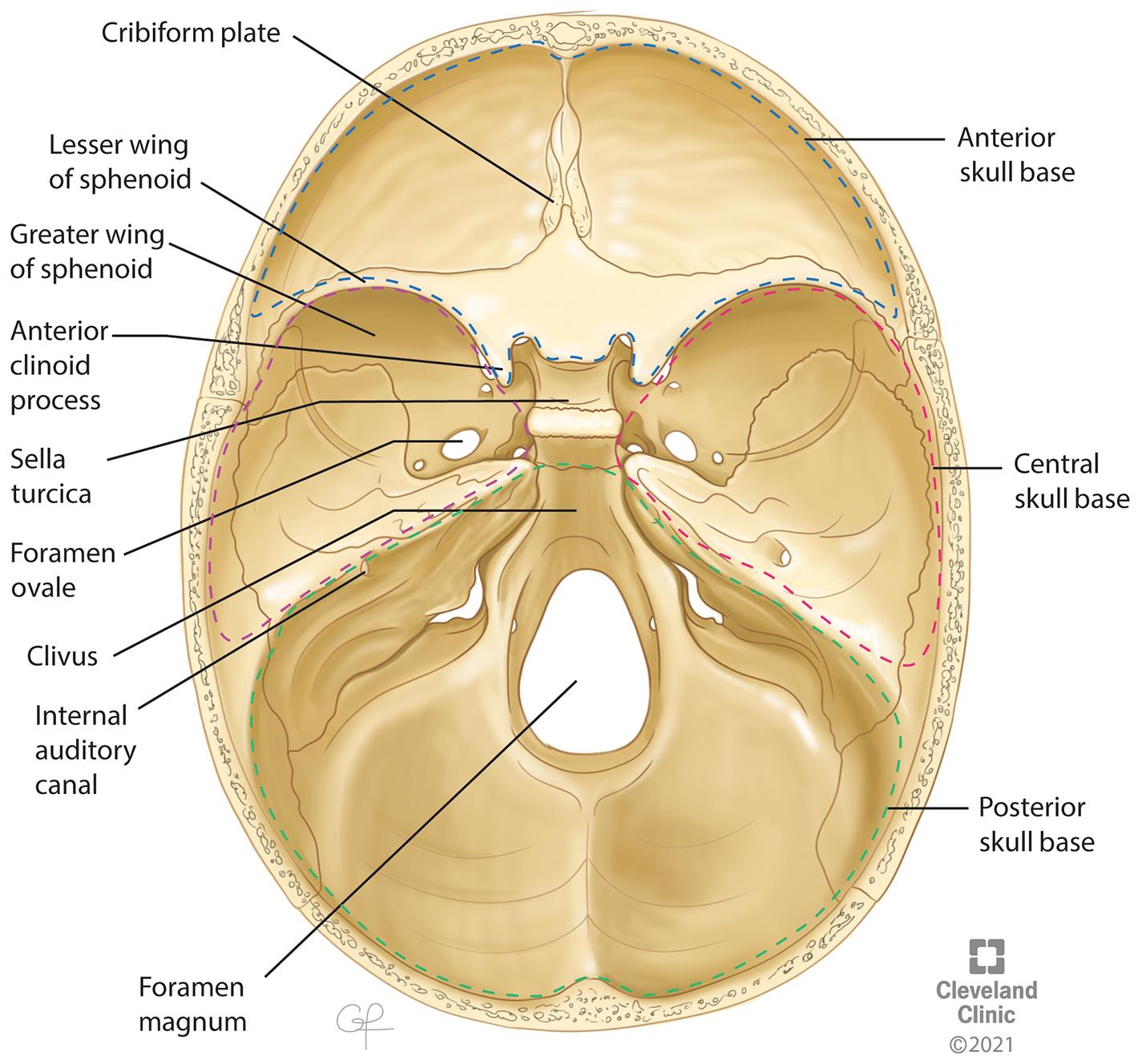
Anterior skull base
Boundaries
Formed laterally by the orbital plates of the frontal bone
Formed medially by the cribriform plate and crista galli of the ethmoid bone
Formed posteriorly by the planum sphenoidale and lesser wings of the sphenoid bone
Central skull base ,
Boundaries
The anterior border is formed by the tuberculum sellae, anterior clinoid processes, posterior margin of the lesser sphenoid wings, and anterior and superior rim of the greater sphenoid wings.
The posterior border is formed by the superior borders of the petrous part of the temporal bone and the dorsum sellae of the sphenoid.
Medially, it is formed by the body of the sphenoid bone.
Laterally, it is formed by the sphenoid triangle, squamous part of the temporal bone, and temporomandibular joint.
Posterior skull base ,
Boundaries
The anterior aspect of the posterior skull base is formed by the posterior surface of the clivus.
Laterally, the posterior skull base is formed by the posterior surfaces of the petrous temporal bone superiorly and the condylar part of the occipital bone inferiorly.
Posteriorly, the posterior skull base is formed by the mastoid temporal bone and the squamous occipital bone.
Reconstruction after ablation of malignant lesions is often challenging because of wide resection of stabilizing structures, especially skull base bone. These lesions can be associated with large dural defects and are at risk for high-flow cerebrospinal fluid (CSF) leaks as well. Reconstructive goals are multifactorial but primarily center around the following key tenets outlined next.
Provide structural support for the brain and orbit
Restoration of a watertight and airtight barrier between the intracranial and extracranial contents
Separating the central nervous system from the aerodigestive tract
Safeguard critical structures from injury, infection, and desiccation, including the brain, cranial nerves, intracranial vessels, and orbit
Bringing vascularized tissue to accelerate the healing process, especially in patients who require postoperative adjuvant radiation
Reestablishing the lining of the nasal cavity, nasopharynx, or oropharynx
Preservation of cosmesis and restoration of the three-dimensional appearance of the face and head with bone and soft tissues
The causes of skull base defects are numerous and can include external blunt force or penetrating trauma; surgical iatrogenic trauma; or skull base erosion caused by high intracranial pressure, radiotherapy, infections, or most commonly by malignancy and tumor resections. Given the anatomic complexity of the skull base, multiple factors need to be considered when reconstructing a skull base defect, especially when the defect occurs after resection of a malignant lesion. The following are some of the key factors surgeons must consider when selecting which flap to use in addressing complex reconstructions of the skull base:
Size of defect
Presence of CSF leak: high flow versus low flow
Previous treatment: radiation therapy or previous surgery
Type of pathology: whether it is aggressive, has a strong possibility of recurrence or will need to be closely monitored for recurrence
Recipient vessels available
Plan for adjuvant radiation therapy
Need for structural support and cosmesis: orbital defects or support for intracranial contents
Need to obliterate sinus
Surgical approach to tumor resection
The method of reconstruction is heavily influenced by the surgical approach used for resection of a mass in addition to the nature and location of the mass. When a mass is intradural, it can be either extra-arachnoidal or intra-arachnoidal. In extra-arachnoidal surgeries, such as pituitary surgery, CSF leaks are not always common. In intra-arachnoidal surgeries, however, a CSF leak should always be expected. These intra-arachnoidal surgeries are then further divided into whether they cause a high-flow (a cistern is directly opened) or a low-flow (no cistern is directly opened) CSF leak. Other factors also increase the risk of postoperative CSF leaks, including an obese body habitus, previous surgery or radiotherapy, Cushing disease, and high-risk pathologies (e.g., craniopharyngioma). ,
Generally, low-flow CSF leaks and smaller defects (<1 cm) can be repaired with multilayered free grafts, allografts, and dural sealants with high success rates. For larger dural defects (>3 cm), high-flow CSF leaks, wide dural or arachnoid dissection, or poorly vascularized tissue defects, vascularized tissue provides a much more successful reconstruction.
Use of microvascular free flaps for reconstruction of skull base defects has greatly improved the outcomes and cosmesis after large craniofacial and skull base tumor ablations. The discovery and utilization of different sources of donor tissue have also provided a wide array of possible flaps that each carry with them their own advantages and disadvantages. A common obstacle that all surgeons must face, however, deals not with the choice of which free flap to use but instead the availability of suitable recipient vessels for their free flaps in the area of the skull base. Defects of the anterior skull base or orbitomaxillary area are remotely located from lower branches of the external carotid system that are most commonly used during head and neck reconstruction. As such, it has become a well-known challenge to identify appropriate recipient vessels in these areas.
Historically, larger caliber vessels of the neck have proven to be the most useful and reliable in head and neck reconstruction. Certain reconstructions, however, may not allow adequate pedicle length to reach the neck; in these situations, vein grafts have been used to allow flap vessels to reach from pedicle to recipient vessels. Previous studies, however, have demonstrated an association between use of vein grafts and increased risk of flap failure. , The facial or angular and superficial temporal vascular systems are located in close proximity to the skull base, scalp, and midface and provide a unique opportunity for reliable, quick, and minimally morbid vascular access without the need for vein grafts. In a study by Revenaugh et al., they described reliable utilization of distal facial vessels, angular vessels, and superficial temporal vessels for reconstructions using minimally invasive access techniques and aesthetically favorable scar location. These methods allow for reliable recipient vessel use in areas close to the skull base. Several other studies have also described the most popular recipient vessels used during skull base reconstruction include the ipsilateral superficial temporal or facial vessels. , These vessel systems offer adequate vessel diameter and a paired arteriovenous system that can accommodate most free flap pedicles.
In some situations, traditional vessel options may be limited by previous neck dissections, radiation, or use of recipient vessels by previous surgeries. In these situations, surgeons can assess for the availability of ipsilateral transverse cervical vessels or contralateral branches of the external carotid artery, including the contralateral facial artery. In extreme circumstances of vessel depletion or reoperation, several salvage techniques have been described, including cephalic vein transposition, thoracodorsal transposition, and the use of the pedicle of a previous flap.
In most cases, however, comfort with the different available types of flaps and methods to use nearby recipient vessels gives surgeons the flexibility to use a particular flap that will provide adequate coverage of the defect without the need for vein grafting or other controversial techniques.
As endoscopic endonasal techniques have evolved over the past couple of decades, there has been an ever-changing landscape when it comes to appropriate reconstructive techniques. Endoscopic techniques, pedicled flaps, and free mucosal grafts are described separately in this book. However, there are often situations when the size and location of the skull base defect to be repaired exceeds the limits of these flaps, and in these instances, especially when open techniques are used during tumor resection, free flaps provide further options.
After the decision has been made to use a microvascular free flap as a method for reconstructing a skull base defect, the next step is to decide which donor site will be used. There have been significant advances in reconstructive techniques, leaving surgeons with many different options depending on the size, shape, and complexity of the defect. We describe commonly used donor tissues as well the risks, benefits, advantages, and disadvantages of each type of free flap.
The anterolateral thigh (ALT) free flap was first described in 1984 by Song et al. as a soft tissue flap that is perfused by the septocutaneous branches of the lateral circumflex femoral artery. Since its discovery, it has become one of the most commonly used free flaps for head and neck reconstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here