Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
How does the principal function of the kidney differ in fetal and neonatal life?
During fetal life the placenta is responsible for fetal water and electrolyte homeostasis. The principal function of the fetal kidney is the continuous excretion of water and electrolytes into the amniotic cavity, which is essential for maintenance of amniotic fluid volume. Normal amniotic fluid volume is essential for normal lung development. After birth the kidneys assume responsibility for maintenance of appropriate total body water and electrolyte homeostasis ( Fig. 9-1 ).
What is the urine flow rate in the fetus?
Urine is made by the fetus in increasing amounts as gestation advances. In fact, fetal urine output is quite high—in the range of 25% of body weight per day, approximately 750 to 1000 mL per day near term. Fetal urine, along with pulmonary secretions, is an important contributor to amniotic fluid. The process is dynamic, with amniotic fluid being produced continuously, then swallowed and reabsorbed from the gastrointestinal tract ( Fig. 9-2 ). Fetal oliguria may produce oligohydramnios. Obstruction in the gastrointestinal tract or neurologic impairment of swallowing may result in polyhydramnios.
When does nephrogenesis begin, and when is it complete?
The first definitive nephrons form at 8 weeks of gestation. Renal function adequate to sustain extrauterine life develops by approximately 23 weeks of gestation. Nephrogenesis is complete at 36 weeks.
When are the fetal kidneys detectable by ultrasound?
By 22 weeks’ gestation 95% of fetal kidneys are detectable.
What proportion of cardiac output does the fetal kidney receive?
Fetal renal blood flow (RBF) increases steadily from approximately 4% at 17 to 18 weeks of gestation to 6% at term. Adult kidneys receive between 20% and 25% of cardiac output. The low RBF in the fetus is due to high renovascular resistance caused by increased activity of the renin-angiotensis-aldosterone and sympathetic nervous systems.
How does RBF change after birth? When does RBF reach adult levels?
RBF increases sharply after birth at term to between 8% and 10% of cardiac output. Adult levels of RBF are not achieved until approximately 2 years of age.
How does glomerular filtration rate (GFR) change in fetal life and after birth?
Fetal GFR slowly increases during fetal life until approximately 36 weeks, when nephrogenesis is complete. Thereafter little increase occurs until birth, at which time there is a dramatic increase in GFR. The increase in GFR with birth is less dramatic in preterm infants ( Fig. 9-3 ).
What are the factors that cause the postnatal increase in GFR?
Increase in net filtration pressure, which is the difference between hydrostatic pressure and oncotic pressure across glomerular capillaries
Increase in the ultrafiltration coefficient, which is a function of total glomerular capillary surface area and capillary permeability per unit of surface area
What are normal values for GFR in a newborn infant?
 ) and term neonates (
) and term neonates ( ) is schematically represented.
) is schematically represented.
See Table 9-1 .
What are normal values for serum creatinine concentration ([Cr]) in a newborn infant?
| GESTATIONAL AGE | POSTNATAL AGE | ||
|---|---|---|---|
| 1 WEEK | 2-8 WEEKS | >8 WEEKS | |
| 25-28 weeks | 11.0 ± 5.4 | 15.5 ± 6.2 | 47.4 ± 21.5 |
| 29-34 weeks | 15.3 ± 5.6 | 28.7 ± 13.9 | 51.4 ± 20.7 |
| 38-42 weeks | 40.6 ± 14.8 | 65.8 ± 24.8 | 95.7 ± 21.7 |
The answer to this question is complicated. In fact, it is the change in serum [Cr]—not a single value—after birth that is relevant. At birth serum [Cr] is largely a function of maternal serum [Cr]. The subsequent change varies with gestational age. In infants younger than 30 weeks’ gestation, serum [Cr] either does not change or it increases 30% to 40% before declining to the birth level during the first 5 to 8 days of age, then subsequently declines before reaching a steady state by 7 to 10 weeks of age. The duration of the plateau is inversely related to gestational age; the rate of decline is directly related to gestational age. In infants at 30 weeks’ gestation or older, serum [Cr] declines from birth to reach a steady value at 3 to 6 weeks of age. The rate of decline is directly related to gestational age.
What determines urine output in the postnatal period?
The volume of urine in the postnatal period is determined by water and sodium intake, GFR, the ability to maintain a concentration gradient in the renal medullary interstitium, and the presence or absence of antidiuretic hormone (ADH).
What are the important differences in the regulation of sodium ion (Na + ) and potassium ion (K + ) balance?
The vast majority of total body Na + is extracellular, whereas the vast majority of total body K + is intracellular.
Serum [Na + ] is solely a function of total body water and sodium balance. Serum [K + ] is a function of internal (the distribution of K + across cell membranes) and total body (or external) potassium balance. Urinary Na + excretion is a function of the amount of Na + filtered (which depends on the GFR and serum [Na + ]) and the amount of Na + which is reabsorbed along the renal tubules. The amount of K + filtered has little effect on urinary potassium because 5% to 10% of the filtered K + is delivered to the distal nephron regardless of serum [K + ] or total body potassium balance. Urinary K + excretion, then, is a function of the amount of potassium secreted or reabsorbed in the distal nephron.
What factors influence internal K + balance?
Potassium uptake by cells is stimulated by the following:
High [K + ]
Beta 2 -adrenergic agonists
Insulin
Respiratory and metabolic alkalosis
Potassium movement from the intracellular to extracellular space is stimulated by the following:
Low [K + ]
Alpha-adrenergic agonists
Beta 2 -adrenergic antagonist
Respiratory acidosis (metabolic acidosis to a much lesser extent)
Ischemia
Cell damage
Hyperosmolaity
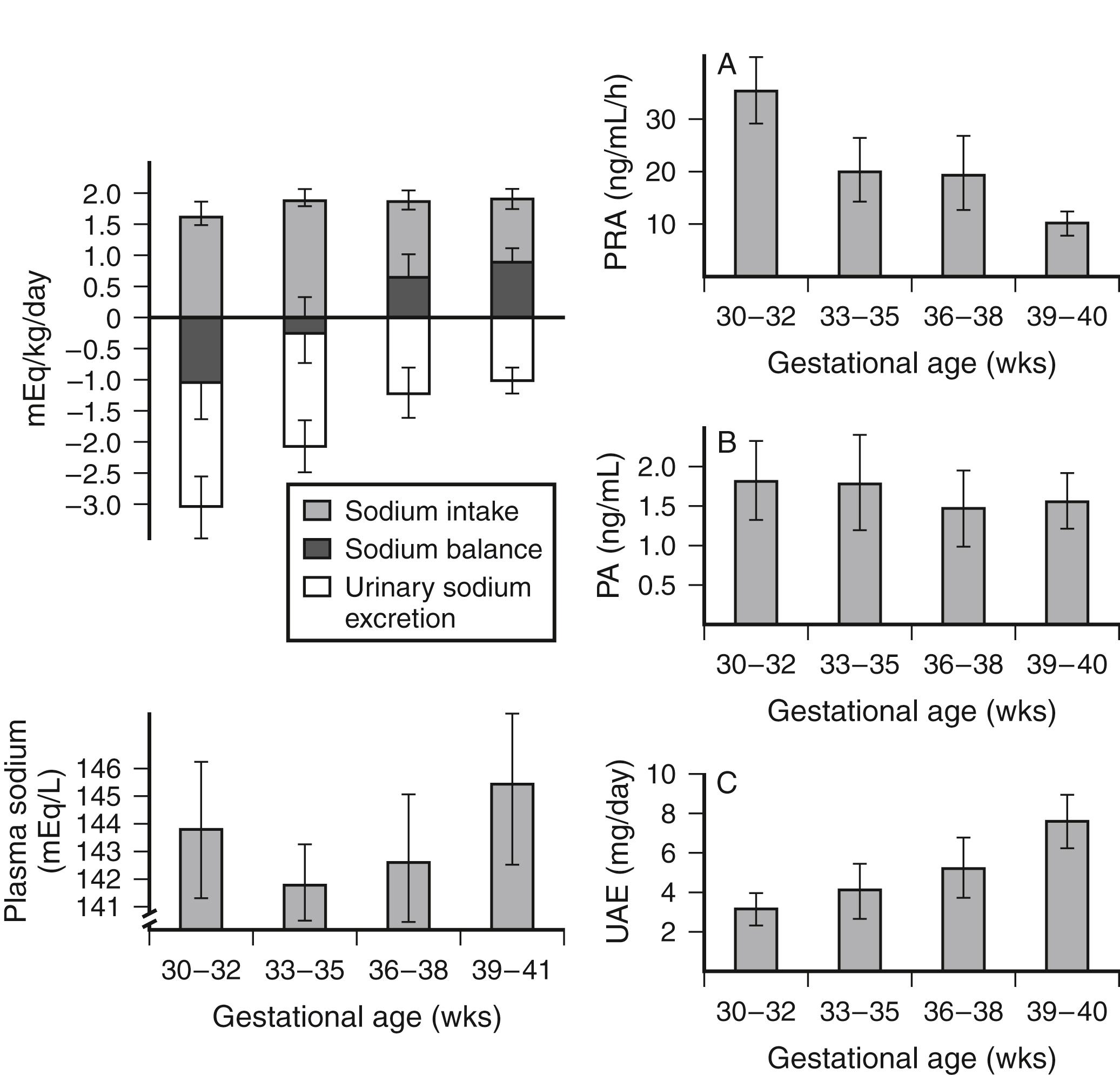
How does the capacity of preterm infants to conserve sodium differ from that of term infants?
Term infants conserve sodium effectively after the first few hours of life (after contraction of the extracellular fluid space). Preterm infants conserve sodium less effectively for the following reasons:
Their proximal tubular capacity for sodium reabsorption is limited.
Their distal tubular response to aldosterone is diminished ( Fig. 9-4 ).
If preterm infants have a limited capacity to conserve sodium, is their ability to excrete a sodium load enhanced?
No. Their ability to excrete a sodium load is limited by their low GFR.
How does the concentrating capacity of the preterm and term infant compare to that of the adult?
Concentrating ability is limited in infants for several reasons. Protein intake by the infant is used to make new cells during this period of rapid growth, and relatively little nitrogen is diverted to urea. Urea is an important component of the tonicity of the medullary interstitium and the osmolality of urine. Additional factors include (1) the relatively short loops of Henle in the neonatal nephrons that limit the surface area available for equilibration with the interstitium and (2) a high level of prostaglandins that can increase medullary blood flow and “wash out” the medullary concentration gradient. The maximum urine concentration in the preterm infant is approximately 600 mOsm/L, in the full term infant is 800 mOsm/L, and in the adult is 1500 mOsm/L.
Preterm infants have a limited capacity to excrete a free water load. Is this because they cannot dilute their urine as much as full-term infants?
Not primarily. Preterm infants are capable of diluting their urine to 75 mOmol/L, compared with that of full-term infants and adults of 50 mOsm/L. The capacity of the newborn to excrete a free water load is limited by their lower GFR.
Why do preterm infants have difficulty excreting a potassium load?
Data in animals suggest that potassium secretion by the immature distal nephron is limited by the following:
A relative paucity of K + channels in the apical membrane of principal cells in the distal nephron
Lower flow delivery to the distal tubule as the result of lower GFR
Lower sensitivity to aldosterone 1 2 3 4 5 6
1 Beall MH, van den Wijngaard J, van Germet M, et al. Water flux and amniotic fluid volume: understanding fetal water flow. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012. p. 3–18.
2 Gallini F, Maggio L, Romagnoli C, et al. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol 2000;15:119–124.
3 Gasser B, Mauss Y, Ghnassia JP, et al. A quantitative study of normal nephrogenesis in the human fetus: its implications in the natural history of kidney changes due to low obstructive uropathies. Fetal Diagn Ther 1993;8:371–84.
4 Guignard J-P, Gouyon J-B. Glomerular filtration rate in neonates. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012. p. 117–35.
5 Lorenz JM. Potassium metabolism. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012. p. 61–73.
6 Sulyok E. Renal aspects of sodium metabolism in the fetus and neonate. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012, p. 31–59.
When should the time of first voiding be considered delayed?
Ninety-seven percent of infants pass urine in the first 24 hours of life and 100% by 48 hours. During the first 2 days of life, infants urinate two to six times per day ( Table 9-2 ).
Why do preterm infants lose weight after birth?
| TERM INFANTS CUMULATIVE | PRETERM INFANTS CUMULATIVE | POSTTERM INFANTS CUMULATIVE | ||||
|---|---|---|---|---|---|---|
| # | % | # | % | # | % | |
| In delivery room | 51 | 12.9 | 17 | 21.2 | 3 | 12 |
| 1-8 hr | 151 | 51.1 | 50 | 83.7 | 4 | 38 |
| 9-16 hr | 158 | 91.1 | 12 | 98.7 | 14 | 84 |
| 17-24 hr | 35 | 100 | 1 | 100 | 4 | 100 |
∗ In 395 term infants, 80 preterm infants, and 25 postterm infants.
This decrease in weight is the result of catabolism secondary to low caloric intake and a physiologic decrease in the extracellular water (ECW) volume that is independent of caloric intake. Most premature babies manifest a natriuretic diuresis in the first few days of life, which results in negative net total body water and sodium balance and, therefore, a decrease in extracellular fluid (ECF) volume.
Why is the reduction in ECW volume in preterm infants considered physiologic?
The diuresis occurs in spite of large variation in water and sodium intake.
Relatively large differences in water and sodium intake are required to moderate this reduction.
It occurs even if caloric intake mitigates postnatal weight loss.
When the body weight initially lost postnatally is regained, the proportion of body weight that is ECW remains stable at the new lower level. Thus the decrease in extracellular volume relative to body weight in the immediate postnatal period is not a transient phenomenon.
Water and sodium intakes high enough to prevent or markedly attenuate this decrease in extracellular volume have been associated with increased morbidity in premature newborns (e.g., patent ductus arteriosus, necrotizing enterocolitis, chronic lung disease).
Which should be used, birth weight or daily weight, to calculate water and sodium requirements during the first week of life?
The clinician should use what the attending physician requests. After the first day of life, however, it is important to understand that it is the absolute fluid and electrolyte intake (milliliters or millimoles per day) relative to that in the previous 8 to 24 hours that is relevant. In other words, should the absolute fluid or electrolyte intake be more or less than it was previously? The answer depends on what fluid and electrolyte balances resulted from the previous intakes and on what water and electrolyte losses are anticipated. There is no magic amount of water per kg/day that is appropriate for all infants, even infants at the same weight, gestational age, postnatal age, and in the same environment. If the infant loses more water (and therefore weight) than you judge to be appropriate and you anticipate that water losses will remain approximately the same, the absolute amount of water (milliliters per day) given should be increased. However, if the current weight is used to calculate fluid requirements, the absolute amount of water administered may be only slightly more or even less than the amount given the day before. For example, an 860-g infant loses 110 g (approximately 13% of birth weight) in the first day of life after receiving 100 mL/kg/day (86 mL/day). You decide this rate of weight loss is too great and increase water intake by 20% to 120 mL/kg/day. Based on the current weight of 750 gm, however, this is only 90 mL/day, which is barely more than that given the previous day. If water losses remain the same, weight loss will be only slightly less over the next 24 hours despite an increase in water intake per kilogram current body weight.
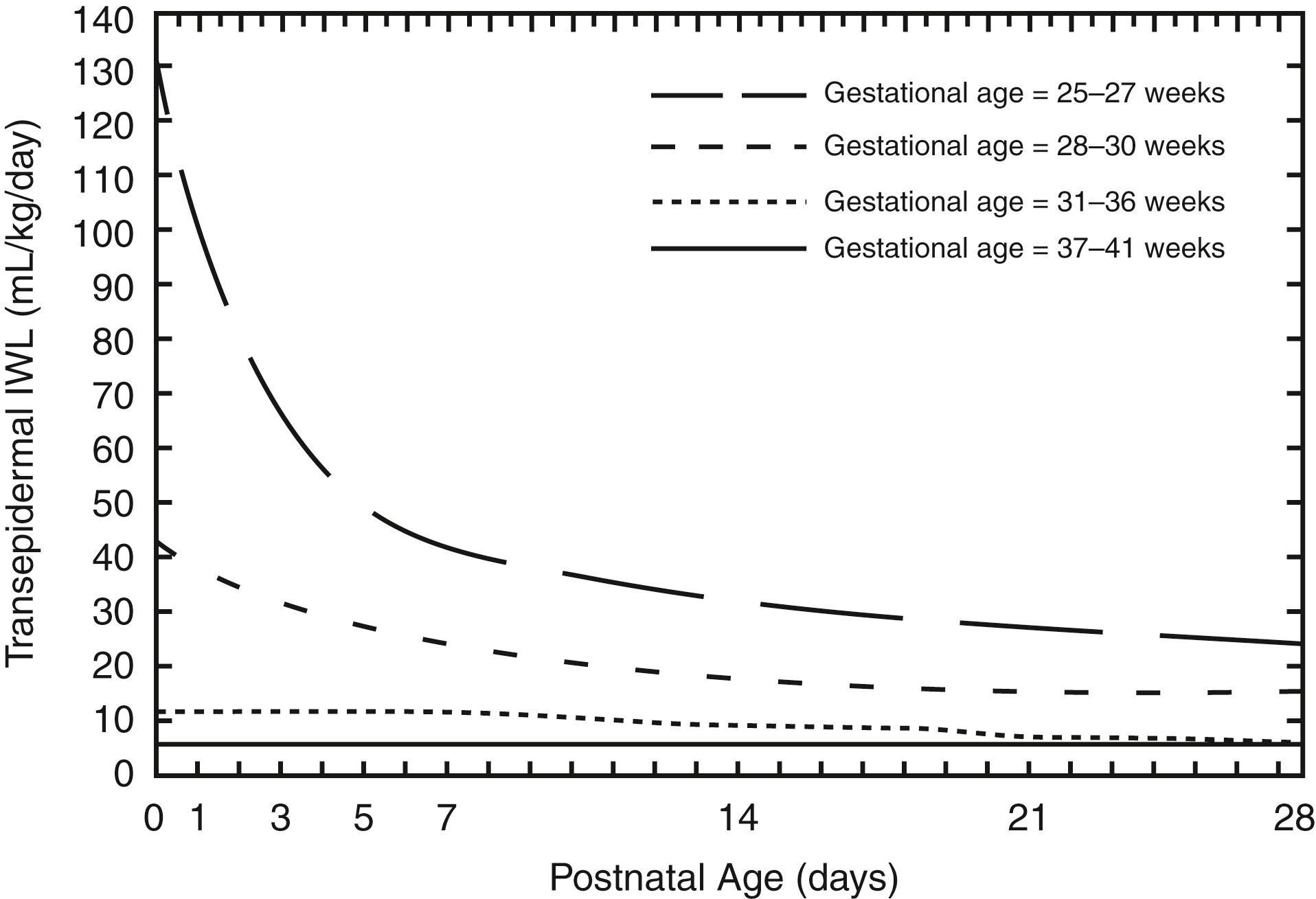
What are the main variables to consider when estimating insensible water loss (IWL)?
The most important determinants of IWL are gestational age, postnatal age, antenatal steroids, and environment. IWL decreases with increasing gestational and postnatal age ( Fig. 9-5 ), exposure to antenatal steroids, and increasing ambient humidity.
Why is accurate estimation of IWL so important in estimating fluid administration rate in preterms?
It is the major route of water loss in very preterm infants.
It is not under feedback control—there is nothing the infant can do to reduce the rate of IWL if water intake to too low.
What concentration of dextrose is needed for infants dependent on intravenous intake?
The concentration of dextrose in the intravenous solution is an irrelevant number. The relevant variable is the dextrose administration rate. Neonates normally produce 4 to 8 mg/kg/min of glucose endogenously. Administration at this rate usually maintains serum glucose concentration in the normal range and conserves glycogen stores. Once the rate of water administration is determined, a dextrose concentration is selected that provides somewhere between 4 and 8 mg/kg/min. In some infants higher glucose infusion rates may be necessary, occasionally exceeding even 12 to 14 mg/kg/min in some circumstances to maintain appropriate blood glucose levels.
Once the fluid administration rate is determined, how can the dextrose concentration necessary to provide a target dextrose administration rate be calculated?
Is there a simple way to calculate the dextrose administration rate that will be provided with a given dextrose concentration and administration rate of the intravenous fluid?
Yes.
The specimen used for bedside glucose measurement by point of care (POC) analyzers is whole blood. Is it necessary to “correct” the glucose concentration determined with point of care analyzers to reflect the plasma concentration?
No. Although plasma blood glucose concentrations are approximately 10% to 18% higher than whole blood concentrations (because the water content of plasma is higher than that of blood cells), most POC analyzers are calibrated to plasma and therefore provide plasma glucose concentrations. However, POC analyzers have limited accuracy, with a tendency to read falsely lower than the actual plasma glucose concentration. Variation from the actual plasma glucose concentration may be as much as 10 to 20 mg/dL, with the greatest variation at low glucose concentrations. Because of limitations with rapid POC testing methods, any abnormal plasma glucose concentration must be confirmed by laboratory testing.
How much sodium (Na + ) should be given on the first day of life?
In the absence of unusual Na + losses (e.g., loss of gastrointestinal or cerebrospinal fluid), no Na + should be given. Under these conditions the kidney is the principal route of Na + loss. During the first day of life, urine Na + excretion is low (0.5 to 2 mEq/kg/day). With the onset of the postnatal diuresis (when the rate of net water loss often exceeds net Na + loss), the serum sodium [Na + ] level often rises. Therefore it is usually best to withhold Na + initially, especially in extremely premature infants, in whom IWL is quite high and quite variable, therefore placing these infants at particular risk for developing significant hypernatremia in the first few days of life.
When should maintenance potassium be started in an extremely premature infant?
The main route of potassium loss is in the urine. Urine potassium losses are low initially because GFR and urine output are relatively low after birth. Moreover, serum potassium concentration ([K + ]) may rise in extremely premature infants even in the absence of exogenous potassium. Therefore potassium should be withheld until it can be ascertained that renal function is normal and, in extremely premature babies, the serum [K + ] is normal and not increasing.
Baby R is a 22-hour-old, 25-weeks’-gestation male infant in a humidified incubator. He has received 150 mL/kg/day of fluid during the first day of life. Serum sodium concentration ([Na]) is 128 mmol/L. Should sodium intake be increased?
Not necessarily. Serum [Na] is the concentration, not the amount, of sodium in the ECF space. If it is abnormally low, the amount of sodium in the ECF space is “insufficient” for the amount of water in the ECF space. Thus serum [Na] may be low because there is too little extracellular sodium, too much ECW, or both. The most common cause of hyponatremia in neonates in the first 1 or 2 days of life is excessive fluid administration. In such situations free water intake should be restricted. 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
7 Agren J, Sjors G, Sedin G. Transepidermal water loss in infants born at 24 and 25 weeks of gestation. Acta Paediatr 1998;87:1185–90.
8 American Academy of Pediatrics. Clinical report—postnatal glucose homeostasis in late-preterm and term infants. Pediatr 2011;127:575–79.
9 Bauer K, Boverman G, Roithmaier A, et al. Body composition, nutrition, and fluid balance during the first two weeks of life in preterm neonates weighing less than 1500 grams. J Pediatr 1991;118:615–20.
10 Bauer K, Versmold H. Postnatal weight loss in preterm neonates < 1599 g is due to isotonic dehydration of the extracellular volume. Acta Paediatr Scand Suppl 1989;360:37–42.
11 Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2008;1:CD000503.
12 Bell EF, Warburton D, Stonestreet BS, et al. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med 1980;302:598–604.
13 Bell EF, Warburton D, Stonestreet BS, et al. High-volume intake predisposes premature infants to necrotizing enterocolitis [letter]. Lancet 1979;2:90.
14 Clark DA. Time of first void and first stool in 500 newborns. Pediatrics 1977;60:457–59.
15 Costarino AT, Gruskay JA, Corcoran L, et al. Sodium restriction versus daily maintenance replacement in very low birth weight premature neonates: a randomized, blind therapeutic trial. J Pediatr 1992;120:99–106.
16 Dimitriou G, Kavvadia V, Marcou M, et al. Antenatal steroids and fluid balance in very low birthweight infants. Arch Dis Child 2005;90:F509–F513.
17 Guignard J-P, Drukker A. Why do newborns have elevated plasma creatinine? Pediatr 1999;103:e49. www.pediatrics.org/cgi/content/full/103/4/e49 .
18 Hammarlund K, Nilsson GE, Öberg PA, et al. Transepidermal water loss in the newborn I. Relation to ambient humidity and site of measurement and estimation of total transepidermal water loss. Acta Paediatr Scand 1977;66:553–62.
19 Hammarlund K, Sedin G, Strömberg B. Transepidermal water loss in newborn infants VIII. Relation to gestational age and post-natal age in appropriate and small for gestational age infants. Acta Paediatr Scand 1983;72:7.
20 Hartnoll G, Bétrémieux P, Modi N. Randomized controlled trial of postnatal sodium supplementation on body composition in 25 to 30 week gestation age infants. Arch Dis Child 2000;82:F24–F28.
21 Hawdon JM, Ward Platt MP, Aynsley-Green A. Prevention and management of neonatal hypoglycemia. Arch Dis Child 1994;70:F54–F65.
22 Heimler R, Doumas BT, Jendrzejcak BM, et al. Relationship between nutrition, weight change, and fluid compartments in preterm infants during the first week of life. J Pediatr 1993;122:110–14.
23 Kavvadia V, Greenough A, Dimitriou G, Forsling ML. Randomized trial of two levels of fluid input in the perinatal period—effect on fluid balance, electrolyte and metabolic disturbances in ventilated VLBW infants. Acta Paediatrica 2000;89:237–41.
24 Kelly LK, Seri I. Renal developmental physiology: relevance to clinical care. Neoreviews 2008;9:e150–e161.
25 Lorenz JM. Fluid and electrolyte therapy in the very low birth weight neonate. NeoReviews. 2008;9:e102–e108.
26 Lorenz JM, Kleinman LI, Ahmed G, et al. Phases of fluid and electrolyte homeostasis in the extremely low birth weight infant. Pediatr 1995;96:484–89.
27 Lorenz JM, Kleinman LI, Kotagal UR, et al. Water balance in very low birth weight infants: relationship to water and sodium intake and effect on outcome. J Pediatr 1982;101:423–32.
28 Omar SA, DeCristofaro JD, Agarwal BI, et al. Effect of prenatal steroids on water and sodium homeostasis in extremely low birth weight infants. Pediatr 1999;104:482–8.
29 Shaffer SG, Bradt SK, Hall RT. Postnatal changes in total body water and extracellular volume in preterm infants with respiratory distress syndrome. J Pediatr 1986;109:509–14.
30 Singhi S, Sood VI, Bhakoo NK, et al. Composition of postnatal weight loss and subsequent weight gain in preterm infants. Indian J Med Res 1995;101:157–62.
31 Stonestreet BS, Bell EF, Warburton D, et al. Renal response in low-birth-weight neonates: results of prolonged intake of two different amounts of fluid and sodium. Am J Dis Child 1983;137:215–19.
32 Shaffer SG, Bradt SK, Meade VM, et al. Extracellular fluid volume changes in very low birth weight infants during the first 2 postnatal months. J Pediatr 1987;111:124–8.
33 Shaffer SG, Meade VM. Sodium balance and extracellular volume regulation in very low birth weight infants. J Pediatr 1989;115:285–90.
34 Tammela OKT, Koivisto ME. Fluid restriction for preventing bronchopulmonary dysplasia? Reduced fluid intake during the first weeks of life improves the outcome of low-birth-weight infants. Acta Paediatrica 1992;81:207–12.
35 Van der Wagen A, Okken A, Zweens J, et al. Composition of postnatal weight loss and subsequent weight gain in small for dates newborn infants. Acta Paediatr Scand 1985;74:57–61.
36 Verma RP, Shibil S, Fang H, et al. Clinical determinants and utility of early postnatal maximum weight loss in fluid management of extremely low birth weight infants. Early Hum Dev 2009;85:59–64.
Like the lung, the premature renal function is immature; there is a diminished capacity to excrete a free water load, to maintain plasma electrolyte homeostasis, to acidify the urine, and to concentrate the urine.
All premature neonates lose weight after birth. This results from a physiologic postnatal decrease in ECW volume. However, when more than 10% to 15% of body weight is lost in the first week of life, the possible pathophysiologic process should be considered and investigated.
Sodium and potassium should not usually be given on the first day of life, but glucose is necessary. Preterm infants may also require calcium.
What is the definition of hyperkalemia in the newborn?
Hyperkalemia is defined as a serum potassium concentration that is equal to or greater than 6.7 mEq/L from a nonhemolyzed whole blood sample.
What are the two ways that hyperkalemia develops in premature infants?
Hyperkalemia is caused by perturbations in internal or external K + balance:
Internal K + balance: shift of potassium from the intracellular to extracellular space
and/or
Positive external K + balance caused by either impaired renal potassium excretion or (less commonly) excessive intake. Increased K + intake of a magnitude sufficiently severe to cause hyperkalemia is usually the result of a dosing error.
What is nonoliguric hyperkalemia?
Nonoliguric hyperkalemia is a rise in the serum potassium concentration equal to or greater than 6.7 mEq/L in the absence of a falling or low urine output.
What is the pathophysiology of nonoliguric hyperkalemia?
Nonoliguric hyperkalemia may develop in the first 24 to 36 hours of life even in the absence of potassium intake. In fact, most infants who develop nonoliguric hyperkalemia are in negative potassium balance. Therefore nonoliguric hyperkalemia is caused by a shift of potassium from the intracellular fluid to the extracellular space. Although the mechanisms responsible for this shift are unknown, infants with nonoliguric hyperkalemia are believed to have lower levels of Na + /K + ATPase. It is noteworthy that serum [K + ] increases after birth in nearly all extremely preterm infants, even those who do not develop hyperkalemia. The etiology of this shift is unknown, but it is only clinically significant in very preterm infants.
What is the incidence of nonoliguric hyperkalemia in premature infants?
When originally reported in the literature in the early 1980s, the prevalence of nonoliguric hyperkalemia ranged from 25% to 50% of infants below 1000 g birth weight or younger than 28 weeks’ gestational age. However, it is much less common now, even in infants younger than 25 weeks’ gestational age. This is probably the result of the increased prevalence of antenatal steroid therapy and more aggressive nutrition, which have been shown to reduce the risk of nonoliguric hyperkalemia.
What are the consequences of hyperkalemia in a premature infant?
Hyperkalemia increases the ratio of extracellular [K + ] to intracellular [K + ], depolarizing cells with excitable membranes, most importantly myocardial cells. This may causes bradyarythmias. These are uncommon with serum [K + ]s lower than 7 to 8 mmol/L. If serum [K + ] continues to rise, asystole may occur. However, asystole is uncommon with serum [K + ]s lower than 8 to 9 mmol/L.
What are the indications for treatment of nonoliguric hyperkalemia?
There is no consensus regarding this issue. Treatment may be considered if serum [K + ] is equal to or greater than 7 meq/L or there are electrocardiographic changes resulting from hyperkalemia. It is important to know that nonoliguric hyperkalemia normally resolves without treatment with the onset of physiologic natriuretic diuresis.
How is nonoliguric hyperkalemia managed?
Nonoliguric hyperkalemia is managed in the following ways:
By antagonizing the arrythmagenic effect of hyperkalemia
0.5 to 1 mEq/kg elemental calcium (1 to 2 mL/kg of 10% calcium gluconate solution) by slow intravenous push
Correct acidosis
By stimulating cellular uptake of potassium
Correct respiratory and metabolic acidosis
Nebulized albuterol therapy—rapid effect; effectiveness documented in extremely premature infants with nonoliguric hyperkalemia in one randomized controlled trial; experience insufficient to confirm safety
Exogenous insulin—effective but takes time to initiate, and titration of insulin and glucose to avoid hypoglycemia is difficult
By increasing renal potassium secretion
Furosemide does increase potassium excretion but is unproven in the management of hyperkalemia
Peritoneal dialysis is rarely required except with hyperkalemia caused by renal failure.
Is the use of a cation exchange resin (e.g., Kayexalate) an effective and safe way to treat hyperkalemia?
Use of this method to treat hyperkalemia is no longer considered safe and effective.
Insulin therapy has been shown as more effective in lowering serum [K + ] in extremely premature infants with nonoliguric hyperkalemia in a randomized controlled trial. Moreover, cation exchange resins have been associated with intestinal injury.
Nonoliguric hyperkalemia (potassium >6.7 mEq/L in plasma from a nonhemolyzed whole blood specimen) still occurs in extremely premature infants, but the current prevalence is less than originally reported (15% to 50%). This probably results from the increased prevalence of antenatal steroid exposure. Therefore the clinician should be particularly vigilant in checking for nonoliguric hyperkalemia in extremely preterm infants whose mothers have not received antenatal steroids before their birth.
Other causes of hyperkalemia of which to be cognizant are oliguric acute renal failure and drugs that inhibit K excretion (e.g., spironolactone and enalapril).
Hyperkalemia may be a life-threatening emergency. In this circumstance inhalation treatment with albuterol is most rapidly effective.
37 Fukuda Y, Kojima T, Ono A, et al. Factors causing hyperkalemia in premature infants. Am J Perinatol 1989;6:76–9.
38 Hu PS, Su BH and Peng CT, et al. Glucose and insulin infusion versus kayexalate for early treatment of non-oliguric hyperkalemia in very-low-birth-weight infants. Acta Paediatr Taiwan 1999;40:314.
39 Lorenz JM, Kleinman LI, Markarian K. Potassium metabolism in extremely low birth weight infants in the first week of life. J Pediatr 1997;131:81.
40 Malone TA. Glucose and insulin versus cation-exchange resin for the treatment of hyperkalemia in very low birth weight infants. J Pediatr 1991;118:121–3.
41 O’Hare FM, Molloy EJ. What is the best treatment for hyperkalemia in a preterm infant? Arch Dis Child 2008;93;174–6.
42 Omar SA, DeCristofaro JD, Agarwal BI, et al. Effect of prenatal steroids on potassium balance in extremely low birth weight neonates. Pediatr 2000;106:561–7.
43 Sato K, Kondo T, Iwao H, et al. Internal potassium shift in premature infants: cause of nonoliguric hyperkalemia. J Pediatr 1995;126:109–13.
44 Singh DS, Sadiq HF, Noguchi A, et al. Efficacy of albuterol inhalation in treatment of hyperkalemia in premature infants. J Pediatr 2002;141:16–20.
45 Yaseen H, Khalaf M, Dana A, et al. Salbutamol versus cation-exchange resin (Kayexalate) for the treatment of nonoliguric hyperkalemia in preterm infants. Am J Perinatol 2008;25:193–8.
How are acid–base measurements made in blood gas analyzers?
Hydrogen ion concentration ([H + ]) is measured potentiometrically using a complicated system that employs two electrodes (usually Ag/AgCl) designed such that the potential between them is sensitive to the [H + ] in the intervening medium. [H + ] is expressed as pH, which equals the negative logarithm of [H + ] (− log[H + ] ). Because pH is defined as the negative log [H + ], pH decreases as [H + ] increases and increases when [H + ] decreases.
Note: Unfortunately, as a result of expressing pH as −log[H + ], the proportional change in [H + ] is masked. To understand logarithm, think of “power.” Thus 10 3 = 1000 and log (1000) = 3. When the pH changes by 0.3 units (e.g., from 7.4 to 7.1), the hydrogen ion concentration nearly doubles from 40 to 79 nanoMol/L.
The partial pressure of oxygen is measured amperometrically by the Clark electrode. The reduction reaction of interest is: 4e − + O 2 + 2H 2 O = 4OH − . Current flows in linear proportion to the activity of dissolved oxygen.
The partial pressure of carbon dioxide (CO 2 ) is measured using an electrode with a semipermeable membrane that allows only CO 2 to diffuse into the electrode compartment, where it is converted to carbonic acid. The hydrogen ions produced by this reaction are then measured as previously described. The semipermeable membrane ensures that this measurement is completely independent of blood pH.
Plasma bicarbonate concentration
is not measured; it is calculated from the Henderson–Hasselbalch equation:
The concentration of carbonic acid ([H 2 CO 3 ]) is not measured, but it is proportional to the dissolved carbon dioxide (CO 2 ), with which it is in equilibrium. Dissolved carbon dioxide equals the product of the solubility coefficient of carbon dioxide in plasma at 37° C (0.031) and the partial pressure of carbon dioxide (pCO 2 ), which is measured. Therefore the
can be calculated from the measured pH and measured pCO 2 as follows:
The equation uses pK′ (the apparent pK), to account for the equilibrium between dissolved CO 2 and
. The pK′ for H 2 CO 3 dissociation in adult human plasma is 6.1.
What is base excess? How is it measured?
The blood buffer bases of human adults are plasma
, plasma proteins,
in red blood cells, and hemoglobin (Hgb). The effect of these buffers is to establish and stabilize a pH of blood at approximately 7.4.
When measured , buffer base is the number of millimoles of strong base or strong acid needed to titrate 1 L of blood (Hgb = 15 g/dL) to pH = 7.4 at 37° C while pCO 2 is held at 40 mmHg (at which point the addition of base or acid has restored the total blood buffers to normal values). Base excess (BE) in the blood (actual BE) is the difference between what actually is measured in the test sample and the sum of these values.
In clinical practice it is the BE in the extended extracellular space (red blood cells + plasma + interstitial fluid [ISF]) that is of interest. The concentration of buffer base in the ISF is lower than whole blood, primarily because ISF contains no Hgb. Therefore the algorithm used in blood gas autoanalyzers to calculate ECF BE uses a model of the blood volume diluted with ISF. There are minor variations in the algorithm used to calculate ECF BE, but the most common one is as follows:
The normal total of extracellular buffers for infants is 3 to 4 mEq/L lower, so infants normally have a base deficit in this range.
Serum  and BE are both measures of metabolic acid–base status. Which is better?
and BE are both measures of metabolic acid–base status. Which is better?
This has been subject of some debate for some time. However, BE is easier to interpret than serum
because it is less dependent on pCO 2 . When an increase in [H + ] is not due to an increase in respiratory acid (i.e., carbonic acid produced from the combination of CO 2 and H 2 O), the added H + is bound by the buffer anions and base excess falls in direct proportion to the added H + . However, when an increase in [H + ] is due to an increase in CO 2 , serum
increases (because it is in equilibrium with CO 2 ) and the other buffer anions decrease in direct proportion; thus BE remains unchanged. Therefore at a pH of 7.4, what serum
is normal depends on the measured pCO 2 ( Table 9-3 ).
Why is serum  lower (and BE mildly negative) in newborns compared with that of the adult under baseline conditions? What serum
lower (and BE mildly negative) in newborns compared with that of the adult under baseline conditions? What serum  is considered normal?
is considered normal?
| pCO 2 (mmHg) | PLASMA  (mmol/L) (mmol/L) |
|---|---|
| 20 | 12 |
| 25 | 15 |
| 30 | 18 |
| 35 | 22 |
| 40 | 24 |
| 50 | 30 |
| 60 | 36 |
The serum
in preterm infants is normally 17 ± 1.2 mEq/L, with two standard deviations encompassing values as low as 14.5 mEq/L. During the first week of life, term infants have a serum
of 20 ± 2.8 mEq/L. During the first year of life, the serum
is still only approximately 22 ± 1.9 mEq/L, compared with 23 ± 1 mEq/L and 26 ± 1 mEq/L in older children and adults.
These differences in normal serum bicarbonate levels with maturation result from the increased capacity of the mature kidney to conserve bicarbonate and to excrete [H + ]. These factors also impair the capacity of the newborn to excrete acid loads. This is the result of immaturity of carbonic anhydrase in the renal tubules and the intercalated cells of the collecting duct.
Do infants excrete more or less titratable acid and ammonia per kilogram of body weight compared with older children?
Titratable acid is a term to describe acids such as phosphoric acid and sulfuric acid, which are involved in acid excretion. It excludes ammonium (NH 4 + ) as a source of acid and is part of the calculation for net acid excretion. The titratable acid excretion rate in term infants younger than 1 month old is about one half of adult values, and the ammonium excretion rate about two thirds that of older children and adults. Preterm infants have even lower rates. After 1 month of age the net acid excretion rate in term infants is similar to that in older children and adults when expressed per 1.73 m 2 . Preterm infants also increase their rates of titratable acid and ammonium excretion with maturation, but these rates still remain lower than in term infants, even up to the age of 4 months. After 1 month of age the amount of ammonia excreted also depends on diet (ammonia production is increased in infants fed cow’s milk compared with that of infants fed breast milk).
How low can a premature infant reduce the urine pH?
During the first 3 weeks of life a premature infant can reduce the urine pH only to 6 ± 0.1. After 1 month, the urine pH can be reduced to 5.2 ± 0.4.
How can the oxygen saturation value reported with the blood gas be used clinically?
It is not useful. The oxygen saturation reported with the blood gas is calculated from the measured pO 2 using an algorithm based on the adult oxygen–Hbg desaturation curve.
In acid–base disorders in human biology, in which body compartment are measurements made? What is the significance of this?
Acid–base measurements are made in the blood, but they are the same in the ISF space. Because
is calculated from pH and pCO 2 , it represents the plasma concentration of
. As calculated by blood gas autoanalyzers, the reported BE reflects the difference between the normal and actual buffer base concentrations. However, intracellular acid–base status cannot be measured in clinical practice. Because CO 2 diffuses across cell membranes much more rapidly than
, it is possible for rapid changes in pCO 2 to change the acid–base profile of the two compartments in different directions and at different rates. When attempting to treat acid-base disorders, the clinician must consider the possible consequences, such as worsening intracellular acidosis in the face of alkalinization of the ECF with infusions of bicarbonate.
What is the difference between acidemia (or alkalemia) and acidosis (or alkalosis)?
Strictly speaking, acid emia and alkal emia refer to the actual pH of the blood relative to a pH of 7.4. A lower pH indicates acidemia; a higher pH indicates alkalemia. Acid osis and alkal osis refer to the respiratory and metabolic components of acid–base status compared with normal:
A pCO 2 greater than 40 indicates respiratory acidosis; a pCO 2 less than 40 indicates respiratory alkalosis.
A BE less than 0 mmol/L indicates metabolic acidosis; a BE greater than 0 mmol/L indicates metabolic alkalosis
What are the principal mechanisms whereby infants compensate for abnormal acid–base abnormalities?
Metabolic alkalosis compensation: hypoventilation
Metabolic acidosis compensation: hyperventilation
Respiratory acidosis compensation: increased renal absorption of bicarbonate
Respiratory alkalosis compensation: diminished renal absorption of bicarbonate
What is the importance of the volume of distribution in correcting acid–base derangements?
After a known amount of solute is introduced into a solution, the concentration is measured, and the apparent volume in which the solute is distributed is calculated. This volume is called the volume of distribution, and it is used to estimate how much solute is needed to change the measured concentration of that solute. For simple single compartments and inert solutes, the calculation measures the true volume. For multicompartment systems and unstable solutes, the solute may be distributed unevenly (i.e., either concentrated in or excluded from various compartments), metabolized, or otherwise eliminated. In these systems the calculated volume is different from the true volume in which the solute is distributed. Because of its interaction with a number of buffer systems, both intracellular and extracellular, and its elimination as CO 2 through the lungs, the volume of distribution of bicarbonate is larger than the ECF space. For metabolic acidosis in neonates the dosage should be based on the following formula if blood gases and pH measurements are available:
(mEq) = 0.3 × weight (kg) × base deficit (mEq/L). The usual dosage is 1 to 2 mEq/kg/dose.
What is the anion gap?
The anion gap is the difference in the serum between the concentrations of cations and anions. It its calculated as follows:
The [K + ] may be excluded from the calculation because wide variation in serum potassium is lethal. With [K + ] in the equation the normal anion gap is approximately 12, and without [K + ] in the equation it is approximately 8.
If there is an increase in unmeasured cations, the anion gap will be increased. If there is an increase in unmeasured anions, the anion gap will be decreased.
Is the anion gap useful in the differential diagnosis of acid–base disturbances?
Theoretically, it should be. Distinguishing between metabolic acidosis caused by bicarbonate loss and metabolic acidosis caused by increases in organic acid (an unmeasured cation) is a common and important problem. The anion gap will be normal when acidosis is caused by bicarbonate loss (because it is associated with increased renal tubular reabsorption of Cl − and therefore increases in serum [Cl − ] without change in unmeasured anions. On the other hand, when metabolic acidosis results from an increase in organic acid (e.g., lactic acid), the sum of unmeasured cations will be higher. Unfortunately, there is so much overlap between a normal anion gap (no change in unmeasured cations or anions) and abnormal anion gaps resulting from a change in unmeasured cations or anions that the anion gap is clinically useful only in cases of extreme changes in unmeasured cations and anions. Because the serum lactic acid concentration can be readily measured clinically and because it is by far the organic acid most likely to be increased, the anion gap has fallen into disuse. It can be helpful, though, with marked elevation in organic acids whose measurements are less readily available.
What do we know about the efficacy of the therapeutic use of sodium bicarbonate to correct acidemia in pediatric patients?
As a general rule, replacement of bicarbonate when body losses of bicarbonate are excessive (e.g., through the stool or urine) is appropriate. On the other hand, there is minimal evidence documenting the value of sodium bicarbonate infusions to correct acidemia resulting from many, if not most, other causes (e.g. lactic acidosis). In fact, data in animals, children, and adults suggest that correction of lactic acidosis with sodium bicarbonate infusions may be detrimental. Although it is relatively easy to make the pH of the ECF change in the desired direction, it is much more difficult to know in what direction the associated change in intracellular pH will be, which is the relevant issue for cell function. Moreover, if the cause of the acidemia is ongoing, the improvement in pH will be temporary; the primary cause of the acidemia must be corrected. Therefore sodium bicarbonate treatment should be used cautiously, if at all. 46 47 48 49 50 51 52 53 54
46 Ammari AN, Schulze KF. Uses and abuses of sodium bicarbonate in the neonatal intensive care unit. Curr Opin Pediatr 2002;14:151–6.
47 Aschner J, Poland RL. Sodium bicarbonate: basically useless therapy. Pediatr 2008;122(4):831–5.
48 Berg CS, Barnette AR, Myers BJ, et al. Sodium bicarbonate administration and outcome in preterm infants. J Pediatr 2010;157:684–7.
49 Higgins C. An introduction to acid-base balance in health and disease. June 2004. Available at http://www.acutecaretesting.org/en/articles/an-introduction-to-acidbase-balance-in-health-and-disease . Accessed August 4, 2012.
50 Higgins C. Lactate and lactic acid. Oct 2007. Available at http://acutecaretesting.org/en/articles/lactate-and-lactic-acidosisosis . Accessed Aug 4, 2012.
51 Kecskes ZB, Davies MW. Rapid correction of early metabolic acidaemia in comparison with placebo, no intervention or slow correction in LBW infants. Cochrane Database Sys Rev. 2009;CD002976.
52 Kofstad J. All about base excess—to BE or not to BE. July 2003. http://www.acutecaretesting.org/en/articles/all-about-base-excess--to-be-or-not-to-be . Accessed August 4, 2012.
53 Lorenz JM, Kleinman LI, Markarian K, et al. Serum anion gap in the differential diagnosis of metabolic acidosis in critically ill newborns. J Pediatr 1999;135:751–5.
54 Seri I. Acid-base homeostasis in the fetus and newborn. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012. p. 105–12.
A primary acid–base disturbance is always defined by the pH. A pH less than 7.3 indicates a primary acidemia, whereas a pH greater than 7.5 indicates a primary alkalemia.
The clinician must then turn to the pCO 2 ,
, or base excess, and the underlying clinical situation to define the the primary disturbance.
Sodium bicarbonate should be used sparingly to treat acidemia. The clinician should never administer bicarbonate until the ability to excrete carbon dioxide is ensured; otherwise, the clinical acid–base disturbance may be worsened.
Diuretics therapy may be complicated by hyponatremia. How should hyponatremia be managed in this situation?
Hyponatremia is the result of too little sodium relative to water in the ECW compartment; therefore it may be caused by a deficit of sodium or an excess of water in the ECW compartment. The primary action of loop diuretics is to inhibit chloride (and thereby sodium) reabsorption in the loop of Henle. Therefore hyponatremia with loop diuretics could be caused by a sodium deficit resulting from excessive diuretic therapy or from excessive administration of free water. The former is managed by decreasing the frequency of loop diuretic administration, the latter by decreasing free water intake.
What damage can chronic administrative loop diuretics inflict on the kidneys, urinary tract, or both?
Loop diuretics induce hypercalciuria by inhibiting renal tubular calcium reabsorption. Therefore chronic administration of these agents can cause nephrocalcinosis, calcium nephrolithiasis, or both.
What is the best way to treat the hypokalemic, hypochloremic metabolic alkalosis that occurs in newborns who receive chronic diuretic therapy?
Diuretics cause metabolic alkalosis by increasing potassium secretion. This results from the increased delivery of water and sodium to the distal tubule and is treated with potassium chloride (KCl) supplementation. Diuretic therapy also induces a reduction in effective intraarterial volume and thereby activates the renin-angiotensin-aldosterone system, which stimulates secretion of potassium in the distal tubule. Blocking the effect of aldosterone on the distal tubule therefore counteracts the metabolic consequences of pharmacologic diuresis. Accordingly, adding spironolactone, a competitive inhibitor of aldosterone, to the diuretic regimen may prevent or improve derangements in serum bicarbonate and potassium concentrations. Caution should be used in adding spironolactone to the diuretic regimen and supplementing with KCl. 100
100 Guignard J-P, Gouyon J-B. Use of diuretics in the newborn. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012. p. 233–50.
Infants receiving loop diuretics may require increased potassium and chloride intake to prevent potassium depletion and metabolic alkalosis.
Loop diuretics tend to be more effective in neonates but cause calciuresis, which can result in osteopenia, nephrocalcinosis, renal stone formation, or a combination of these.
Routine use of diuretic therapy has not been shown to significantly alter the course of bronchopulmonary dysplasia, although it may provide some immediate assistance in improving ventilation. The benefits should be carefully weighed against the risks of metabolic, bone, and renal complications.
The nurse reports that a 3-day-old infant is oliguric. You wonder, “How does she know the infant is oliguric?” What qualifies as oliguria?
Your question is not so naïve, given the wide range of urine volumes from the most dilute to the most concentrated. The nurse likely responds on the basis of physical evidence; for example, he or she may say that the infant had only three wet diapers over the past 24 hours. If the baby is in an intensive care unit and the urine volume is being quantified, urine flow rate can be calculated. Within the first 24 hours after delivery the volume may be as low as 0.5 to 0.7 mL/kg/h, but beyond this period it is usually greater than 1 mL/kg/h. Oliguria is defined as a urine output persistently below 1 mL/kg/h.
If an infant is found to be oliguric, what are the possible causes? How should it be evaluated and treated?
In considering what the causes of oliguria are, you need to remember the determinants of urine flow rate (see Question 6). The primary causes of oliguria are as follows:
Dehydration. Dehydration is defined as an inappropriately negative decrease in total body water, sodium, or both caused by insufficient water and sodium intake. If urine can be obtained for analysis, urine [Na + ] will be low and urine osmolality will be high with dehydration. Treatment depends on the cause but always includes replenishment of total body water and sodium.
Acute renal failure (ARF). Renal failure by definition is a decrease in GFR below normal for gestational and postnatal age. Evaluation of serum [Cr] is required to judge whether GFR is reduced. However, a single value, especially in the first days of life when serum [Cr] is largely a function of maternal serum [Cr], will not be sufficient. The pattern of change over time, taking into account gestational and postnatal age, is more relevant. Treatment of ARF depends on the underlying etiology, but the principles of management are shown in Table 9-4 .
| Monitor weight. |
| Monitor urine output and fluid balance. |
| Monitor serum electrolytes, blood urea nitrogen, and creatinine. |
| Remove potassium from intravenous fluids until renal output is adequate. |
| Adjust doses of drugs excreted by the kidney. |
| Provide adequate nutrition. Adjust protein intake based on blood urea nitrogen to avoid overload. Add calories as carbohydrate and fat. |
| Correct acidosis with supplemental acetate, citrate, or bicarbonate. |
| Attempt a trial of furosemide to promote and maintain urine output. |
| Support blood pressure with dopamine. |
| Attempt dialysis, if necessary. |
Syndrome of inappropriate antidiuretic hormone secretion (SIADH). SIADH is the secretion of antidiuretic hormone by the hypothalamus in the absence of volume or osmolar stimuli. Treatment is restriction of free water.
It is important to note that all the aforementioned causes may occur without associated oliguria, but urine output should be relatively low. There is an extensive differential diagnosis for each of these primary causes, and the clinical context is important in narrowing the differential diagnosis.
An oliguric term infant had a serum creatinine of 0.7 mg/dL at birth and 1 mg/dL at 48 hours of life. How do you interpret these levels?
This would be consistent with ARF because serum [Cr] should fall after birth in a term newborn.
In the face of an abnormal change in serum [Cr], how may the urine sodium concentration be helpful in evaluating oliguria?
Urinary indices to separate prerenal ARF from intrarenal ARF are not as useful in neonates as in older children and adults. However, the best index is the fractional excretion of sodium (FENa), which is calculated as follows:
In prerenal failure in which renal tubular function is normal, FENa is 2.5% to 3%. A FENa above 2.5% to 3% indicates intrarenal ARF with associated renal tubular injury. Because of overlap between the two groups, specificity is limited. Note that during postnatal natriuretic diuresis or extracellular volume (ECV) expansion, FENa will be high; in this case, however, urine output should also be high. However, in the polyuric phase of ARF, urine output and FENa are abnormally high. The former and the latter can be differentiated by urine osmolality. With ECV expansion the urine is hypoosmolar; in the polyuric phase of ARF the urine is isoosmolar ( Table 9-5 ).
A 2-day-old baby is requiring significant ventilator support. His condition is complicated by bilateral pneumothoraces, and he has been anuric since birth. In reviewing his chart, you note that his mother had oligohydramnios. The baby is defined as “funny looking” in the admission note. Can you formulate an armchair differential diagnosis before going to see the baby?
| URINE OSMOLALITY(MOSM/L) | URINE SODIUM(MMOL/L) | FRACTIONAL EXCRETION OF SODIUM (%) | |
|---|---|---|---|
| Prerenal failure (newborn/preterm Infant) |
>350 | <20 to 30 | <2.5 |
| Renal tubular injury (acute tubular necrosis) |
<350 | >30 to 40 | >2.0 |
The most likely diagnosis is oligohydramnios sequence.
How should this infant be evaluated?
The most helpful initial test would be abdominal sonography concentrating on the kidneys, ureters, and bladder. 55 56 57 58 59 60
55 Andreoli SP. Kidney injury in the neonate. In: Oh W, Guignard J-P, Baumgart S, editors. Nephrology and fluid/electrolyte physiology: neonatology questions and controversies. 2nd ed. Philadelphia: Saunders; 2012. p. 285–303.
56 Ellis EN, Arnold WC. Use of urinary indexes in renal failure in the newborn. Am J Dis Child 1982;136:615–7.
57 Miall LS, Henderson MJ, Turner AJ, et al. Plasma creatinine rises dramatically in the first 48 hours of life in preterm infants. Pediatr 1999;104:e76. Available at www.pediatrics.org.cgi.content/full/104/6/e76 . Accessed July 28, 2012.
58 Mathew OP, Jones AS, James E, et al. Neonatal renal failure: usefulness of diagnostic indices. Pediatrics 1980;65:57–60.
59 Pitkin RM, Reynolds A. Creatinine exchange between mother, fetus, and amniotic fluid. Am J Physiol 1975;228:231–7.
60 Rudd PT, Hughes EA, Placzek MM, et al. Reference ranges for plasma creatinine during the first month of life. Arch Dis Child 1983;58:212–5.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here