Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the late 1980s the late Dr. David J. P. Barker used historical birth records to pioneer the concept that the origins of adult disease could be strongly associated with fetal environmental exposures in pregnancy that resulted in low birth weight (BW) and modified both structure and function of tissues regulating human diseases later in life. Over the past several decades the fetal programming or developmental origins of health and disease (DOHaD) hypothesis has been validated epidemiologically and mechanistically using human and animal models. DOHaD has taught us about the role of a mismatch between a constrained prenatal and a plentiful postnatal environment in the pathogenesis of obesity (i.e., the “thrifty” pathway) likely operating in populations undergoing rapid transition. Another developmental pathway to obesity and its comorbidities, more novel evolutionarily and likely more important in Western societies, is developmental overnutrition. This pathway reflects the effects of hypernutrition during fetal and/or early postnatal life and creates the conditions for the later pathophysiologic effects of an obesogenic environment ( Fig. 13.1 ). Both phenomena lead to the same phenotypic ends: adolescent and adult-onset obesity and metabolic syndrome.
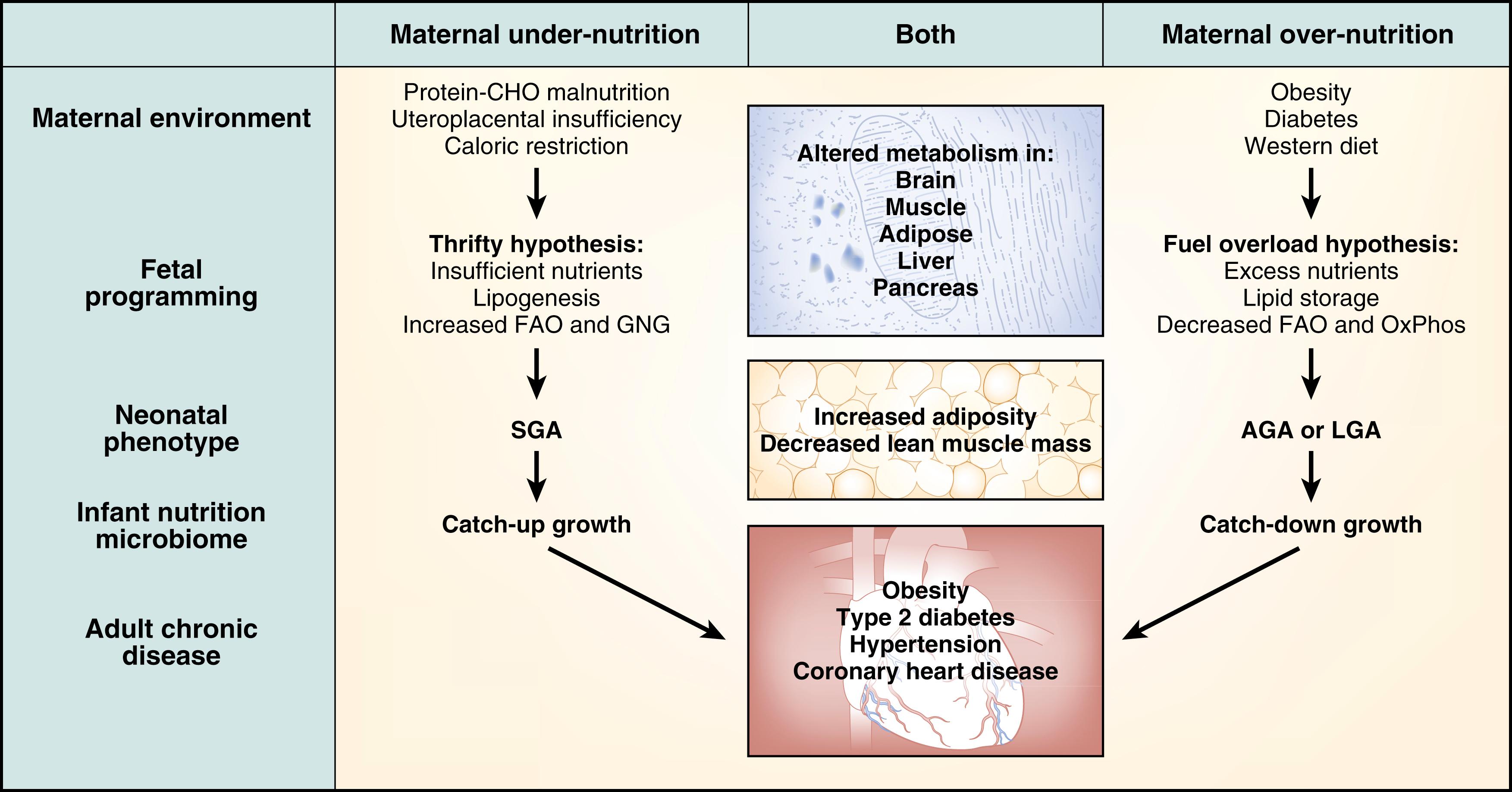
Fetal developmental programming can occur fundamentally in two ways: first, via gene-environment interactions that may produce persistent epigenetic events, and secondly, by impacting normal organ development to impart risk for developing chronic disease(s). Data showing that maternal nutritional challenges have epigenetic or molecular effects that underlie chronic metabolic diseases are ongoing. The mechanisms underlying how poor maternal health and diet imparts risk for future metabolic disease beginning in utero remain understudied in humans but are beginning to emerge in nonhuman primate (NHP) models. Rodent studies and human clinical data indicate that maternal and early infant nutrition affects multiple organ systems, including the brain, pancreatic islets, liver, adipose, muscle, and the immune system. Human studies suggest that reduced dietary fat intake and improved exercise can slow down the transmission of metabolic risk. , The effects of modifying adult lifestyle, when formally tested in randomized trials in humans, have been disappointingly small and lack longitudinal results. Importantly, as we move into an era of higher technological capacity, “omics” and “big data” analyses, and larger sample sizes, particularly in longitudinal cohorts, we will be able to discover new pathways and potential nutritional strategies for interventions in high-risk mothers and the next generation of infants.
Early exploration of the DOHaD hypothesis required systematic studies of BW and adult-onset chronic disease. In Hertfordshire, United Kingdom, starting in 1911, birthing mothers were attended by midwives, who recorded the BW. , A health visitor went to the babies’ homes at intervals throughout infancy, and weights at 1 year were recorded. Table 13.1 lists the findings for 10,636 men born between 1911 and 1930. Hazard ratios for coronary heart disease increase with decreasing BW. Stronger trends with weight are seen at 1 year. Table 13.2 shows findings for a sample of men following glucose tolerance tests. The percentage with impaired glucose tolerance or type 2 diabetes mellitus increased steeply with decreasing BW. The association between low BW and coronary heart disease has now been replicated among men and women in Europe, North America, and India. Low BW has been shown to predict altered glucose tolerance in studies of men and women around the world.
| Birth Weight (Pounds) | Death From CHD | |
|---|---|---|
| Before 65 yr | All Ages | |
| At Birth | ||
| ≤5.5 | 1.50 (0.98–2.31) | 1.37 (1.00–1.86) |
| 6.5 | 1.27 (0.89–1.83) | 1.29 (1.01–1.66) |
| 7.5 | 1.17 (0.84–1.63) | 1.14 (0.91–1.44) |
| 8.5 | 1.07 (0.77–1.49) | 1.12 (0.89–1.40) |
| 9.5 | 0.96 (0.66–1.39) | 0.97 (0.75–1.25) |
| >9.5 | 1.00 | 1.00 |
| P -value for trend | .001 | .005 |
| Age 1 yr | ||
| ≤18 | 2.22 (1.33–3.73) | 1.89 (1.34–2.66) |
| 20 | 1.80 (1.11–2.93) | 1.58 (1.15–2.16) |
| 22 | 1.96 (1.23–3.12) | 1.66 (1.23–2.25) |
| 24 | 1.52 (0.95–2.45) | 1.36 (1.00–1.85) |
| 26 | 1.36 (0.82–2.26) | 1.29 (0.93–1.78) |
| ≥27 | 1.00 | 1.00 |
| P -value for trend | <.001 | <.001 |
| Birth Weight (Pounds) | Men With 2-hr Glucose of ≥7.8 mmol/L (%) | Odds Ratio (95% Confidence Interval) a |
|---|---|---|
| ≤5.5 | 40 | 6.6 (1.5–28) |
| 6.5 | 34 | 4.8 (1.3–17) |
| 7.5 | 31 | 4.6 (1.4–16) |
| 8.5 | 22 | 2.6 (0.8–8.9) |
| 9.5 | 13 | 1.4 (0.3–5.6) |
| >9.5 | 14 | 1.0 |
| P -value for trend | <.001 |
The association between coronary heart disease and poor weight gain in infancy, first shown in Hertfordshire, was later confirmed in the Helsinki Birth Cohort. 19 This cohort of 8760 people born in Helsinki during 1934–1944 demonstrated that heart disease developed later in life in boys and girls who were small at birth and remained small in infancy. They experienced accelerated gain in weight and body mass index (BMI) (weight/height 2 ) after 2 years of age. Heights remained below average, which is consistent with the known association between the disease and short adult stature. Table 13.3 is based on 2997 patients treated for hypertension and 698 patients treated for type 2 diabetes mellitus in the Helsinki Birth Cohort. These two disorders are associated with the same general pattern of growth as for coronary heart disease. Small size at birth is followed by accelerated weight gain in childhood. The highest risk for each disease occurs among men and women who had low BW but were in the highest BMI group at 11 years. As with coronary heart disease, the risk for obesity is determined by body size at birth and BMI in childhood. , It is the tempo of weight gain in childhood as well as the attained body size that determines risk.
| Birth Weight (kg) | Body Mass Index (kg/m 2 ) at 11 yr | |||
|---|---|---|---|---|
| 15.7 | 16.6 | 17.6 | >17.6 | |
| Hypertension | ||||
| ≤3.0 | 2.0 (1.3–3.2) | 1.9 (1.2–3.1) | 1.9 (1.2–3.0) | 2.3 (1.5–3.8) |
| 3.5 | 1.7 (1.1–2.6) | 1.9 (1.2–2.9) | 1.9 (1.2–3.0) | 2.2 (1.4–3.4) |
| 4.0 | 1.7 (1.0–2.6) | 1.7 (1.1–2.6) | 1.5 (1.0–2.4) | 1.9 (1.2–2.9) |
| >4.0 | 1.0 | 1.9 (1.1–3.1) | 1.0 (0.6–1.7) | 1.7 (1.1–2.8) |
| Type 2 Diabetes | ||||
| ≤3.0 | 1.3 (0.6–2.8) | 1.3 (0.6–2.8) | 1.5 (0.7–3.4) | 2.5 (1.2–5.5) |
| 3.5 | 1.0 (0.5–2.1) | 1.0 (0.5–2.1) | 1.5 (0.7–3.2) | 1.7 (0.8–3.5) |
| 4.0 | 1.0 (0.5–2.2) | 0.9 (0.4–1.9) | 0.9 (0.4–2.0) | 1.7 (0.8–3.6) |
| >4.0 | 1.0 | 1.1 (0.4–2.7) | 0.7 (0.3–1.7) | 1.2 (0.5–2.7) |
More recent epidemiologic studies have linked excess weight gain in utero, as well as exposure to maternal obesity and gestational diabetes, as risk factors for later metabolic dysfunction. Maternal BMI and glucose homeostasis are thought to be the most important determinants of fetal growth, , and the association between maternal glycaemia and increased BW has been long documented in diabetic pregnancies. However, macrosomia is not uncommon in diabetic pregnancies even with strict glycemic control, , and among offspring of obese women with normal glucose tolerance. , , This suggests that mechanisms other than maternal hyperglycemia contribute to fetal growth. There are clear links between maternal obesity and the risk for early-onset obesity , ; altered immunity, altered stem cell metabolism, increased risk for inflammation and cardiovascular disease, , and type 2 diabetes mellitus. Strong positive associations between infant BW and later BMI support that larger newborns are more likely to become obese adults, and females born large for gestational age (LGA) (≥90th percentile) have a doubled risk for delivering an LGA infant themselves. Even in normal weight mothers the occurrence of gestational diabetes and potential fetal overnutrition leads to increased risk for both gestational and overt type 2 diabetes mellitus in the offspring as early as the second decade. ,
And so, these two polar opposite maternal conditions lead to similar offspring risk for chronic metabolic disease in adulthood, creating a U-shaped distribution of risk versus infant BW. This potentially indicates two separate mechanisms of fetal programming that lead to metabolic dysfunction. Although the clinical phenotypes seem nearly identical, the biochemistry and effect on cellular functions are likely quite different.
Dr. Barker’s initial discovery, that people who develop coronary heart disease grew differently than other people during fetal life and childhood, has led to a new “developmental” model for the disease. The interplay between genes and the environment, and an overview of mechanisms involved in fetal programming of adult-onset metabolic disease, can be found in Fig. 13.2 . Like other living creatures in early life, human beings are “plastic” and able to adapt to their environment. Phenotypic plasticity is the phenomenon by which one genotype can give rise to a range of different physiologic or morphologic states in response to different environmental conditions during development. Gene-environment interactions are ubiquitous in development. Their evolutionary benefit is that, in a changing environment, they enable the adaptation of phenotypes that are better matched to their in utero environments, which may be much different than encountered in extrauterine environment. To what extent “nutritional mismatch” can account for metabolic disease in adult life is uncertain; however, it is clear that both undergrowth and overgrowth increase the risk for metabolic syndrome.
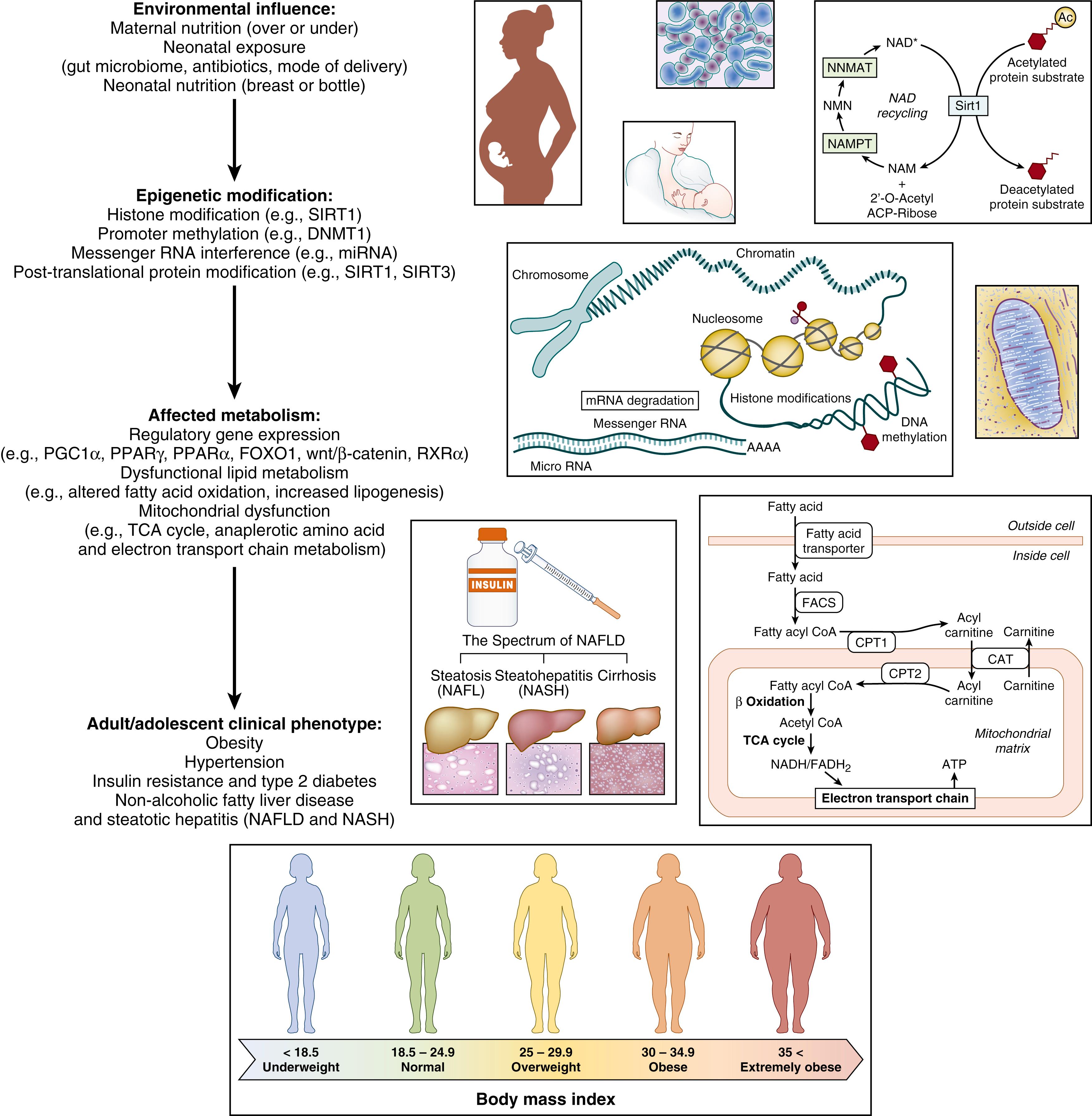
It may be advantageous, in evolutionary terms, for the body to remain plastic during development. Why this plasticity is ultimately lost in most tissues and systems remains to be fully explained. The fetal/neonatal state may originate in a developmentally regulated epigenetic program, whereas the adult-like epigenetic state may be acquired in some tissues within the first week of life. Other tissues (e.g., the trabeculae of bone) remain epigenetically plastic throughout life. It has been suggested that plasticity during intrauterine life enables animals, and humans, to receive a “weather forecast” from their mothers that prepares them for the type of world in which they will have to live. If the mother is poorly nourished, she signals to her unborn baby that the environment is harsh. The baby responds to these signals by adaptations, such as reduced body size and altered metabolism, which help it to survive a life of food shortage after birth. The transition from the fetal state to adult-like state could be regulated in several possible ways. For example, the fetal tissue precursors may be intrinsically programmed to a defined time in ontogeny. Alternatively, external signals from the maturing environment may promote methylation post birth. In this way some neonatal cells may be poised epigenetically to develop dominant responses based on the stage of ontogeny and the differentiation state of the organism, whereas in some tissues the acquisition of the dynamic phase of DNA methylation is acquired by signals from the maturing environment. What determines “the rules” for programming methylation during neonatal life and the mechanisms underlying these changes have yet to be determined. Whatever the mechanism, this plasticity gives a species the ability to make short-term adaptations, within one generation, in addition to long-term genetic adaptations that may span generations.
Although clinical relationships have been well proven on a population-based scale, the cellular mechanisms by which these plastic changes occur in the fetus, and continue to play out over the life of the organism, has only begun to be investigated. The role of epigenetics in regulating the timing and tissue specific nature of gene expression is paramount. Several well-established mechanisms play a critical role in creating a “cellular memory” of life in utero, which have great bearing on offspring physiology and the development of chronic adult conditions. Histone modification (acetylation, methylation, ubiquitylation, phosphorylation, sumoylation, ribosylation, or citrullination) may inhibit or facilitate transcription by structurally altering chromatin (hypercoiled bundles of DNA that uncoil when transcription of a particular gene is needed). Local DNA modifications like promoter methylation (via enzymes called demethylases ; e.g., DMNT1) may either silence or activate the expression of a given gene at a given time and place, which extend beyond the traditional regulation of imprinting centers in the human genome. Expression of bioactive small RNA molecules also act to regulate and modify gene expression posttranscriptionally. Finally, posttranslational modification of enzymes (e.g., acetylation, sumoylation, and phosphorylation) through enzymes such as sirtuins allows finer regulation of multiple enzymes, including important pathways in mitochondria. We are still trying to understand when these modifications occur in any particular tissue, as well as which particular in utero exposures lead to epigenetic changes. As a general rule, unmethylated DNA is largely associated with acetylated histones and active chromatin remodeling. However, the timing and regulation of this plasticity, be they through nearby gene promoters (i.e., CpG shores) or far off (i.e., CpG islands) regions of DNA necessary for regulation of transcription, continue to be discovered.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here