Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Despite the widespread recognition of the importance of prenatal detection of congenital heart disease (CHD), screening for fetal heart disease remains one of the most challenging and, unfortunately, least successful aspects of fetal ultrasonography. The rate of prenatal detection of even severe forms of CHD, albeit continually improving, remains disappointingly low. Moreover, when a fetal heart defect or arrhythmia is confirmed, many professionals may not know how best to translate prenatal detection of CHD into improved neonatal outcome.
This chapter aims neither to make everyone involved with fetal ultrasonography into an expert fetal echocardiographer nor to provide an exhaustive, encyclopedic review of the field of fetal cardiology. Instead, the chapter presents a distinctively practical and clinical approach, helping those involved with fetal cardiac imaging (1) become better at screening the fetal heart for CHD, (2) become better at diagnosing and evaluating common forms of fetal cardiac malformations and arrhythmias, (3) understand the basic approach to fetal and neonatal management of the most common forms of fetal cardiac malformations and arrhythmias, and (4) understand the prognosis associated with the most common forms of fetal heart disease.
After a brief discussion of the epidemiology of fetal heart disease, this chapter reviews the basic approach to fetal cardiac screening in low-risk pregnancies. The next section reviews the more detailed technique of formal fetal echocardiography in pregnancies at high risk for fetal CHD. The chapter’s final sections discuss the diagnosis, management, and prognosis of the most common and clinically important forms of fetal cardiac malformations and arrhythmias.
CHD represents by far the most common form of birth defect. , Although most studies suggest an incidence of approximately 8:1000 live births, this figure includes many forms of disease (e.g., secundum atrial septal defect [ASD], mild valvar pulmonary stenosis, and small muscular or membranous ventricular septal defects [VSDs]) that will never require either medical or surgical attention. Nevertheless, 3–4:1000 liveborn infants have CHD that will require surgical or transcatheter intervention in the first year of life. Because many severe cases of fetal heart disease, particularly those associated with aneuploidy or extracardiac malformations, may end in fetal demise, the spectrum of CHD seen prenatally tends to be more severe than that seen postnatally. At the same time, and in part because many VSDs may close spontaneously before birth, the incidence of CHD during the first and second trimesters should be considerably higher than the incidence at term.
On the other hand, some forms of CHD probably have a higher incidence at term than during the first or early second trimesters. Some forms of CHD evolve dramatically from first trimester to term. , Mild aortic or pulmonary valve stenosis, for example, may evolve prenatally into severe forms of critical pulmonary or aortic stenosis or may even adversely affect ventricular development to the point of manifesting as hypoplastic left heart syndrome (HLHS) or hypoplastic right heart syndrome (HRHS) at term. Similarly, cardiac tumors such as cardiac rhabdomyomas or intrapericardial teratomas may not develop significantly until the second trimester, and they may continue to enlarge dramatically from midgestation to term. Ductal constriction or closure of the foramen ovale may not develop until late in gestation, and the same may be said for fetal arrhythmias, valvar regurgitation, myocardial dysfunction, and congestive heart failure (CHF).
Most cases of CHD occur in pregnancies not identified as being at high risk, , so effective screening for CHD in low-risk pregnancies remains tremendously important. Nevertheless, many risk factors (fetal, maternal, and familial) for fetal heart disease have been identified ( Boxes 23.1–23.3 ). , , The familial recurrence of CHD remains well described , , , , but incompletely understood. Pregnancies conceived via in vitro fertilization appear to be at increased risk for CHD. Some anomalies—omphalocele, diaphragmatic hernia, and tracheoesophageal fistula ; cleft lip/palate ; aneuploidies such as trisomies 13, 18, and 21 , and Turner syndrome (45,X); Noonan and other genetic syndromes such as 22q11 microdeletions, VACTERL, or CHARGE—all carry increased risks for fetal heart disease. Other associations—such as a second-degree relative with CHD, maternal diabetes with first-trimester hyperglycemia, , fetal exposure to certain teratogens, , and fetal ureteral obstruction or gastroschisis —appear to carry moderately increased risks for CHD. A single umbilical artery has been associated with CHD as well. Many syndromes (familial, sporadic, or related to known teratogens) convey variable risks for CHD. Elevated maternal anti-SSA antibody titers place the fetus at risk for the development of heart block or cardiomyopathy or both. Finally, increased nuchal translucency thickness in the first trimester (11 to 14 weeks) represents an important risk factor for fetal heart disease, even in the presence of normal fetal chromosomes. For this reason, first-trimester measurement of nuchal translucency can be a component of fetal cardiac screening, despite the introduction of noninvasive cell-free fetal DNA screening.
Abnormal visceral/cardiac situs
Abnormal four-chamber view or outflow tract evaluation
Abnormal three-vessel tracheal view
Arrhythmia
Aneuploidy
Two-vessel umbilical cord
Extracardiac structural malformation
Fetal growth restriction
Polyhydramnios
Pericardial effusion/pleural effusion/ascites
Twins
Increased nuchal translucency thickness
Systemic lupus erythematosus or Sjögren syndrome
Diabetes mellitus
Phenylketonuria
Viral syndrome (mumps, coxsackievirus, rubella, cytomegalovirus)
Congenital heart disease
Teratogen exposure
In vitro fertilization
First-degree relative with congenital heart disease
First-degree relative with cardiac syndrome
DiGeorge syndrome
Long QT syndrome
Noonan syndrome
Marfan syndrome
Tuberous sclerosis
Williams syndrome
Neural tube defect
Hydrocephalus
Absent corpus callosum
Arnold-Chiari malformation
Dandy-Walker malformation
Tracheoesophageal fistula
Cystic adenomatoid malformation of the lung
Diaphragmatic hernia
Holt-Oram syndrome
Apert syndrome
Thrombocytopenia/absent radii
Ellis–van Creveld syndrome
Fanconi syndrome
Omphalocele
Duodenal atresia
Imperforate anus
Gastroschisis
Dysplastic/absent kidney
Horseshoe kidney
Ureteral obstruction
Anticonvulsants
Valproic acid
Carbamazepine
Phenytoin
Phenobarbital
Warfarin
Aspirin/ibuprofen
Tricyclic antidepressants
Lithium
Recreational drugs (including cocaine)
Isotretinoin
A distinction should be recognized between fetal risk factor for CHD and indication for fetal echocardiography. Because not all risk factors for fetal heart disease are equal in strength, the primary midwife, obstetrician, or perinatologist should weigh the likelihood of fetal heart disease when considering whether further evaluation is indicated. However, the expertise and ability to evaluate the fetal heart vary dramatically among fetal imagers, including obstetricians, perinatologists, radiologists, and pediatric cardiologists. Therefore the determination of which pregnancies to refer for formal echocardiography should depend largely upon the suspected likelihood of CHD and the added expertise in fetal cardiac imaging, management of fetal heart disease, and/or counseling for fetal heart disease offered by the consultant. For example, limited visualization of the fetal heart at the time of the screening anatomy survey (in the case of maternal obesity, for example) may, in many cases, represent a valid indication for referral for further cardiac evaluation. Beyond reflecting both the likelihood of fetal heart disease and the additional expertise anticipated from referral, the decision of whom to refer for full fetal echocardiography may also consider financial/logistic stresses that such a referral may represent for the patient’s family.
The prenatal detection of CHD in the low-risk pregnancy has long been a challenge in prenatal sonography because of the need to evaluate the heart in motion, the heart’s complex 3D structure, and the challenge and importance of optimizing image quality. Although CHD occurs more frequently than any other major congenital anomaly, the rate of detection has remained disappointingly low. In the United States, for instance, even recent reports show prenatal detection of only roughly one-third of cases of major forms of heart disease. Although prenatal detection rates for outflow tract abnormalities remain consistently lower than for abnormalities of the four-chamber view (4CV), prenatal detection of even basic 4CV abnormalities remains challenging.
This weakness in fetal screening sonography is a real problem. CHD is responsible for a large share of neonatal mortality from congenital malformations, and prenatal detection and diagnosis have repeatedly been shown to improve the outcome of fetuses and neonates with CHD. In addition, sometimes the detection of CHD may be the only fetal sonographic sign of aneuploidy (e.g., trisomy 21 or 22q11 microdeletions). For these reasons, more improvement needs to be made in the prenatal detection of the patient at low risk than in the detailed fetal diagnosis of CHD. ,
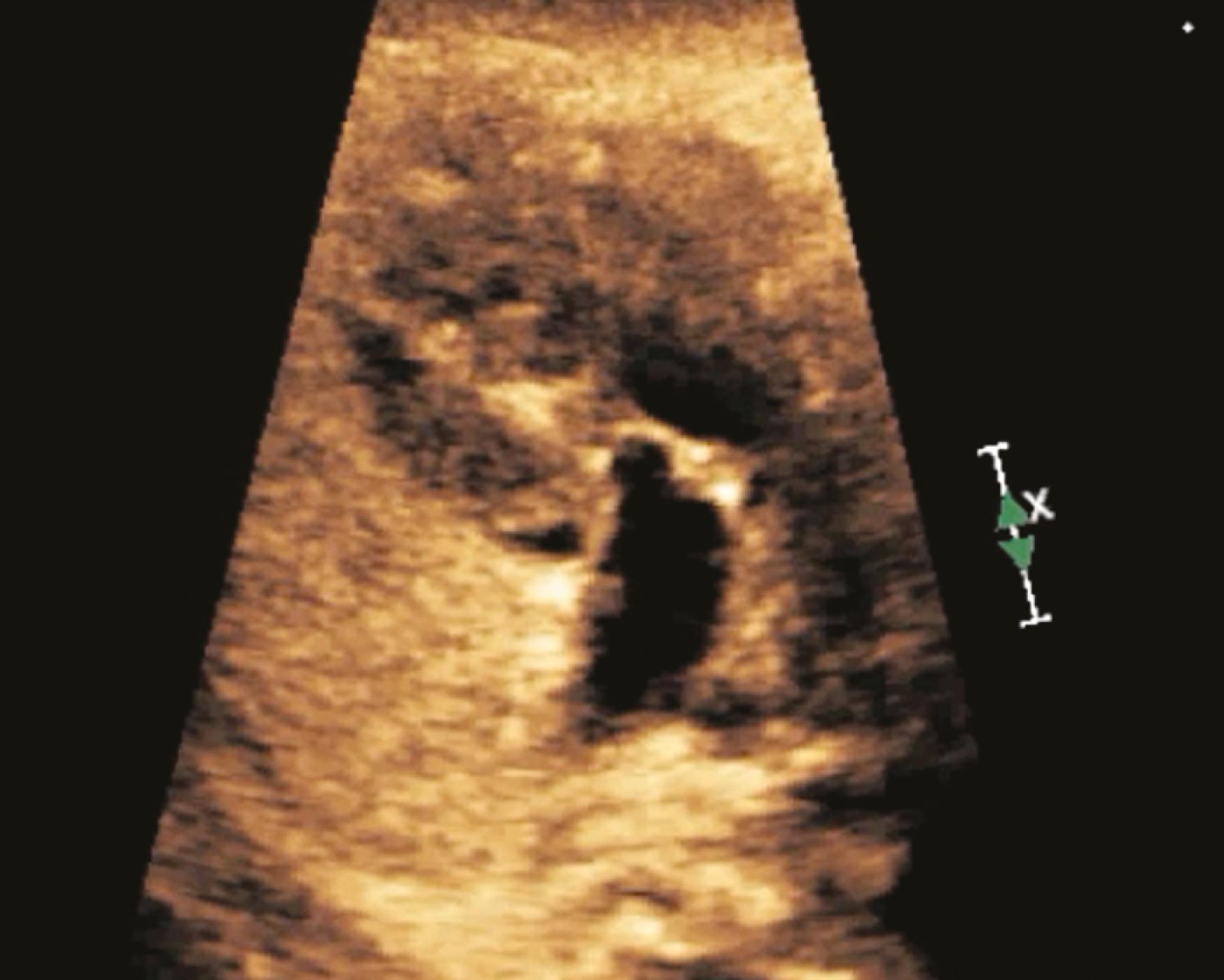
For years, those involved with fetal imaging have attempted to improve the prenatal detection of CHD. Soon after the development of two-dimensional (2D) fetal cardiac imaging, investigators proposed the 4CV as an effective approach to screening the fetus for CHD. For years the 4CV ( ![]() ) remained the standard of care for the screening fetal ultrasound evaluation of low-risk pregnancies—but no longer. , In practice the 4CV has not resulted in the high rates of detection initially anticipated.
) remained the standard of care for the screening fetal ultrasound evaluation of low-risk pregnancies—but no longer. , In practice the 4CV has not resulted in the high rates of detection initially anticipated.
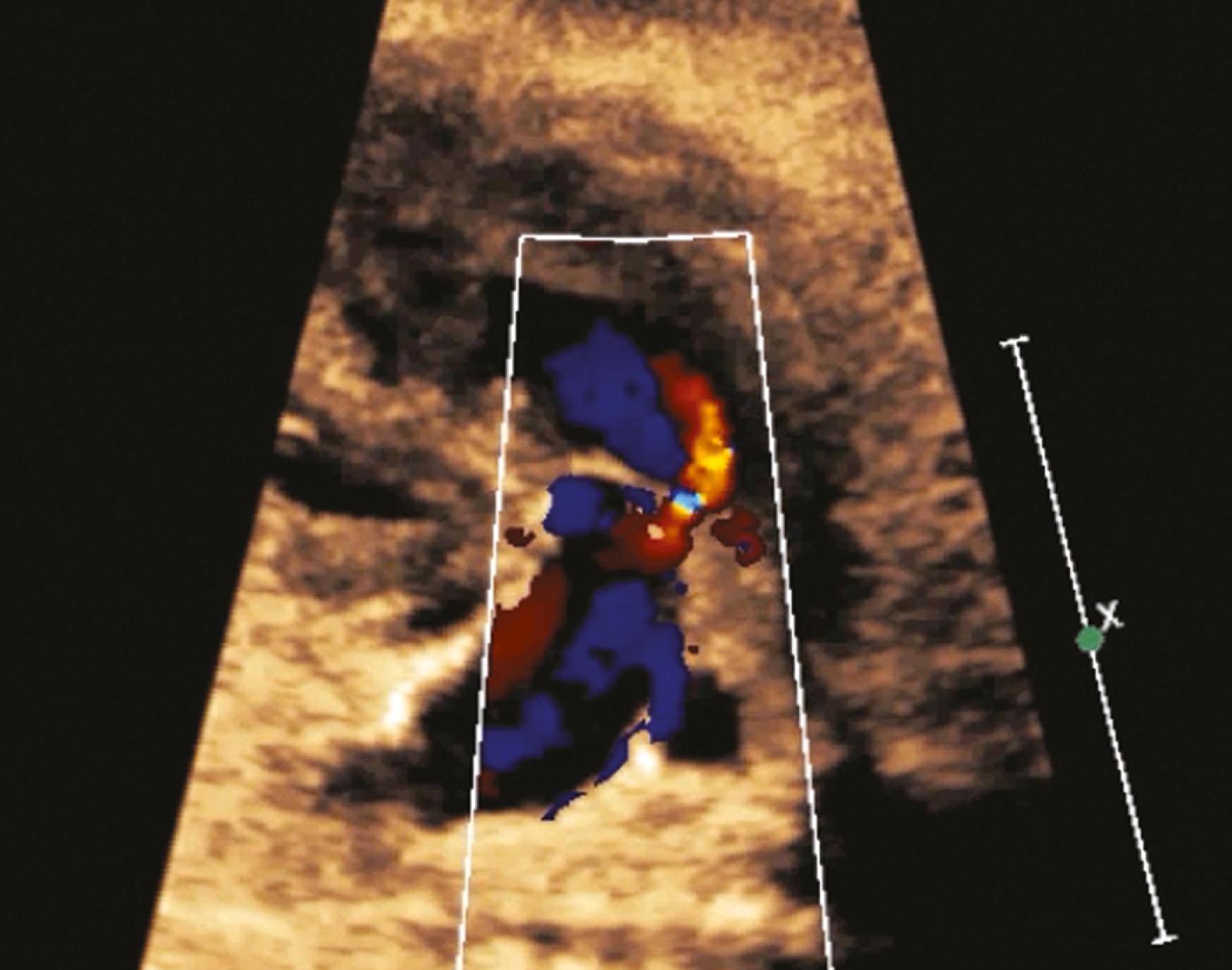
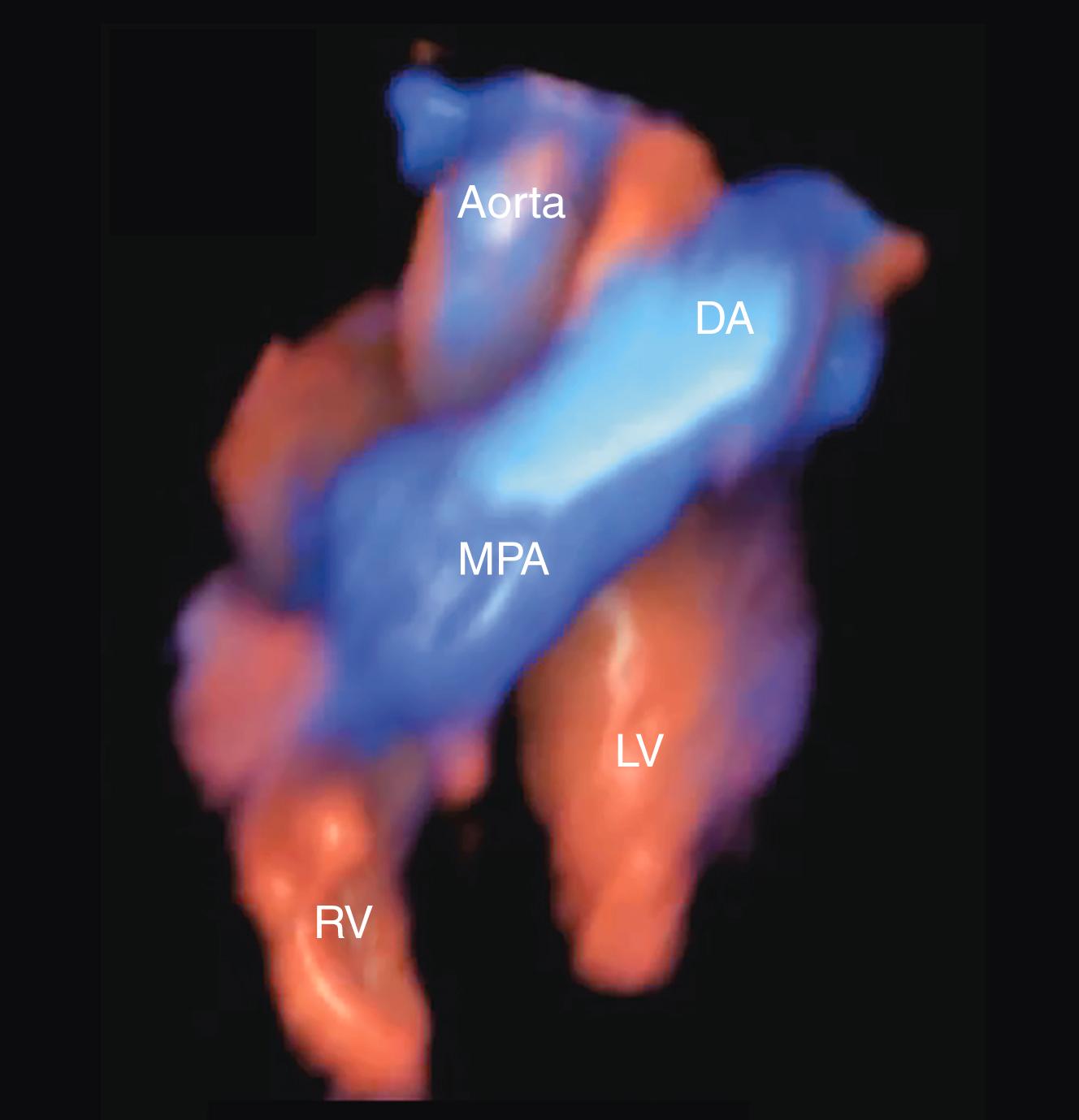
The addition of color flow Doppler to 2D imaging has been found to improve detection rates. Although currently formally recommended predominantly only in high-risk pregnancies, color flow Doppler is increasingly being incorporated into fetal cardiac screening in low-risk pregnancies. Even more important, evaluating outflow tracts along with the 4CV further improves detection rates. , , Many forms of ductal-dependent CHD (e.g., double-outlet right ventricle [DORV], tetralogy of Fallot [TOF], transposition of the great arteries [TGA], truncus arteriosus) have abnormal outflow tracts but generally normal-appearing 4CVs, and it is these ductal-dependent forms of CHD that most commonly require early intervention after delivery. Routine evaluation of the outflow tracts has been problematic, however, in part because the outflow tracts do not reside in a single plane ( ![]() ). Nevertheless, evaluation of outflow tracts along with obtaining the 4CV has now become standard even in low-risk pregnancies.
). Nevertheless, evaluation of outflow tracts along with obtaining the 4CV has now become standard even in low-risk pregnancies.
Some investigators have suggested that 3D imaging may facilitate and improve the evaluation of both the 4CV and the outflow tracts. Algorithms to automate the display of conventional fetal cardiac views from a single volume data set offer some promise. 3D printing of fetal hearts has been successfully performed to help inform counseling and surgical planning. However, image resolution with 3D imaging typically remains lower than with 2D imaging, and considerable expertise is currently required to evaluate volume data sets, despite a wealth of evolving algorithms and display techniques aimed at facilitating and improving analysis.
Given the limitations of ultrasound in evaluating the fetal heart, some investigators have pursued the application of magnetic resonance imaging to the fetal heart. Although fetal cardiac MRI has been limited because of small heart size, motion, and the lack of a readily available means to gate the acquired images to the cardiac cycle, newer algorithms and sophisticated software have enabled some centers to obtain high-quality imaging of the fetal heart, with promise for the future. In the meantime, fetal cardiac MRI has contributed to an improved understanding of fetal cardiovascular physiology and oxygen delivery, including the impact of maternal oxygenation on cerebral blood flow.
Despite these developments, second-trimester screening for major forms of CHD remains suboptimal, for several reasons. , , First, the practice of prenatal screening for CHD remains heavily dependent on the expertise of the sonographer. Second, effective screening requires an experienced reviewer to evaluate the fetal heart. Third, although it makes sense to evaluate static fetal structures as still-frame images, it does not make sense—and does not work—to evaluate the heart as a series of still-frame images. , For these reasons, the beating 4CV and beating outflow tracts have long taken the place of the motionless 4CV as the centerpieces of screening the fetus for CHD. Visualization of valve and myocardial motion facilitates and enhances the evaluation of cardiac structure and makes missing major CHD less likely.
Furthermore, the three-vessel views have more recently been demonstrated to be extremely useful both for screening low-risk pregnancies and for detailed fetal echocardiography. , In fact, an increasing number of regions and practices have demonstrated markedly improved rates of prenatal detection of CHD with a screening program that includes (1) evaluation of the beating heart with both 2D imaging and color flow Doppler and (2) a series of five parallel transverse planes—abdominal situs, four-chamber view, left and right ventricular outflow tracts, and the three-vessel tracheal view.
However, two additional considerations, image quality and angle of acquisition, may help explain the failure of current screening approaches to detect a greater percentage of cases of major forms of CHD. Optimal visualization of the heart requires high-quality windows, appropriate angles of interrogation (e.g., relatively perpendicular to the septum to evaluate for septal defects), and satisfactory fetal lies. In addition, high-quality imaging frequently requires significant transducer pressure; in our practice, to allow us to place both hands on the probe, we utilize a foot pedal to store clips. Optimizing all of these elements requires time and training. Whereas finding the way around the fetal spine to evaluate the crux of the heart may be easier to do with greater scanning expertise, obtaining satisfactory fetal lies still commonly requires extended periods of time to be spent performing various maternal maneuvers aimed at optimizing the fetal lie. The importance of time spent in this way cannot be overstated.
An essential distinction between fetal cardiac screening and fetal echocardiography relates to perspective. Whereas fetal cardiac screening in low-risk pregnancies focuses on views, imaging the heart in high-risk pregnancies focuses on structures. Nevertheless, going further than currently required by formal guidelines for both low-risk and high-risk pregnancies, once an optimal window and image of the heart has been achieved, the sonographer should mentally evaluate the structure and function of each structure during the real-time scan. Optimal visualization of the aortic valve, for example, may require a subtle adjustment in angle of acquisition compared with optimal visualization of the outlet ventricular septum or ascending aorta, just as evaluation of the tricuspid valve may require a subtle adjustment in pressure/angle after evaluation of the mitral valve. Most structures require multiple angles of interrogation to be completely evaluated. The tricuspid valve, for instance, needs to be evaluated perpendicular to the ventricular septum to evaluate for inlet ventricular septal defects but parallel to the septum to evaluate for valvar regurgitation or stenosis using pulsed and color Doppler.
First-trimester fetal cardiac screening, discussed in greater detail elsewhere, has focused on cardiac axis, symmetry, and orientation of the great arteries to each other by the “V sign.” , Second-trimester fetal cardiac screening in low-risk pregnancies should include detailed evaluation of the four-chamber view, outflow tracts, and three-vessel view (preferably from clips, not from still-frame images). Many clinicians find the use of color Doppler to be helpful for fetal cardiac screening, even in low-risk pregnancies. Attempts should be made to optimize image quality and angle of acquisition.
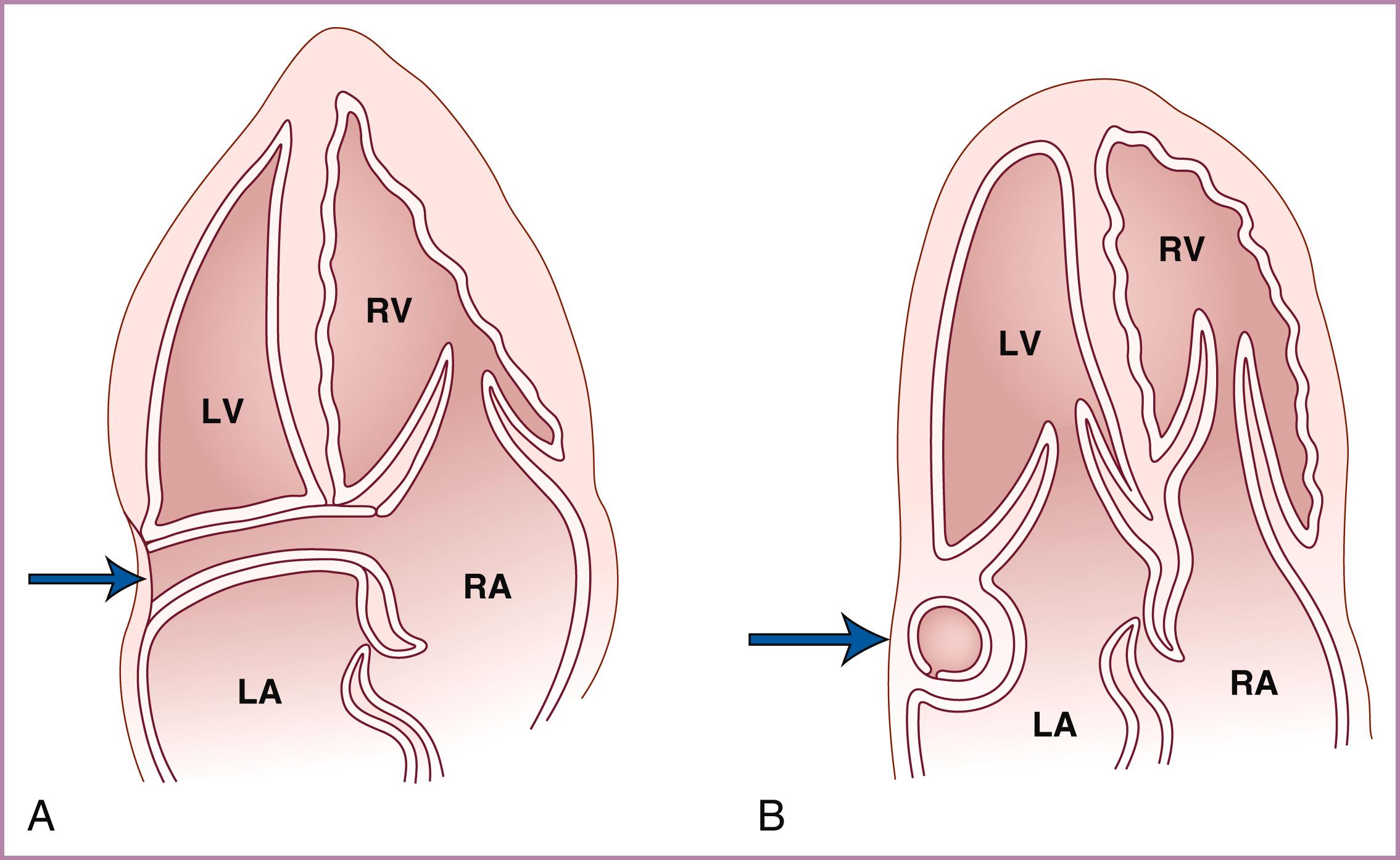
Prenatal screening for CHD should include an evaluation for extracardiac abnormalities associated with CHD. The umbilical cord should have three vessels, the heart and stomach should both be on the fetal left (above and below the diaphragm, respectively), and there should be no ascites ( Fig. 23.1 ) or pericardial or pleural effusions. A trivial amount (up to 2 mm) of pericardial fluid may normally be seen adjacent to the right and left ventricular free walls. Abnormalities of any of these findings should raise the level of suspicion for CHD and prompt more than just a routine evaluation. Before moving to the thorax, evaluation of the fetal abdomen should demonstrate, at a minimum, the stomach on the left and an absence of ascites. More thorough evaluation of the abdomen is recommended even in low-risk pregnancies but is required in high-risk pregnancies (see Visceroatrial Situs, later).
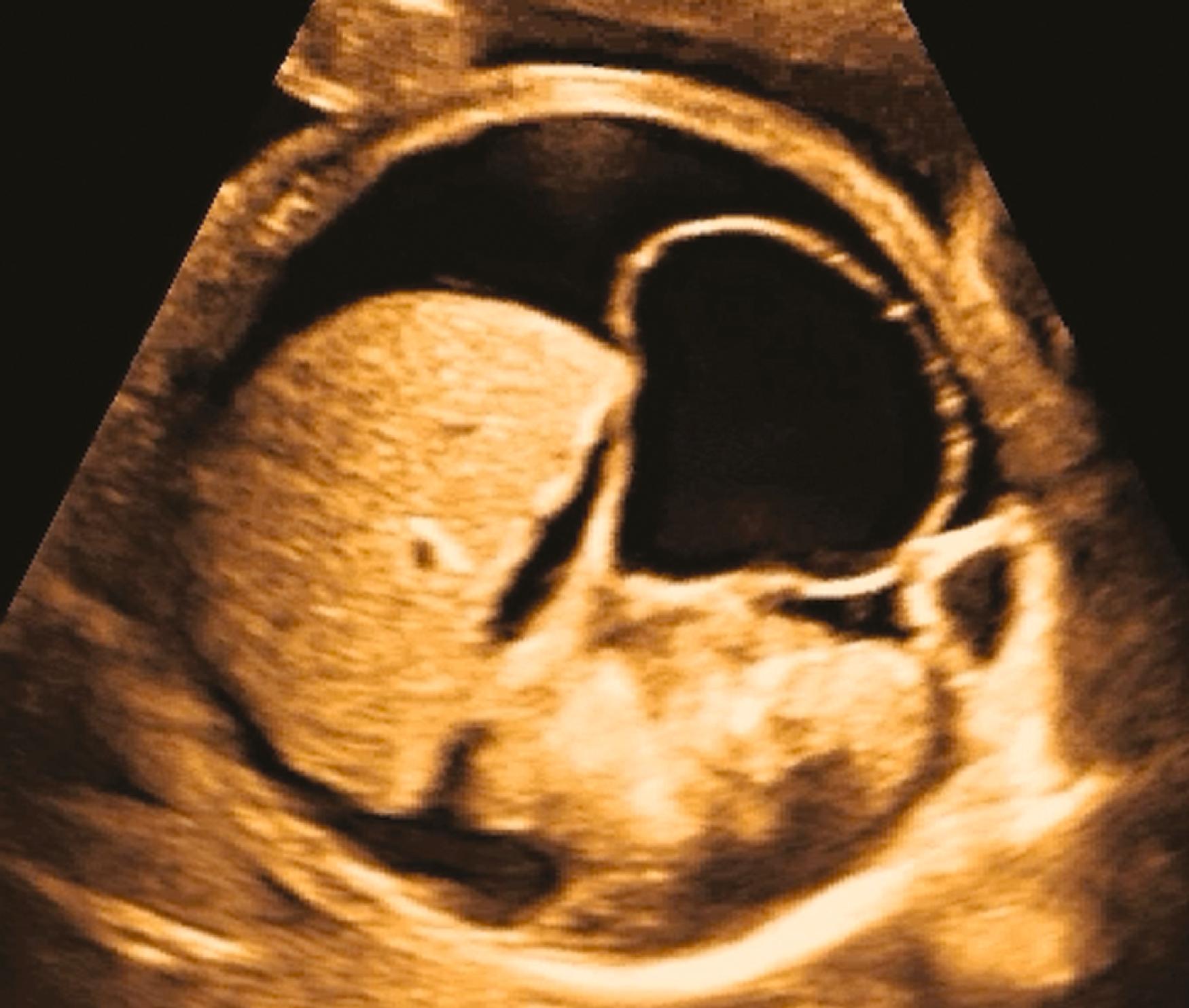
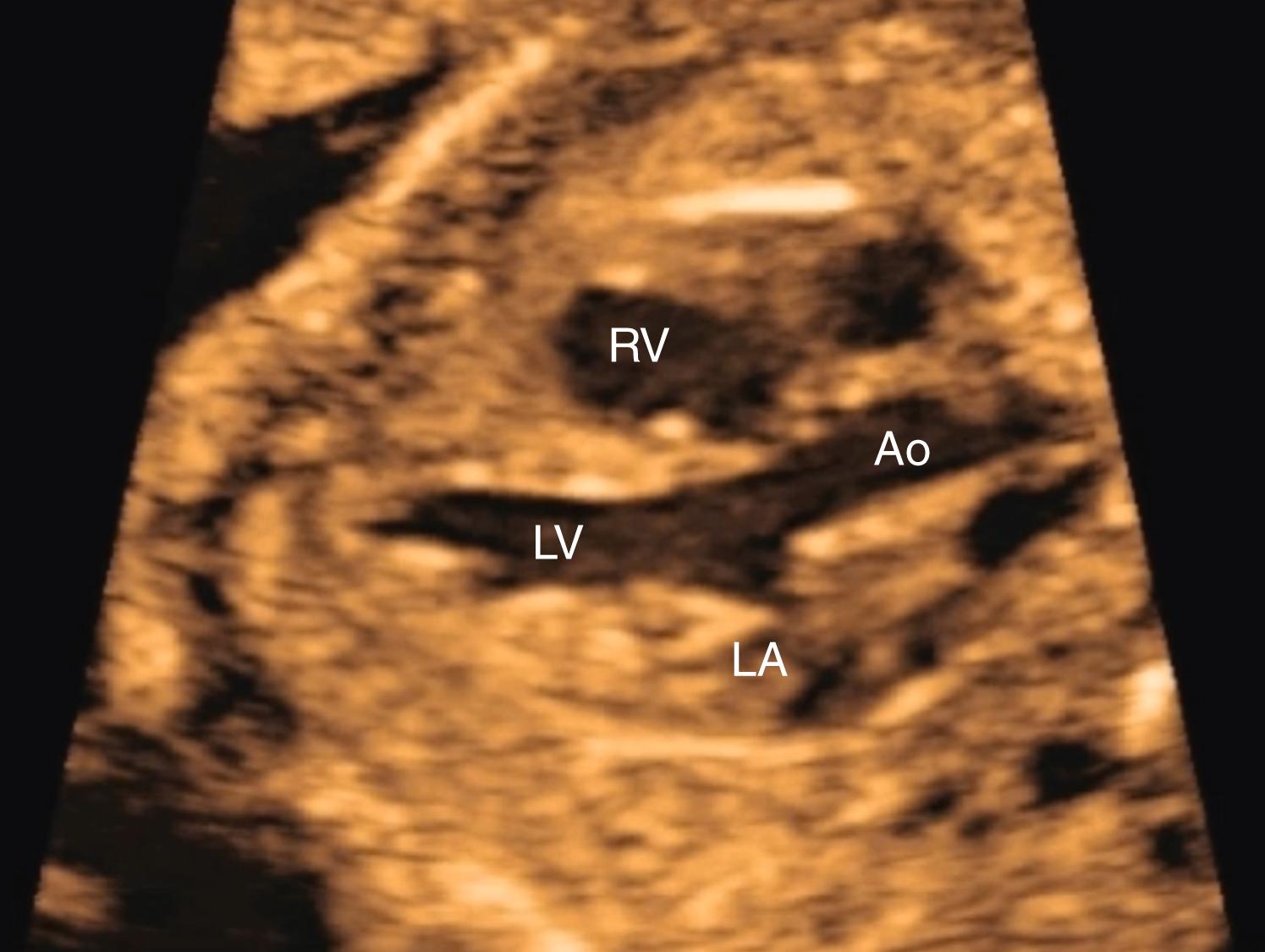
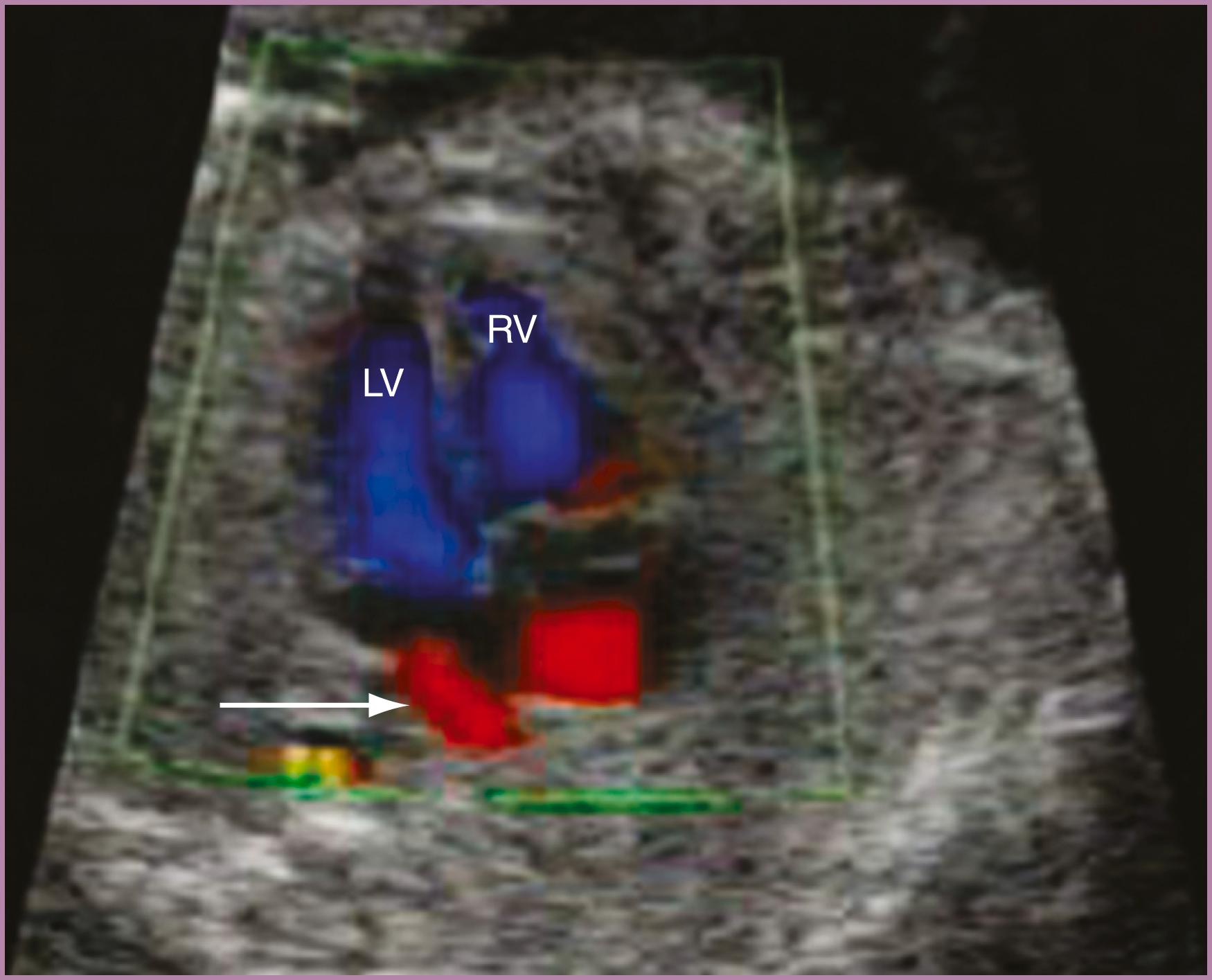
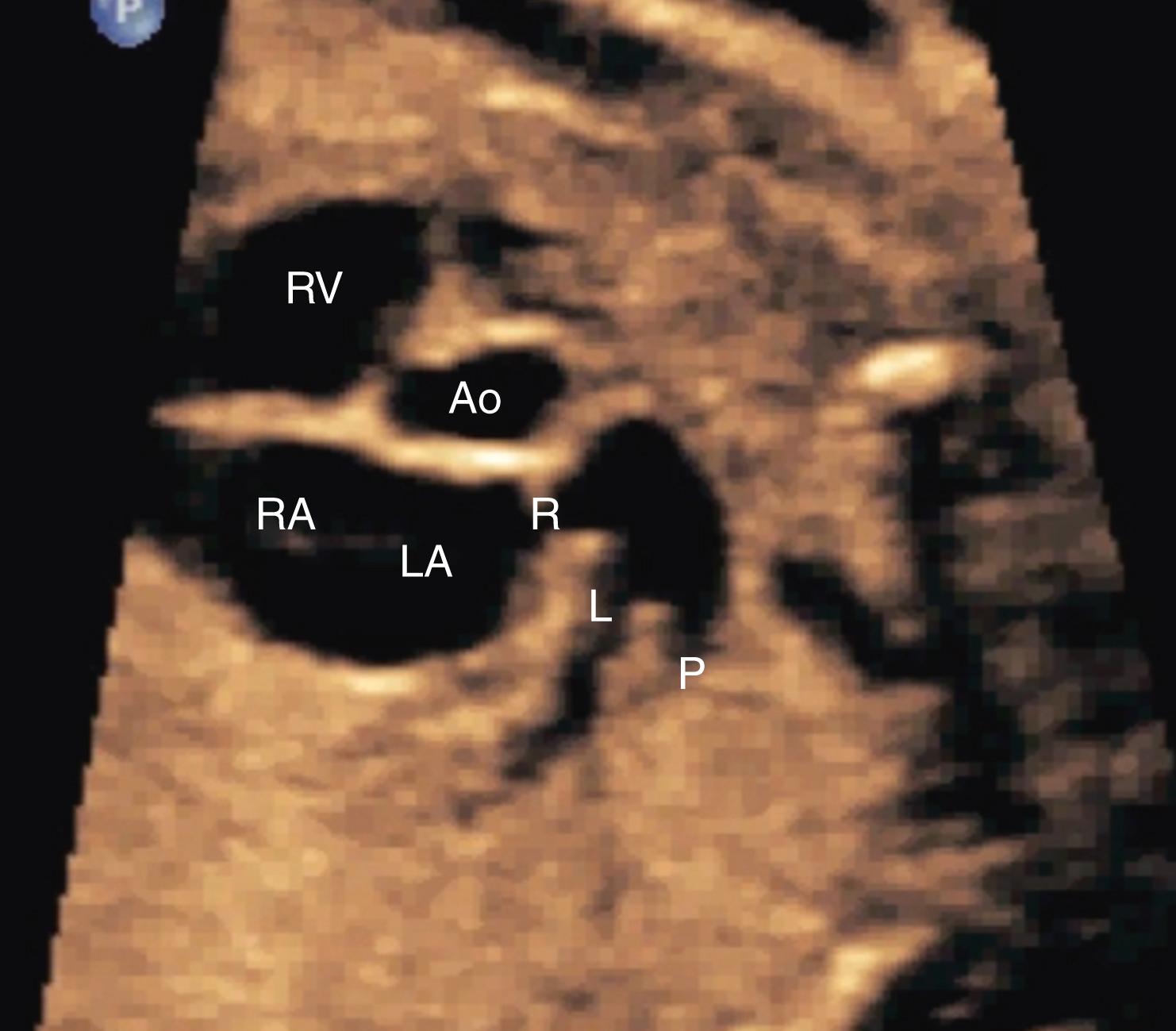
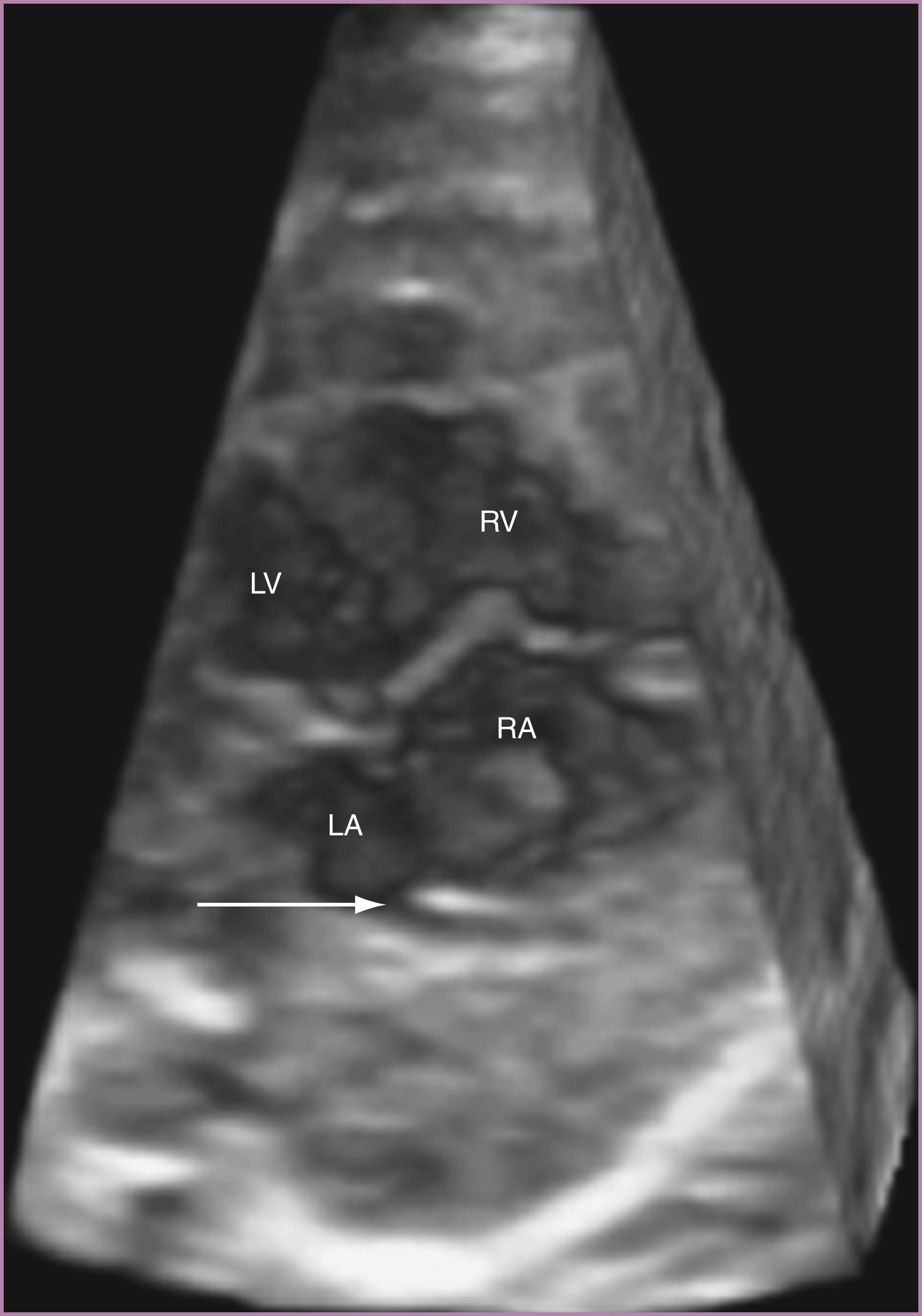
The 4CV ( Figs. 23.2 and 23.3 ; see ) deserves a close, detailed evaluation, even as part of a general screening fetal ultrasound study. Evaluation of the beating 4CV should assess situs, size, symmetry, structure, and squeeze.
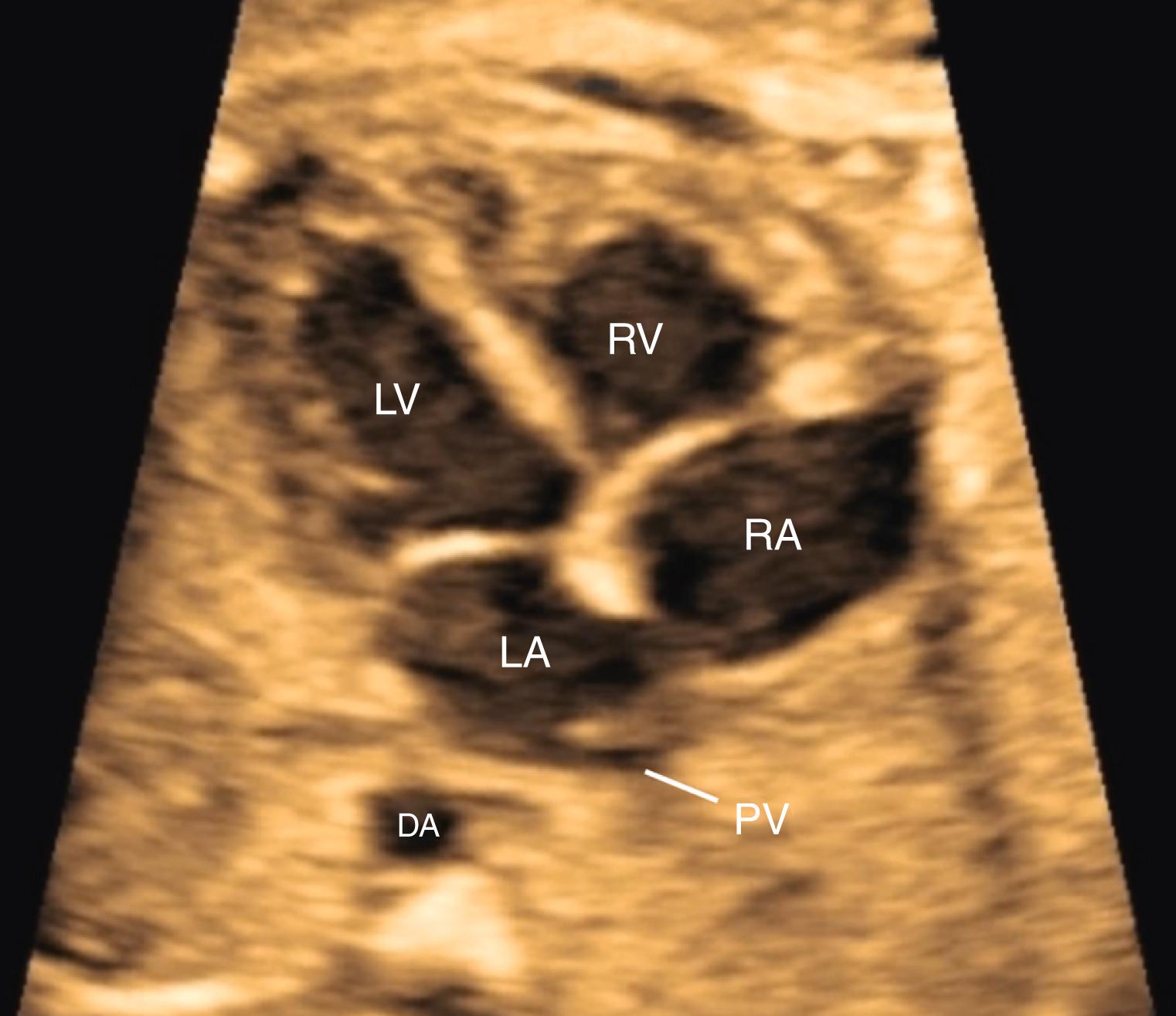
The heart should be located in the left thorax, with the apex directed approximately 45 degrees to the left. Hearts on the right (dextrocardia), in the middle (mesocardia), or with the apex directed overly toward the left (left axis deviation) have all been associated with CHD. Moreover, should the space around the heart be filled with fluid, either circumferentially or with a depth of greater than 2–2.5 mm, further evaluation may be indicated.
The heart should occupy no more than approximately one-third of the cross-sectional area of the thorax. Alternatively, the circumference of the fetal heart should be no more than approximately half the circumference of the thorax. New quantitative approaches to evaluating overall fetal heart size as a component of screening continue to be developed, but none has gained widespread clinical usage. In the end, no quantitative threshold outperforms an experienced eye. An enlarged fetal heart, by any means of measurement, should be considered a strong risk for pathology and should prompt a more detailed evaluation.
The right and left sides of the fetal heart should appear generally symmetric, with the right side typically slightly larger. During the third trimester, this right-sided predominance may become more pronounced. If the left side of the heart appears larger than the right or if the right side appears substantially larger than the left, further evaluation may be prudent.
Frequently there is a question as to whether the right side is enlarged or the left side is small. Commonly, there may be a little of both, with fetal cardiac output simply redistributing. In other cases, however, the pathology is truly one-sided. The use of z values for all four valves and great arteries, as well as other quantitative measurements of cardiac size, can be particularly informative to identify the source of pathology and can be used to follow for progression of disease over time, although not a part of screening scans. Ultimately the important questions to consider may be (1) why is there an abnormal ratio between the right and left sides of the heart and (2) what is the primary lesion, if any?
Even in screening for CHD, several aspects of the structure of the 4CV should be evaluated. Optimal evaluation of the 4CV may require a series of such views because a single 4CV may demonstrate some elements well but other elements less well. For example, defects in the atrial or ventricular septum should be evaluated with imaging relatively perpendicular to the septum, whereas evaluating the atrioventricular valves for regurgitation or an atrioventricular canal defect is best accomplished parallel to the ventricular septum.
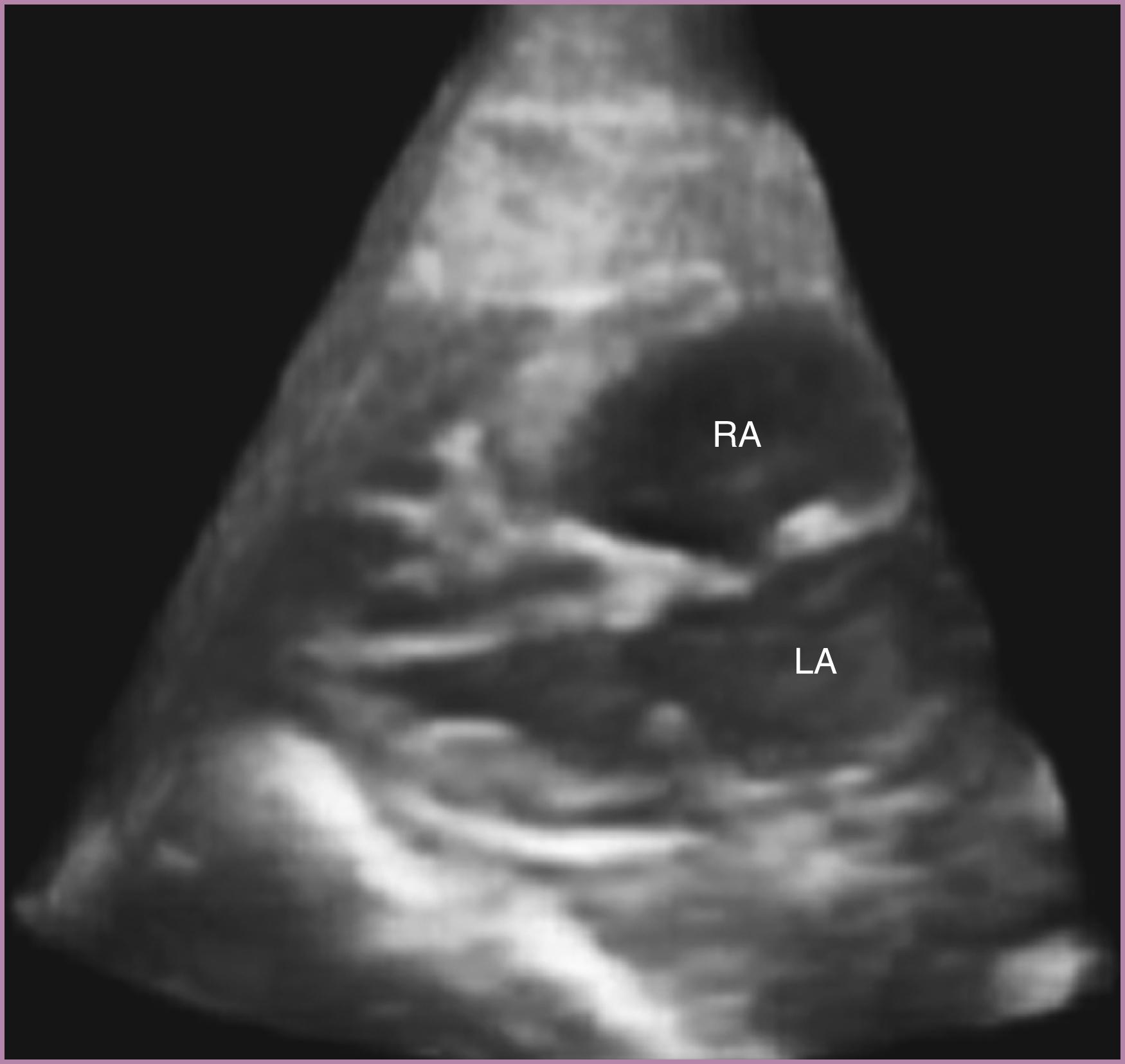
The flap of the foramen ovale should be visualized in the left atrium. This flap may not be well visualized, but a flap that is deviated toward the right atrium should raise the concern for left-sided volume or pressure overload and prompt further evaluation.
The lowest portion of the atrial septum, just above the insertion point of the mitral valve, should always be visualized, ideally relatively perpendicular to the septum.
The septal leaflet of the tricuspid valve should attach to the ventricular septum slightly more toward the apex than does the septal leaflet of the mitral valve. In practice, this normal offset may be subtle and difficult to appreciate.
The mitral and tricuspid valves should be thin and delicate, with the tricuspid valve annulus slightly larger than that of the mitral valve, and the valves should open symmetrically during diastole.
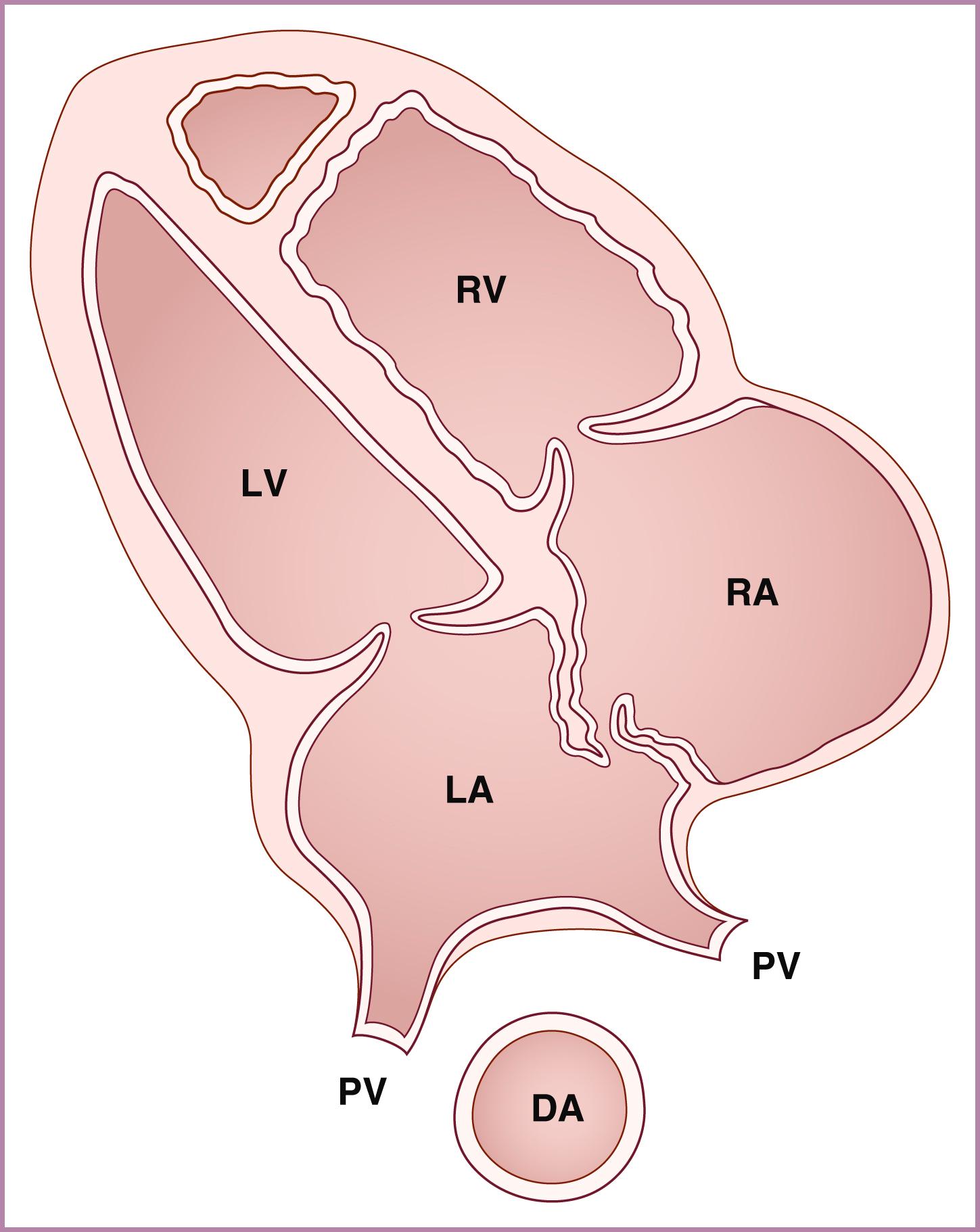
The left ventricle (aligned with the left atrium) should be smooth-walled and extend to the apex. The right ventricle should appear more heavily trabeculated and extend toward the cardiac apex.
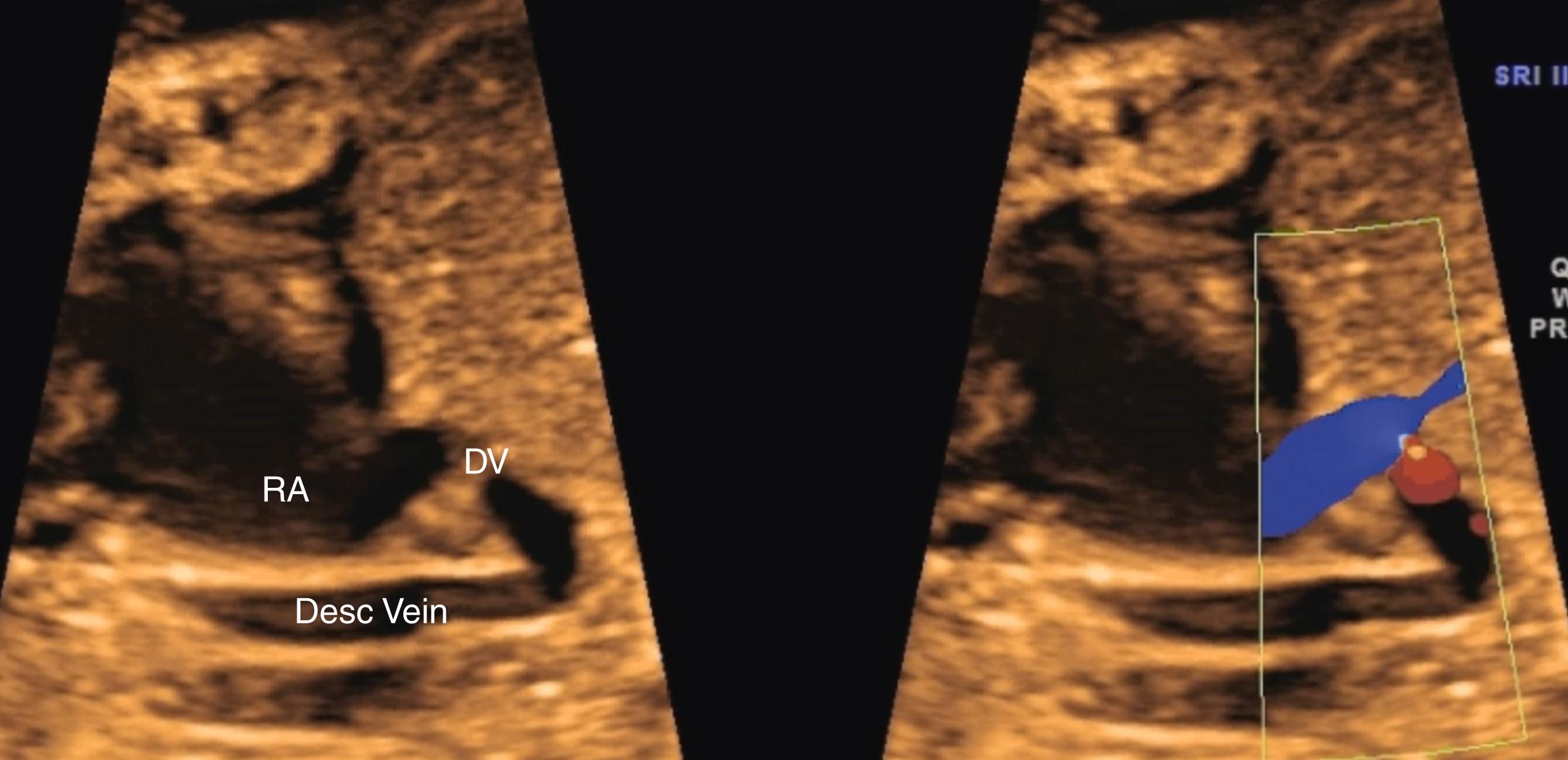
At least one pulmonary vein should be seen to be entering the left atrium, and there should be absence of a prominent venous structure immediately posterior to the left atrium. Such a venous confluence, or “twig sign,” behind the left atrium can be an important sign of total anomalous pulmonary venous return (TAPVR). In addition, attention to should directed to the distance between the cross-sectional descending aorta and the left atrium; an increased distance should raise the suspicion for TAPVR.
The ventricular septum should appear intact with 2D imaging relatively perpendicular to the septum; imaging parallel to the septum increases the likelihood for false-positive diagnosis due to artifactual dropout of the ultrasound signal.
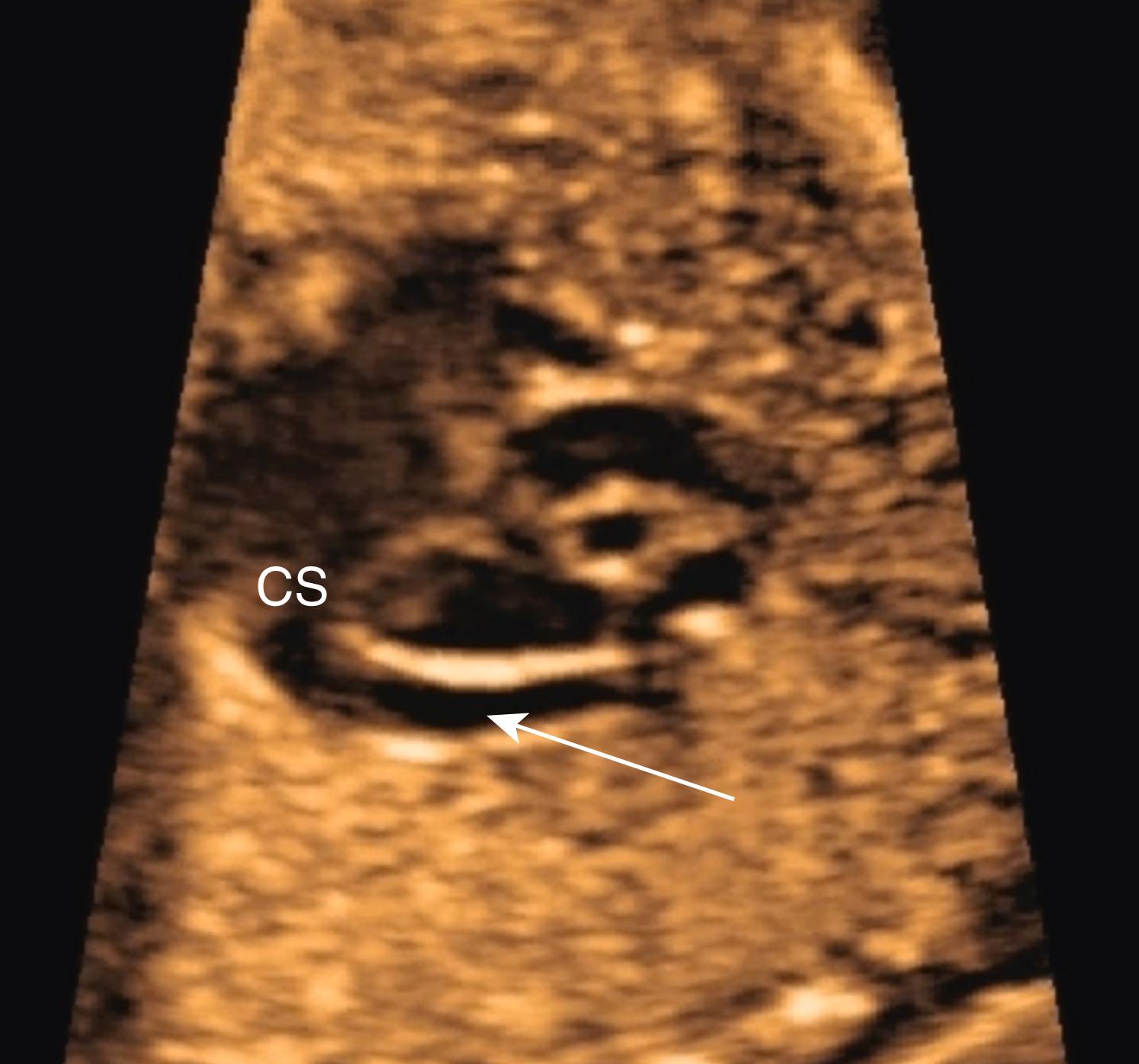
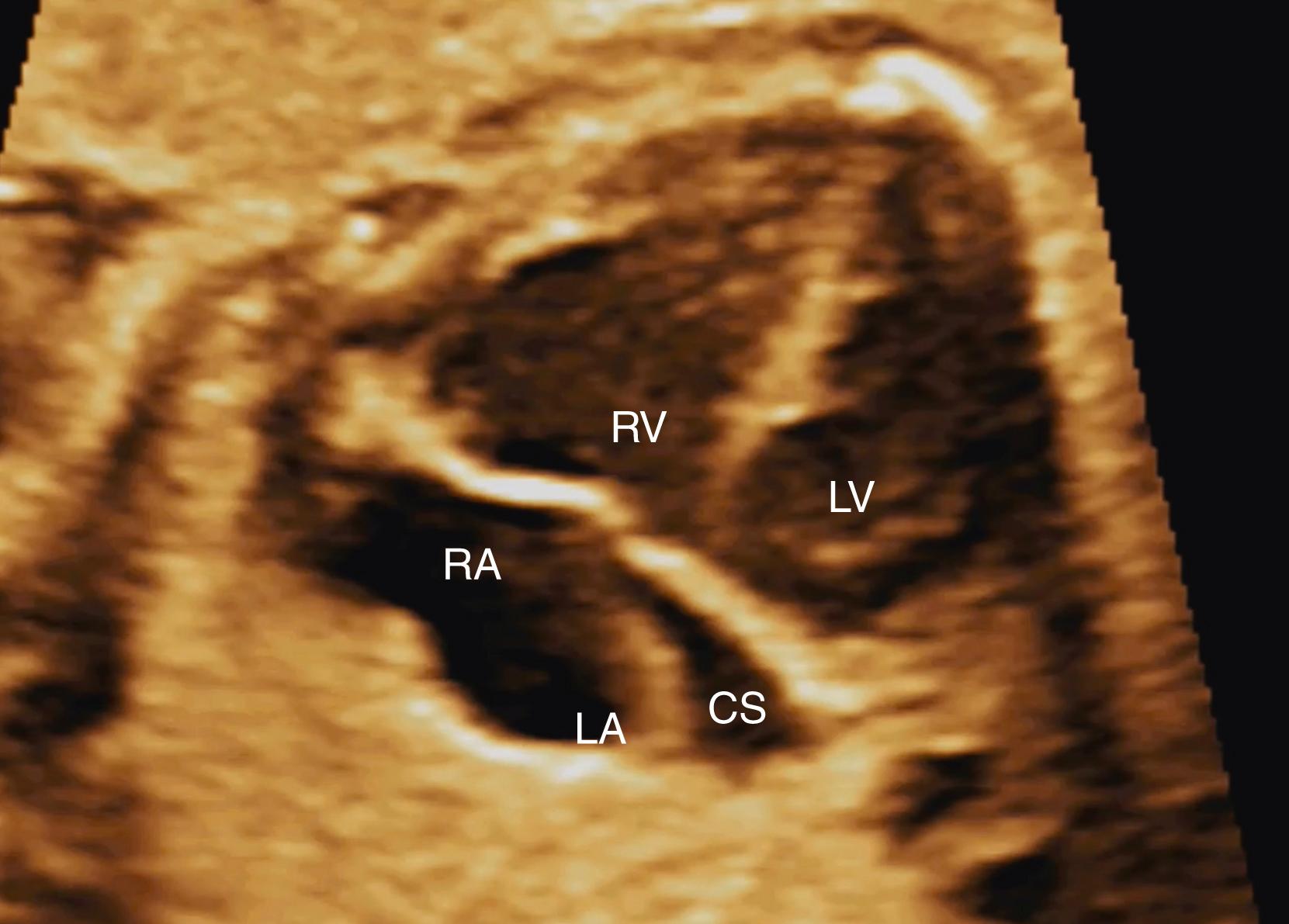
Commonly the coronary sinus is seen in circular cross section in the left atrium, just above the lateral aspect of the mitral valve. When the imaging plane has been shifted to just inferior and posterior to that of the true 4CV, the coronary sinus may be seen longitudinally just above the mitral valve annulus, draining directly into the right atrium. In this plane the mitral valve does not appear to open, and the coronary sinus (particularly, but not exclusively when dilated) may be mistaken for an ostium primum defect.
If available, color flow Doppler ideally can be used even in low-risk pregnancies to evaluate for valvar regurgitation, for a primum atrial septal defect, or for inlet/muscular ventricular septal defects. Color Doppler can also help identify normal return of the pulmonary veins to the left atrium.
The 4CV (as well as the outflow tracts), even for the purposes of screening for CHD, should be evaluated qualitatively in real-time imaging; more detailed, quantitative evaluation of cardiac function should be reserved for high-risk patients (see discussion in Technique of Fetal Echocardiography, later). The heart rate should range roughly between 120 and 160 beats/min, with no pauses and no skipped or extra beats, other than transient bradycardia during deep transducer pressure. The mitral and tricuspid valves should open and close symmetrically, and both ventricles should squeeze well. Abnormalities of rhythm, valvar function, or myocardial motion may reflect important forms of fetal heart disease and may be overlooked with evaluation of only still-frame images. ,
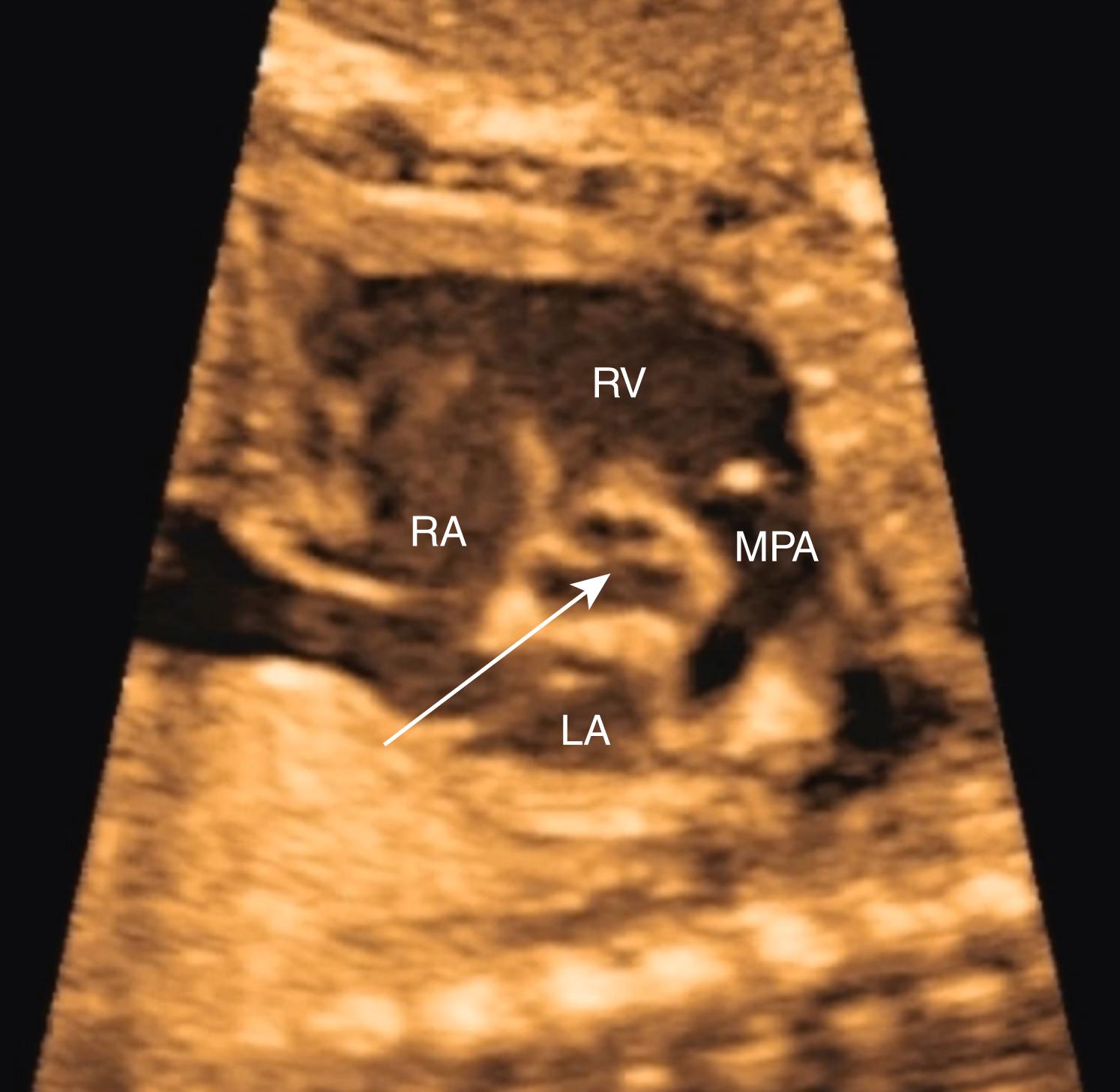
Prenatal screening for CHD should include visualization of both outflow tracts ( ![]() , ; see ). In practice, appropriate evaluation of the outflow tracts may be more challenging than visualization of the 4CV, in part because, unlike the 4CV, the outflow tracts do not lie in a single plane. Nevertheless, because the outflow tract views are part of many critical forms of CHD not visualized with the 4CV, evaluation of both outflow tracts in all pregnancies has appropriately become standard of care throughout much of the developed world. Like imaging of the 4CV, imaging of the outflow tracts requires multiple views and angles for a thorough evaluation.
, ; see ). In practice, appropriate evaluation of the outflow tracts may be more challenging than visualization of the 4CV, in part because, unlike the 4CV, the outflow tracts do not lie in a single plane. Nevertheless, because the outflow tract views are part of many critical forms of CHD not visualized with the 4CV, evaluation of both outflow tracts in all pregnancies has appropriately become standard of care throughout much of the developed world. Like imaging of the 4CV, imaging of the outflow tracts requires multiple views and angles for a thorough evaluation.
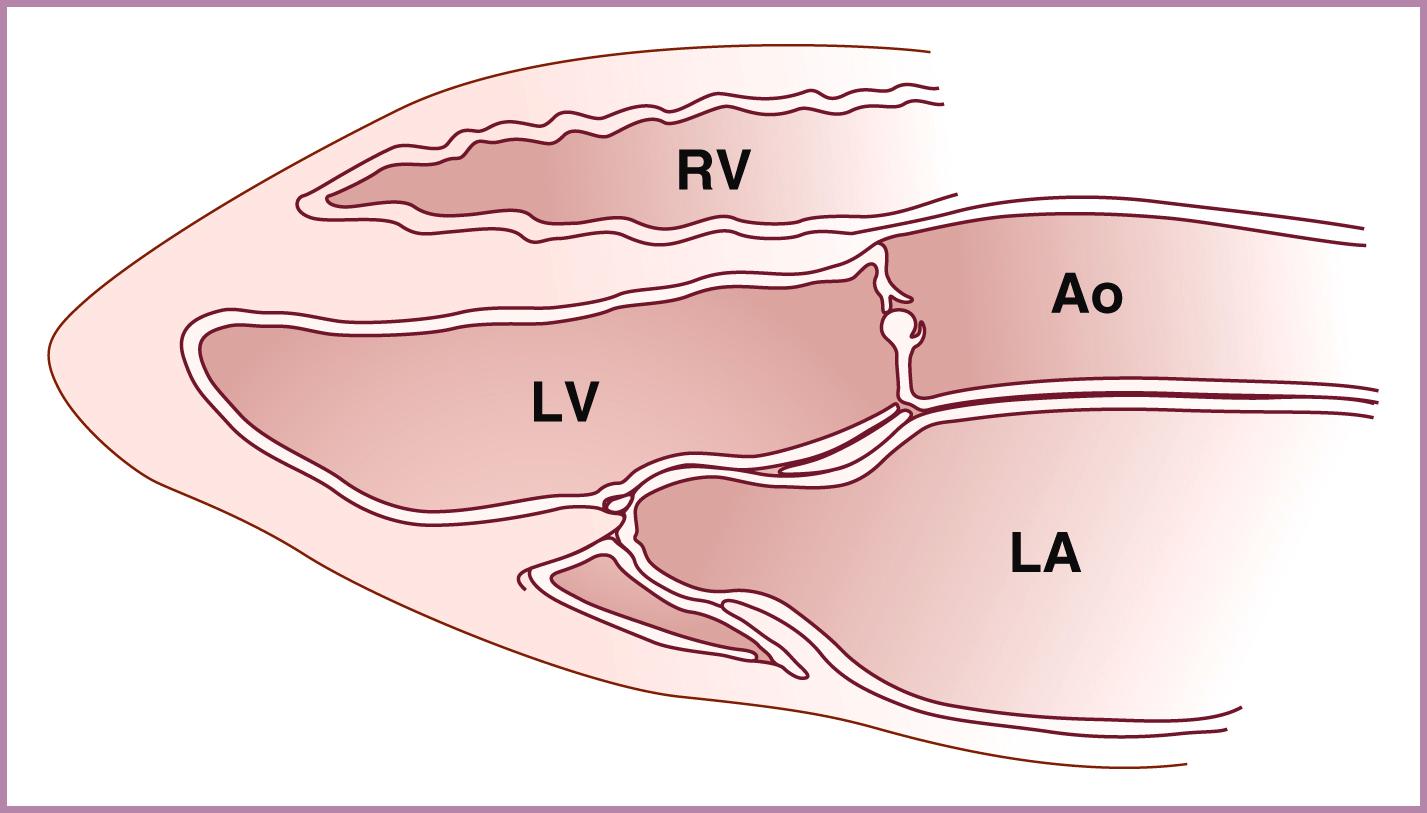
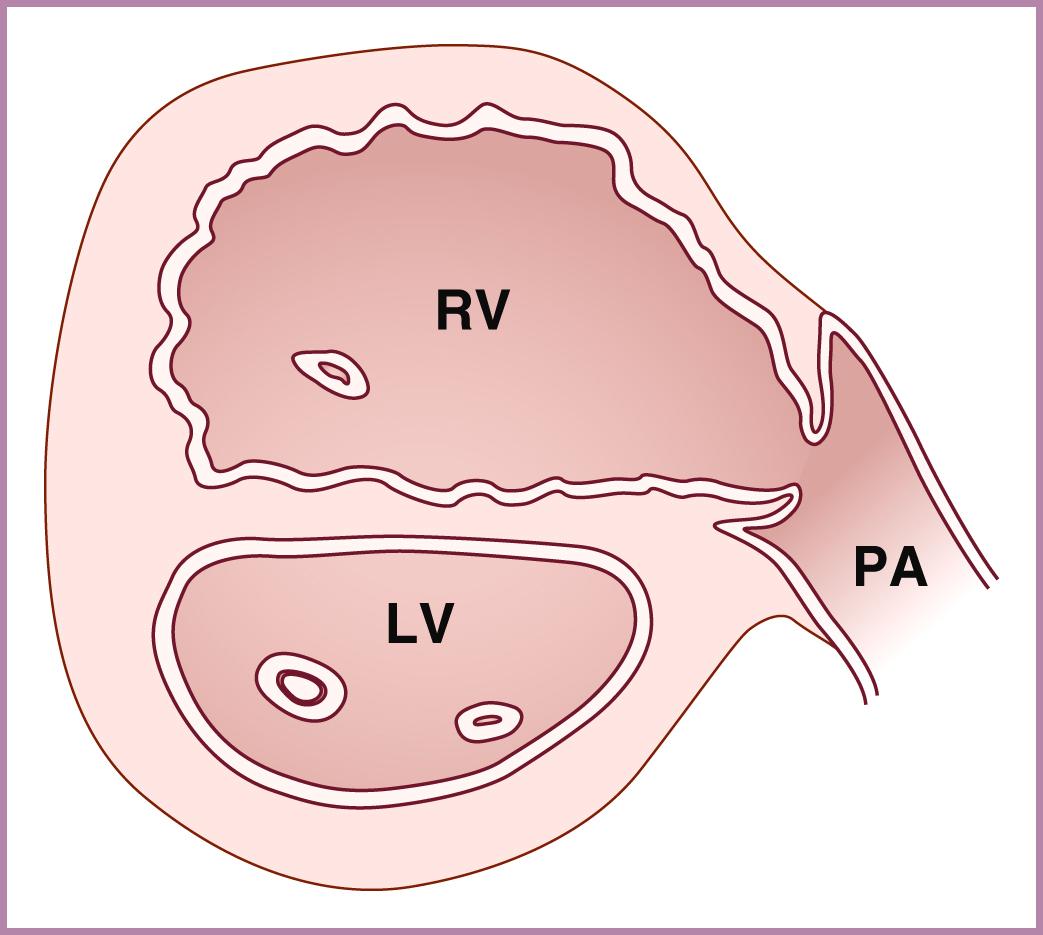
After obtaining the 4CV relatively perpendicular to the ventricular septum, slight angulation of the transducer cephalad from the 4CV should demonstrate the aortic valve and ascending aorta arising from the left ventricle ( Figs. 23.4 and 23.5 ; see , ). The aortic valve should appear thin, symmetric, and delicate, disappearing during systole. The ventricular septum should extend, uninterrupted, from the cardiac apex all the way to the anterior aspect of the ascending aorta. The inlet ventricular septum is not seen with this view. The ascending aorta should point toward the right of the spine. The ascending aorta should be followed up toward the beginning of the aortic arch to rule out posteriorly directed bifurcation (a possible sign of TGA).
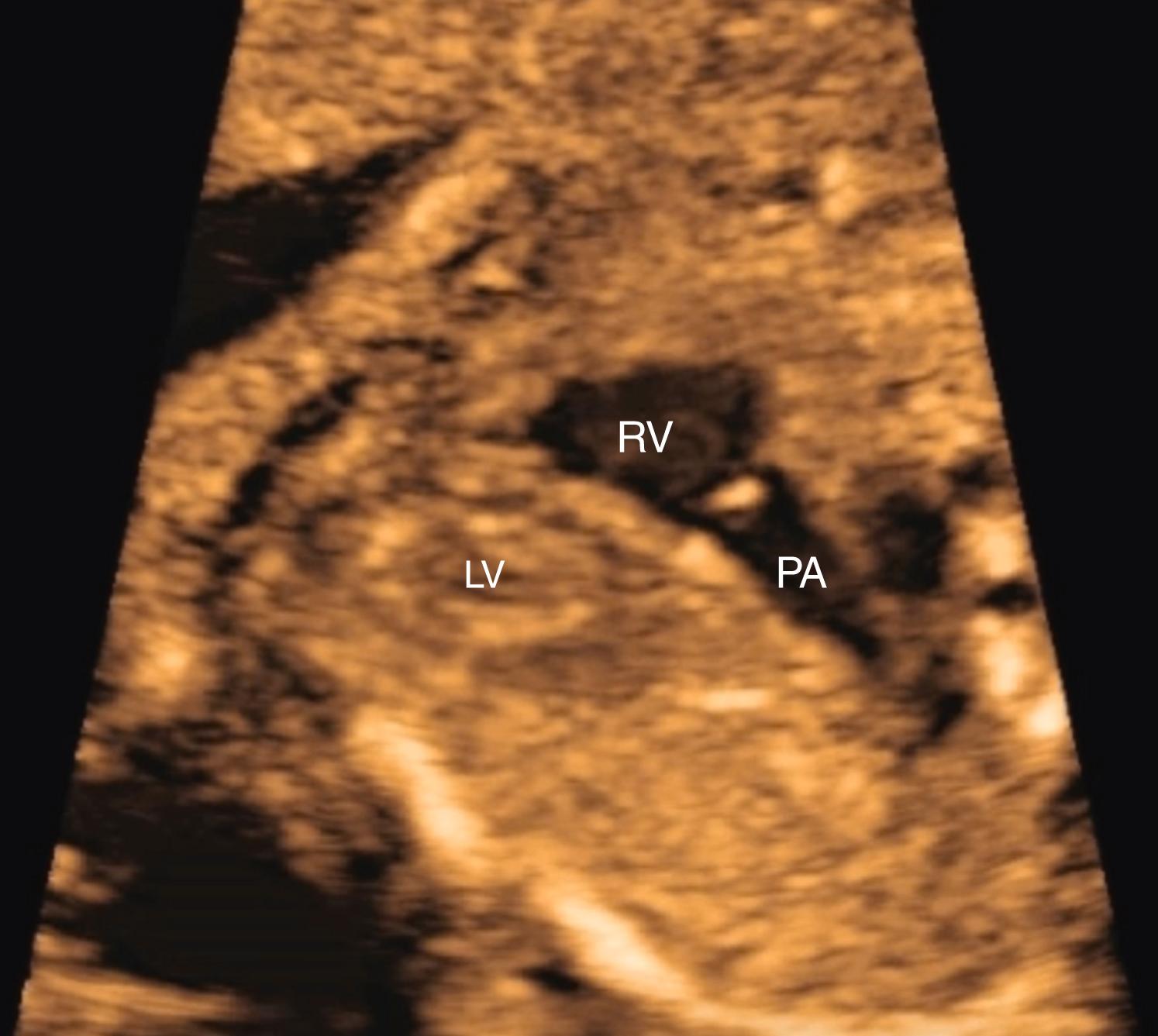
With just slightly further cephalic angulation of the transducer, the right ventricular outflow tract (RVOT) (pulmonary valve and pulmonary artery) can be visualized arising from the right ventricle and crossing the aortic root ( Figs. 23.6 and 23.7 ; ![]() ). The pulmonary valve should be thin, symmetric, and delicate, also disappearing during systole, and the pulmonary valve and main pulmonary artery should be slightly larger than the aortic valve and aortic root. The main pulmonary artery should point to the left of the spine. Evaluation of the aortic and pulmonary valves should be included as part of the outflow tract evaluation.
). The pulmonary valve should be thin, symmetric, and delicate, also disappearing during systole, and the pulmonary valve and main pulmonary artery should be slightly larger than the aortic valve and aortic root. The main pulmonary artery should point to the left of the spine. Evaluation of the aortic and pulmonary valves should be included as part of the outflow tract evaluation.
Although current US guidelines for fetal cardiac screening do not require the use of color flow Doppler or inclusion of the three-vessel tracheal view in low risk-pregnancies, , the use of both can dramatically increase detection rates for CHD. The three-vessel view is discussed in detail in the following section, and its use even in low-risk pregnancies is strongly encouraged. Likewise, cine-loop clips can be helpful, even for low-risk pregnancies (included in 2013 in the International Society of Ultrasound in Obstetrics and Gynecology [ISUOG] practice guidelines but not in 2018 by the American Institute of Ultrasound in Medicine [AIUM] practice guidelines). Sweeps to show the relationship of one portion of the fetal heart to the next, as recommended for high-risk pregnancies in the 2014 scientific statement issued by the American Heart Association, should be strongly considered for low-risk pregnancies as well.
Although the current standard for screening the low-risk pregnancy for CHD uses 2D imaging, 3D/4D fetal cardiac imaging in theory may facilitate the prenatal detection of CHD by allowing automated or virtual evaluation of the entire fetal heart after a simple, short acquisition. Ultimately, 3D fetal cardiac imaging may make acquisition of fetal cardiac data sets less dependent on time and operator skill and facilitate evaluation of the 4CV and outflow tracts offline. , However, 3D and 4D data sets of high quality remain challenging to acquire and interpret. As a result, the promise of 3D/4D fetal cardiac imaging to revolutionize fetal cardiac screening has not yet been fulfilled.
Detailed fetal echocardiography in the pregnancy at high risk for CHD provides an in-depth and comprehensive evaluation of fetal cardiovascular structure and function. , , In experienced hands, and particularly when performed beyond 18 weeks’ gestation, fetal echocardiography has been shown to have high sensitivity for almost all forms of fetal heart disease. ,
First-trimester fetal echocardiography, performed between 10 and 14 weeks’ gestation either transvaginally or transabdominally, does not have the same detection rate for CHD as second-trimester imaging, in part because of resolution considerations when imaging the smaller first-trimester heart and in part because many forms of CHD (e.g., aortic and pulmonary valvar stenosis, ventricular hypoplasia, valvar regurgitation, arrhythmias, cardiac tumors, restriction of the ductus arteriosus or foramen ovale) evolve significantly between the late first trimester and term. , ,
Nevertheless, in experienced hands the clinical role of first-trimester fetal echocardiography has increased along with first-trimester nuchal translucency screening and improvements in image resolution. , , , Early fetal echocardiography provides early reassurance in cases of normal-appearing hearts, the opportunity for early fetal interventions for the small subset of fetal cardiac conditions that may benefit from early invasive intervention, and the opportunity for early termination in cases of severe disease. Many investigators have advocated for routine evaluation of the first-trimester fetal heart by focusing on cardiac axis, symmetry of inflows, and the so-called V sign. , However, detailed first-trimester fetal cardiac evaluation requires specialized training and expertise.
This section reviews the technique of fetal echocardiography as performed transabdominally beyond 16 to 18 weeks’ gestation. Fetal echocardiography is generally performed between 18 and 22 weeks’ gestation, although even better image quality and better detection of late-onset disease occurs between 24 and 26 weeks’ gestation.
Formal fetal echocardiography represents a detailed structural and functional evaluation of the fetal heart and cardiovascular system. In practice the fetal heart and cardiovascular system are best evaluated in an anatomically systematic fashion, using various imaging modalities (i.e., 2D and color Doppler) in combination for each anatomic component of the evaluation. The order in which individual components of the evaluation are performed may vary with case-specific clinical and imaging considerations. Cardiac function should routinely be assessed qualitatively with high-resolution, real-time 2D imaging, spectral Doppler, and color flow Doppler, which is exquisitely sensitive to valvar regurgitation, flow through small VSDs, and mapping pulmonary and systemic venous return.
Various quantitative measurements , , may be performed primarily when an abnormality is suspected, and the AIUM practice standard for fetal echocardiography calls for extensive cardiac Doppler and biometry in all fetal echocardiograms. Spectral Doppler echocardiography is especially useful for flow across vessels or valves with suspected pathology. , , , Any structure that appears small or large should be measured several times, with the best reproducible measurement converted to its respective z -score. More sophisticated tissue Doppler studies , , or other quantitative means of evaluation of ventricular diastolic , or combined diastolic and systolic function may be used by trained individuals in special circumstances. More recently, sophisticated speckle tracking techniques have demonstrated novel quantitative descriptions of fetal heart size, shape, and function, , including assessment of strain and regional wall motion abnormalities, by dividing the heart into multiple segments ( Fig. 23.7 ). As such sophisticated technologies become more widespread and more user-friendly, enhanced quantitative evaluation of fetal heart function may become a more routine component of fetal echocardiography.
This section describes a clinical approach to fetal echocardiography, with an emphasis on the importance of routine 2D and color flow imaging. In contrast to the 2020 guidelines published by the AIUM, this author has not routinely performed cardiac biometry or spectral Doppler.
The formal fetal echocardiogram begins with a determination of the number of fetuses, their levels of activity, their respective positions, and their gestational ages. With multiple-gestation pregnancies, each fetus should undergo its own detailed fetal echocardiogram, and noting the fetal lie or position helps to distinguish one fetus from another. Gestational age determination using fetal biometry enables assessment of fetal growth, may affect counseling or management strategies, and allows assessment of cardiac structures that vary in appearance with gestational age.
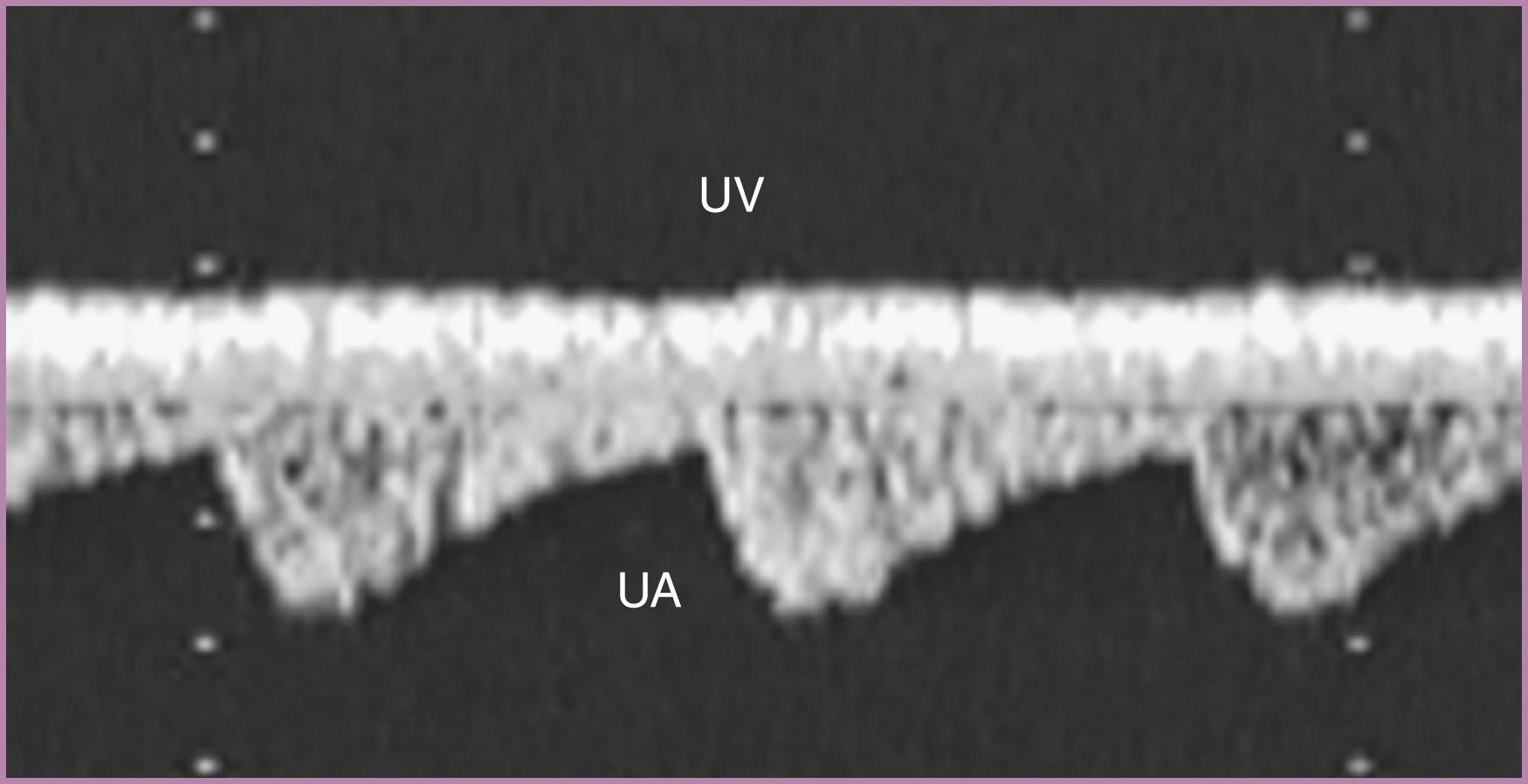
Evaluation of the umbilical cord ( Fig. 23.8 ) may be particularly useful in cases of suspected placental insufficiency or fetal heart failure, but demonstration of a three-vessel cord should be performed routinely. Spectral Doppler evaluation of the umbilical artery provides information on placental impedance, which may affect fetal cardiovascular function and reflect overall fetal well-being, although it is only considered clinically indicated in fetal growth restriction. In cases of suspected placental pathology, calculation of a pulsatility index enables a quantitative assessment of placental impedance. Doppler evaluation of the umbilical vein in the free-floating cord is most useful in cases of suspected fetal heart failure. Normally the umbilical venous waveform pulsates in response to fetal breathing and should not vary with the fetal cardiac cycle. Pulsatile venous flow in the free-floating umbilical cord, reflecting fetal cardiac contractions, suggests elevated fetal right atrial pressure and may represent CHF.
Initial evaluation of the fetus itself should assess for the presence of fluid accumulation suggestive of CHF. Normally, no more than a trivial (loculated and <2 mm) pericardial effusion should be present. A greater accumulation of pericardial fluid (circumferential or >2–2.5 mm) ( Figs. 23.9 and 23.10 ) or the presence of any pleural fluid or ascites (see Fig. 23.1 ) should be considered abnormal, with a differential diagnosis that includes CHF, myopericarditis, viral infection, anemia, and aneuploidy. The significant accumulation of fluid in two or more compartments is considered fetal hydrops.
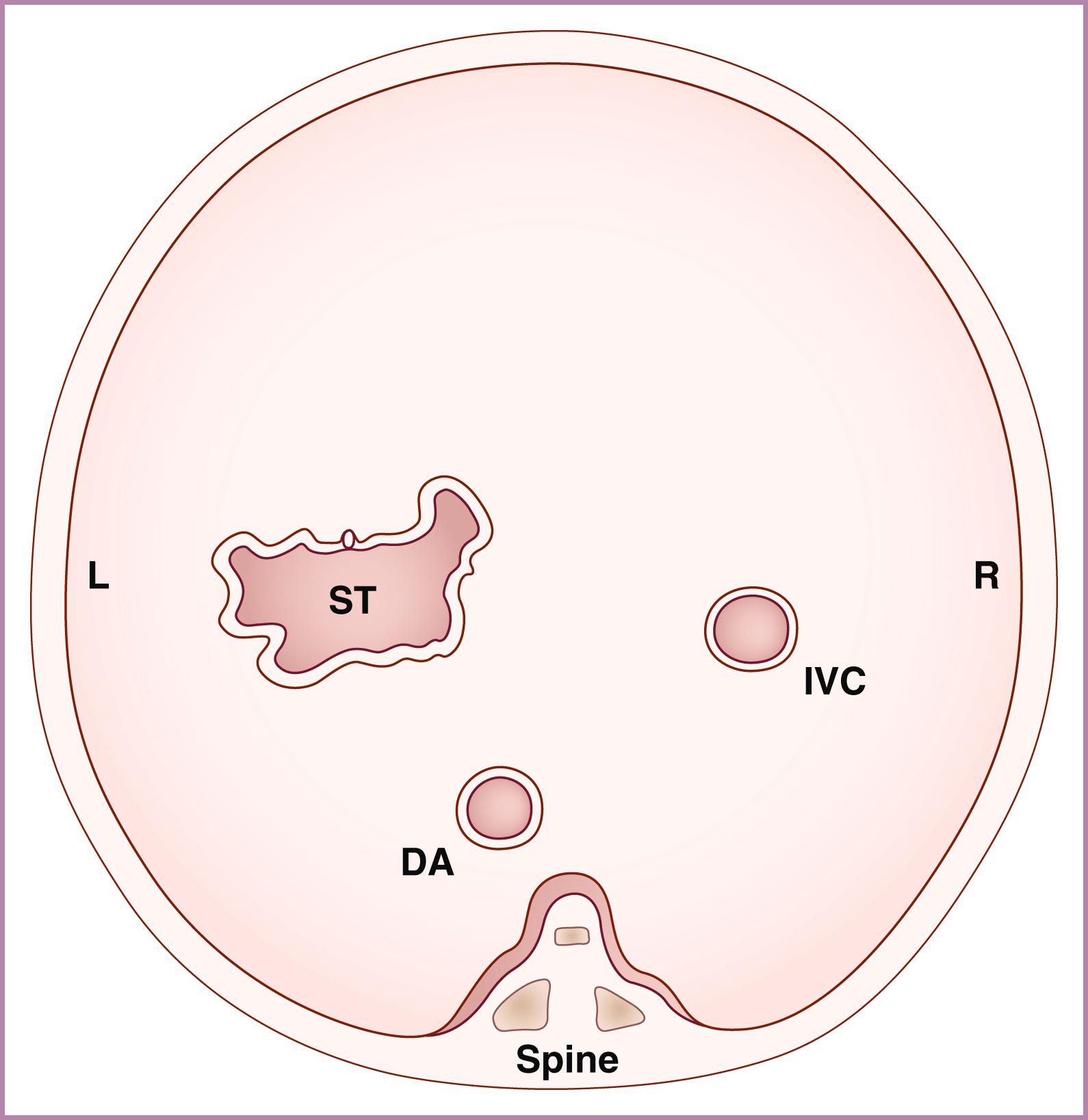
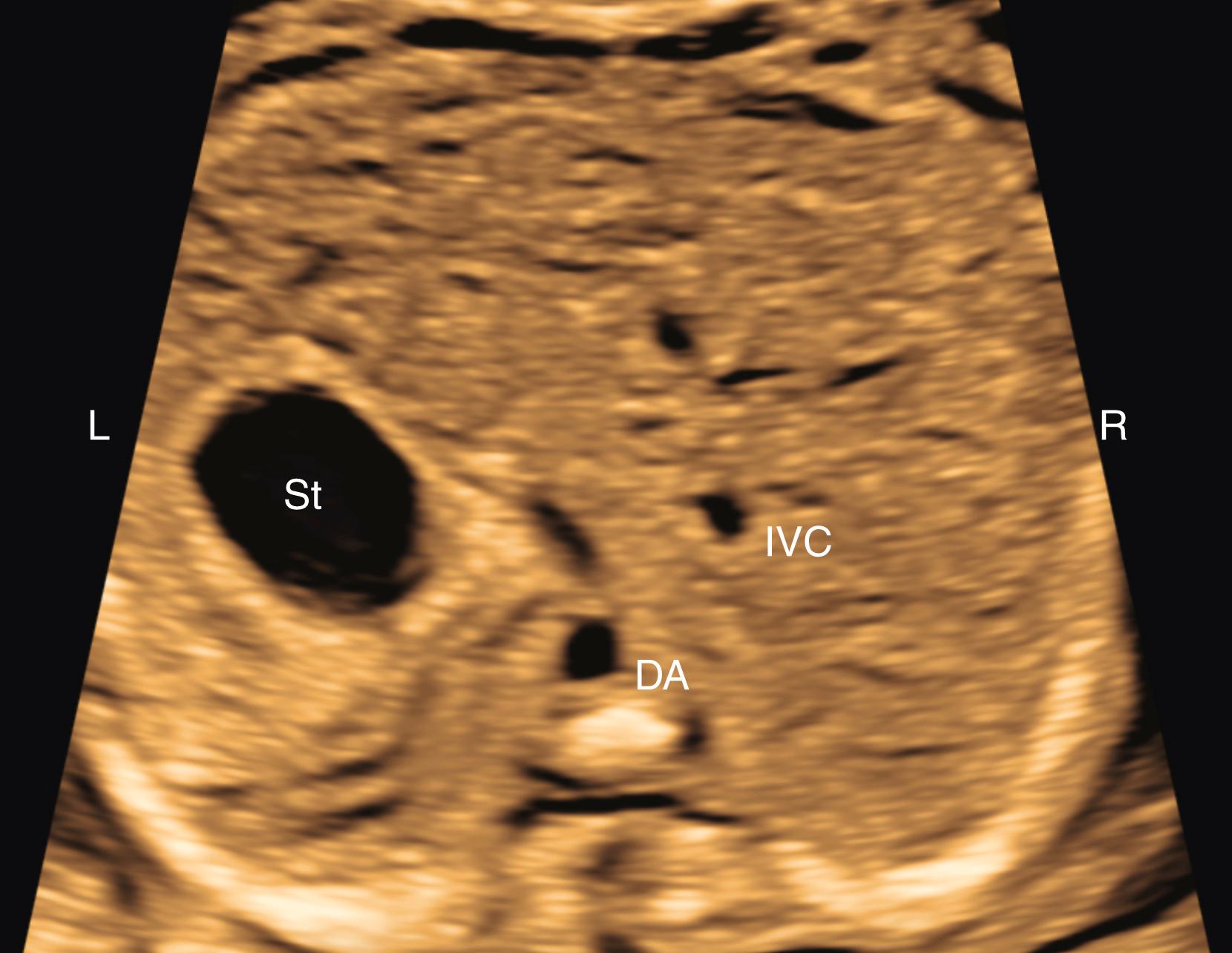
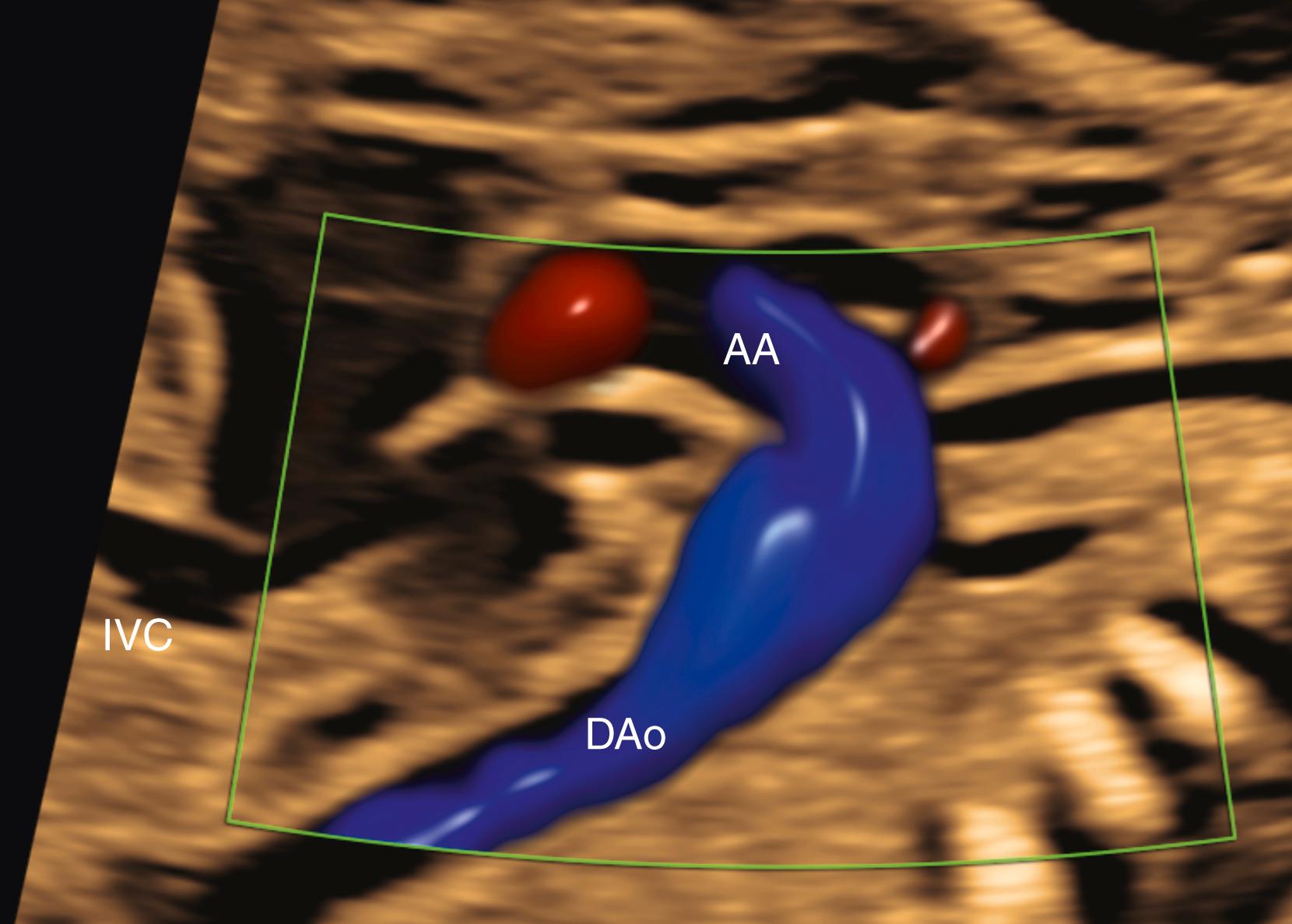
After the determination of fetal number, position, gestational age, and presence or absence of hydrops, attention should be directed toward the structure and function of the fetal cardiovascular system itself. This assessment begins with an abdominal assessment of visceral situs ( Figs. 23.11 and 23.12 ; see ). Transverse imaging of the abdomen should demonstrate the descending aorta, in cross section, just anterior to and slightly leftward of the spine. The stomach should be anterior and leftward, and the inferior vena cava (IVC) and liver should both be anterior and rightward. A large vein (usually the azygos) should not be visualized posterior to the descending aorta ( Fig. 23.13 ); a small azygos vein in this location may be seen in normal fetuses. Angulation of the probe from transverse to sagittal imaging should demonstrate the IVC draining into the right atrium. Finally, transverse imaging of the thorax should demonstrate the heart on the left, with the apex directed anteriorly and leftward. Abnormalities of any of these features should prompt further evaluation for situs abnormalities (heterotaxy), CHD, or both.
As with screening, detailed fetal echocardiography begins by demonstrating the fetal heart in the left thorax with the apex directed approximately 45 degrees to the left (see Figs. 23.2 and 23.3 and ). Left axis deviation has been associated with CHD, particularly abnormalities of the outflow tracts. The heart should fill less than one-third of the area of the thorax, and the heart circumference should be less than half the thoracic circumference. Quantitative measurement of the axis should be considered optional unless an abnormality is suspected.
The fetal heart’s right-sided structures should be equal to or, more commonly, slightly larger than their respective left-sided structures. Mild right heart disproportion may occur in some cases of trisomy 21, even in the absence of structural disease. With advancing gestation the right side of the heart becomes progressively more dominant, making the diagnosis of right heart disproportion much more common during the third trimester than during the second. For this reason, false-positive diagnoses of coarctation of the aorta occur most frequently late in gestation.
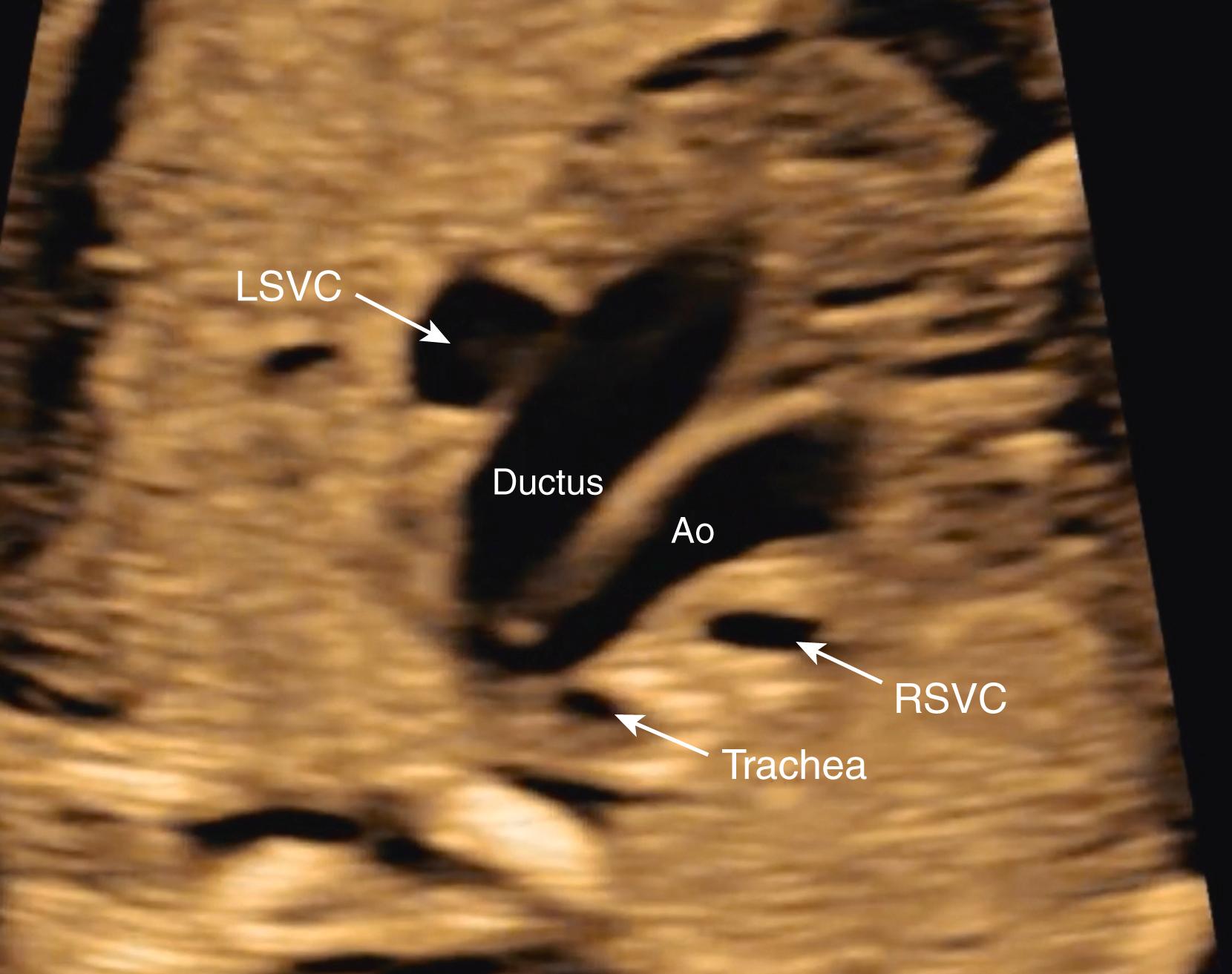
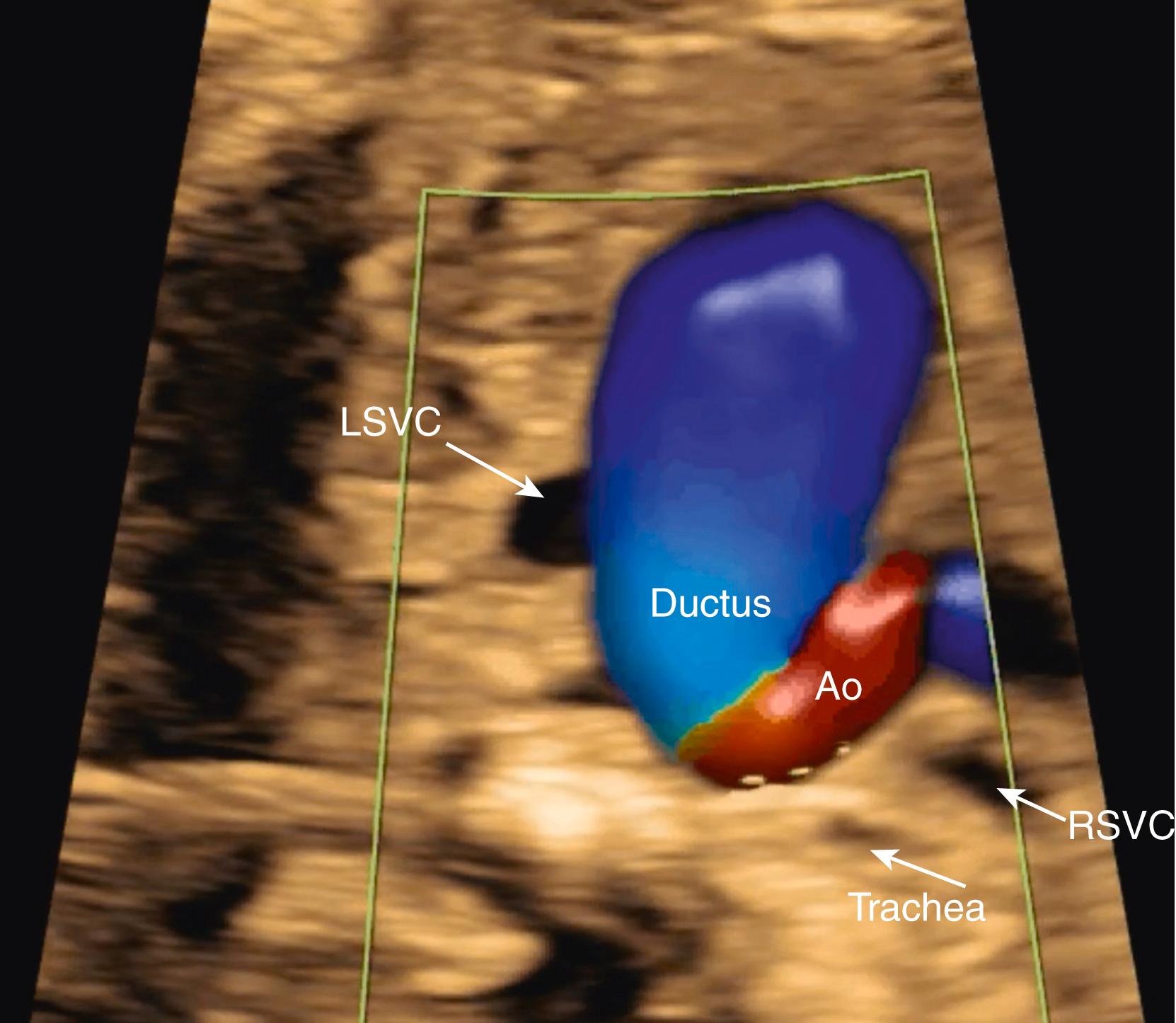
Although evaluation of systemic and pulmonary venous drainage may be accomplished with 2D imaging alone, color flow imaging helps to confirm the anatomy and to demonstrate areas of obstruction, and spectral Doppler may help to assess cardiovascular status still further. , , With slight inferior angulation or tilting of the transducer from the 4CV, the coronary sinus should be seen coursing from left to right just above the mitral groove ( Fig. 23.14 ). At the level of the 4CV the coronary sinus sometimes may be seen in cross section laterally, adjacent to the left atrial free wall just above the mitral annulus. However, enlargement of the coronary sinus ( Fig. 23.15 ) should prompt evaluation for either a left-sided superior vena cava (LSVC) ( Fig. 23.16 ) or anomalous pulmonary venous drainage into the coronary sinus. All four pulmonary veins may be seen, but such visualization can be time consuming and challenging; therefore visualization by color flow Doppler of even one or two pulmonary veins ( Figs. 23.17 and 23.18 ; see Fig. 23.3 ) draining normally to the left atrium is generally sufficient in the absence of suspected disease ( note: the presence of mesocardia/dextrocardia may be associated with partial anomalous pulmonary venous return [PAPVR] in the context of scimitar syndrome). The presence of a ridge of tissue (normal pericardial reflection or infolding of tissue between the left atrial appendage and left pulmonary vein) extending a short distance medially from the posterior and lateral aspect of the left atrium helps to rule out TAPVR, and an increased distance or the presence of a vascular structure between the descending aorta and the left atrium (twig sign) raises suspicion for TAPVR. , Color flow imaging should be performed to confirm normal, unobstructed pulmonary venous return to the left atrium. Spectral Doppler analysis may be useful if color Doppler suggests pathology. Structural abnormalities of pulmonary or systemic venous return raise the possibility of heterotaxy. , ,
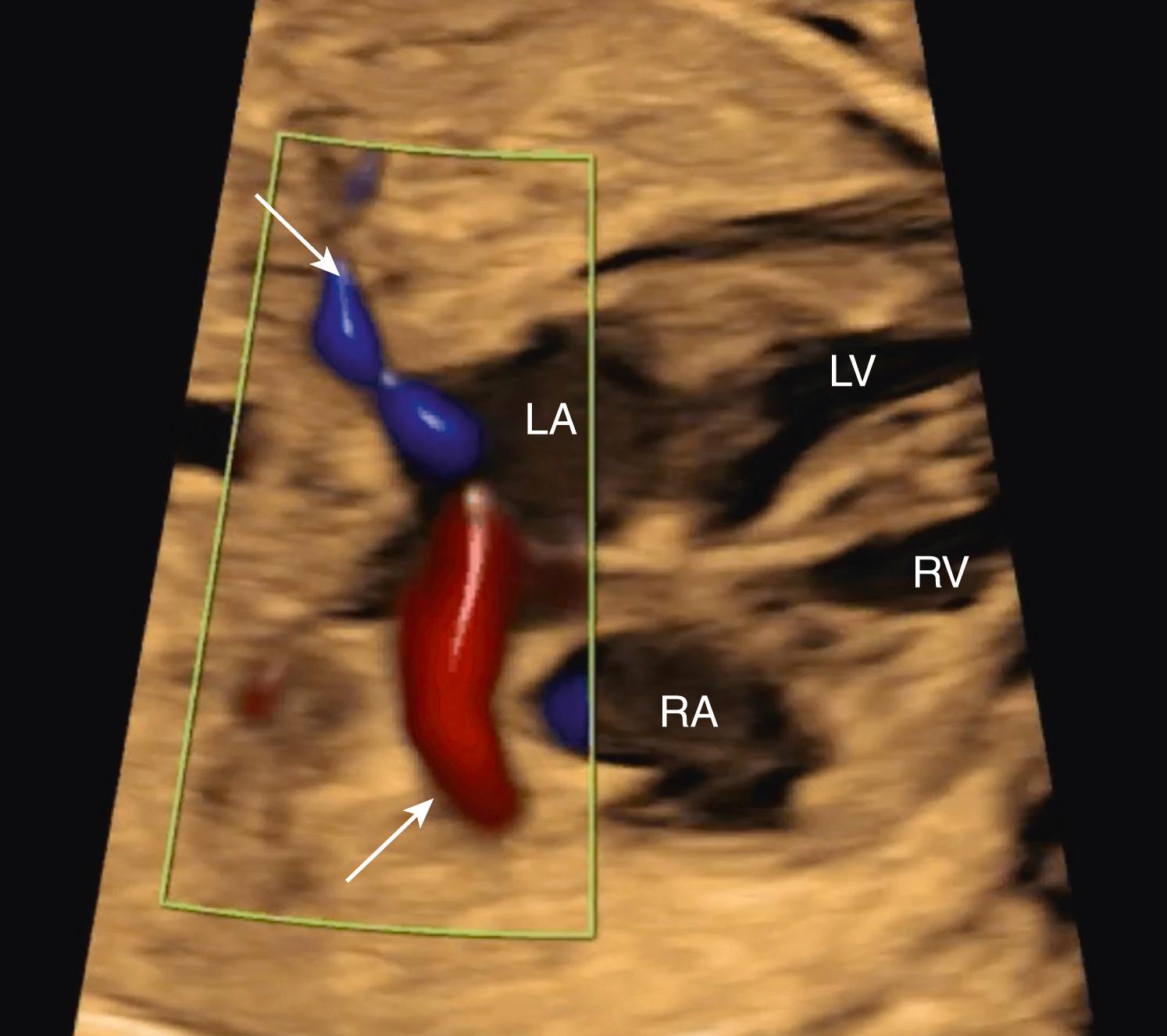
Because the foramen ovale normally shunts oxygenated blood from the right atrium to the left atrium, the flap of the foramen ovale should be deviated toward the left atrium. Although this flap typically moves back and forth during the cardiac cycle, the dominant position should be leftward (see Fig. 23.2 ). The flap itself should occupy a central position in the atrial septum, and it should extend increasingly farther into the left atrium with advancing gestational age. Both the superior and inferior aspects of the atrial septum should appear intact, although the superior rim may not always be seen with routine screening. Occasionally when dilated the coronary sinus (seen longitudinally) may generate the appearance of an absent inferior portion of the atrial septum ( Fig. 23.19 ). For this reason, if this portion of the atrial septum appears deficient, care must be taken to confirm that the image plane has not simply been directed too far posteriorly and inferiorly from the 4CV.
The atrioventricular valve morphology and function should be evaluated, along with the rest of the heart, during real-time motion. Both valves may be evaluated in their entirety from the 4CV (see Figs. 23.2 and 23.3 ), although visualization from other views may be useful, particularly if abnormalities are present or suspected. The tricuspid valve annulus should be the same size as or slightly larger than the mitral annulus. The leaflets should appear thin and delicate, with unrestricted diastolic excursion into their respective ventricles. A subtle abnormality of either valve early in the second trimester may progress to a severe abnormality, or even to hypoplastic right or left heart syndrome, at term. The septal leaflet of the tricuspid valve should insert slightly more apically onto the ventricular septum than the septal leaflet of the mitral valve. The papillary muscles of the tricuspid valve should have attachments to the ventricular septum and the right ventricular free wall. In contrast, the papillary muscles of the mitral valve should have no attachments to the ventricular septum. Whereas evaluation of the crux of the heart should include imaging perpendicular to the ventricular septum, evaluation of the atrioventricular valves should always include color flow imaging parallel to the septum to assess for diastolic turbulence or valvar regurgitation. A trace amount of early systolic tricuspid regurgitation may be normal, although previously many investigators considered any prenatal tricuspid regurgitation to be abnormal. Prenatal regurgitation of any other valve is generally considered abnormal. Fetuses with trisomy may have an increased incidence of tricuspid regurgitation, even in the presence of a structurally normal heart. Measurement of tricuspid and mitral valve annulus size and spectral Doppler evaluation of inflow patterns , should be performed if an abnormality is suspected and are called for routinely in the AIUM practice parameter.
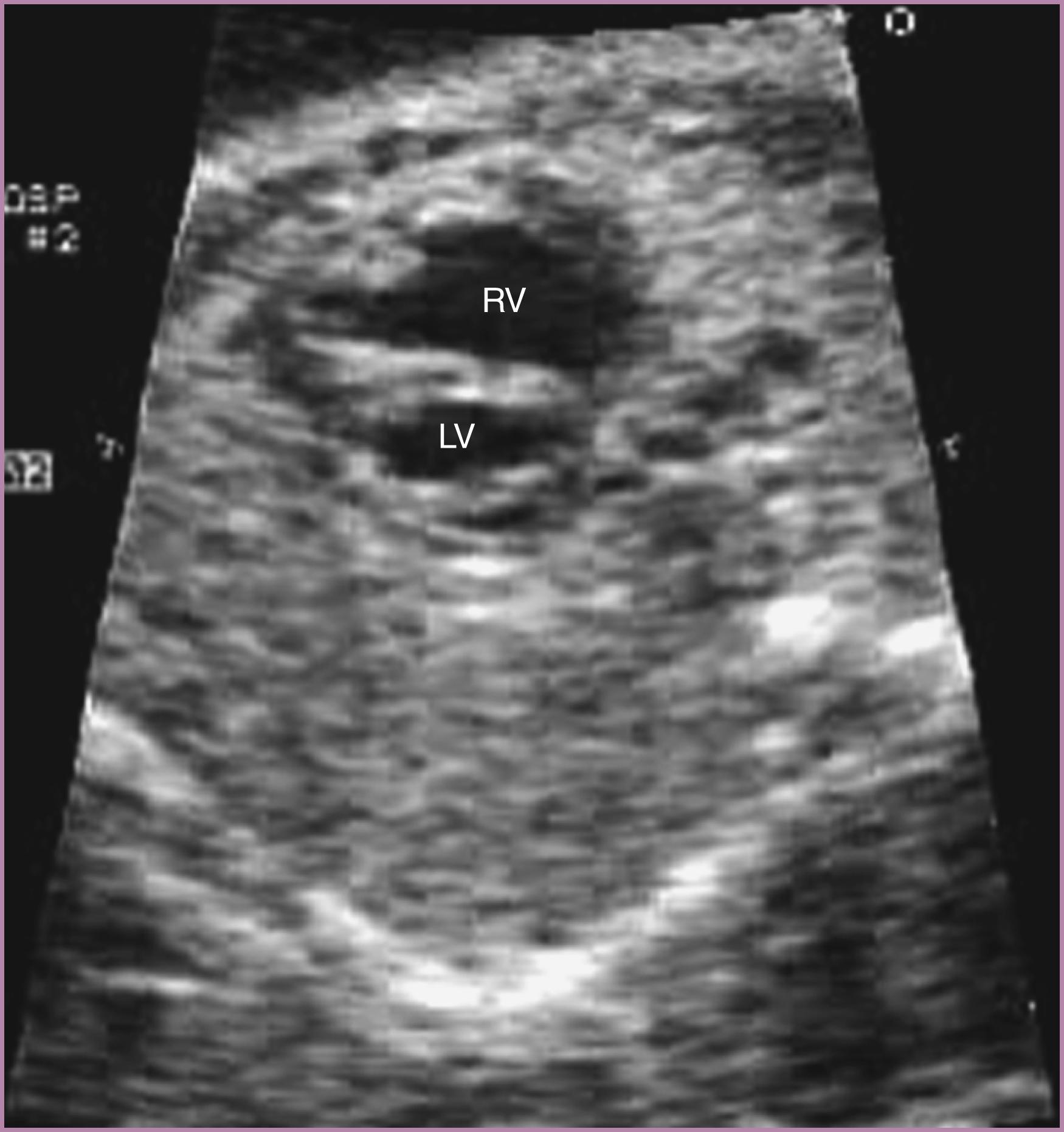
The structure and function of both ventricles should be evaluated in detail. The 4CV provides the single best perspective to evaluate ventricular structure (see Figs. 23.2 and 23.3 ). Both ventricles should extend to the cardiac apex, and both should squeeze symmetrically during systole. The left ventricle should be smooth walled, aligned with the mitral valve, and located leftward and posterior to the right ventricle. The right ventricle should be more heavily trabeculated and should have a moderator band that crosses it near the apex; it should be aligned with the tricuspid valve and located rightward and anterior to the left ventricle. The right ventricle should be equal in size to or, more commonly, slightly larger than the left ventricle, and the right heart predominance increases normally with advancing gestational age. Right heart disproportion ( Fig. 23.20 ) has been associated with trisomy 21. Quantitative assessment of ventricular systolic and diastolic function , , , , is called for routinely in the AIUM practice parameter. Additionally, close attention to ventricular systolic function should always be made qualitatively and from multiple views, including the 4CV and the basal short-axis view (obtained from a sagittal fetal orientation) ( ![]() ). Ventricular dysfunction may be isolated and primary, or it may be associated with structural heart disease.
). Ventricular dysfunction may be isolated and primary, or it may be associated with structural heart disease.
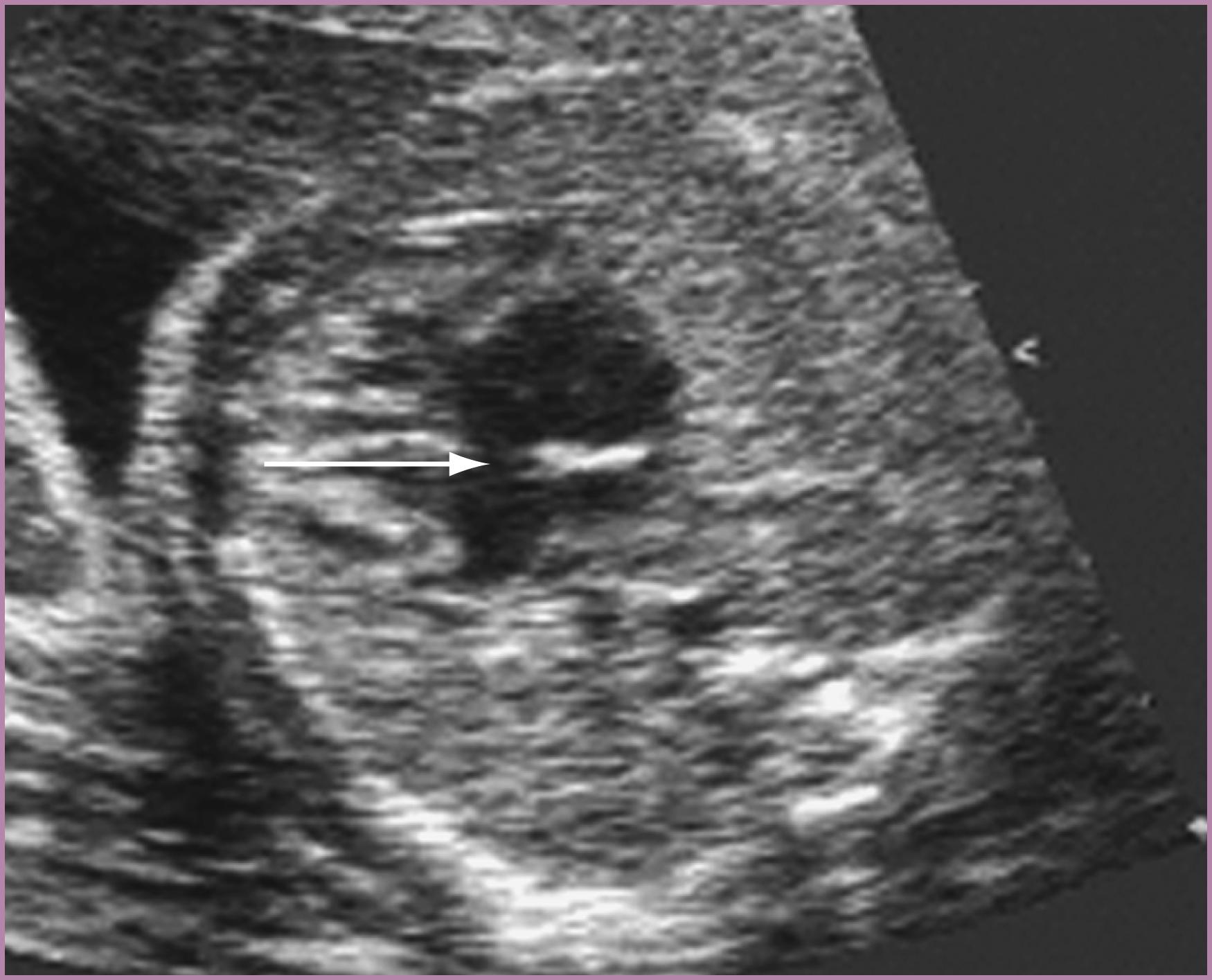
In the absence of related structural/functional pathology, the presence of small, circumscribed, echogenic densities, foci, or reflectors in the chordal apparatus of the mitral valve (or, less commonly, the tricuspid valve) should be considered benign normal variants ( Fig. 23.21 ). If doubt exists regarding the diagnosis, further evaluation should be performed to rule out CHD. Such echogenic foci appear to be more prevalent among fetuses with aneuploidy than among those with normal chromosomes. An important but sometimes subtle distinction should be made between these echogenic reflectors and a more diffuse echogenicity along the mitral and tricuspid valve annuli, throughout the supportive apparatus of the mitral and tricuspid valves, and occasionally more generally in other aspects of the endocardium ( Fig. 23.22 ); this unusual manifestation of fibroelastosis can represent damage related to maternal anti–SS-A/SS-B antibodies.
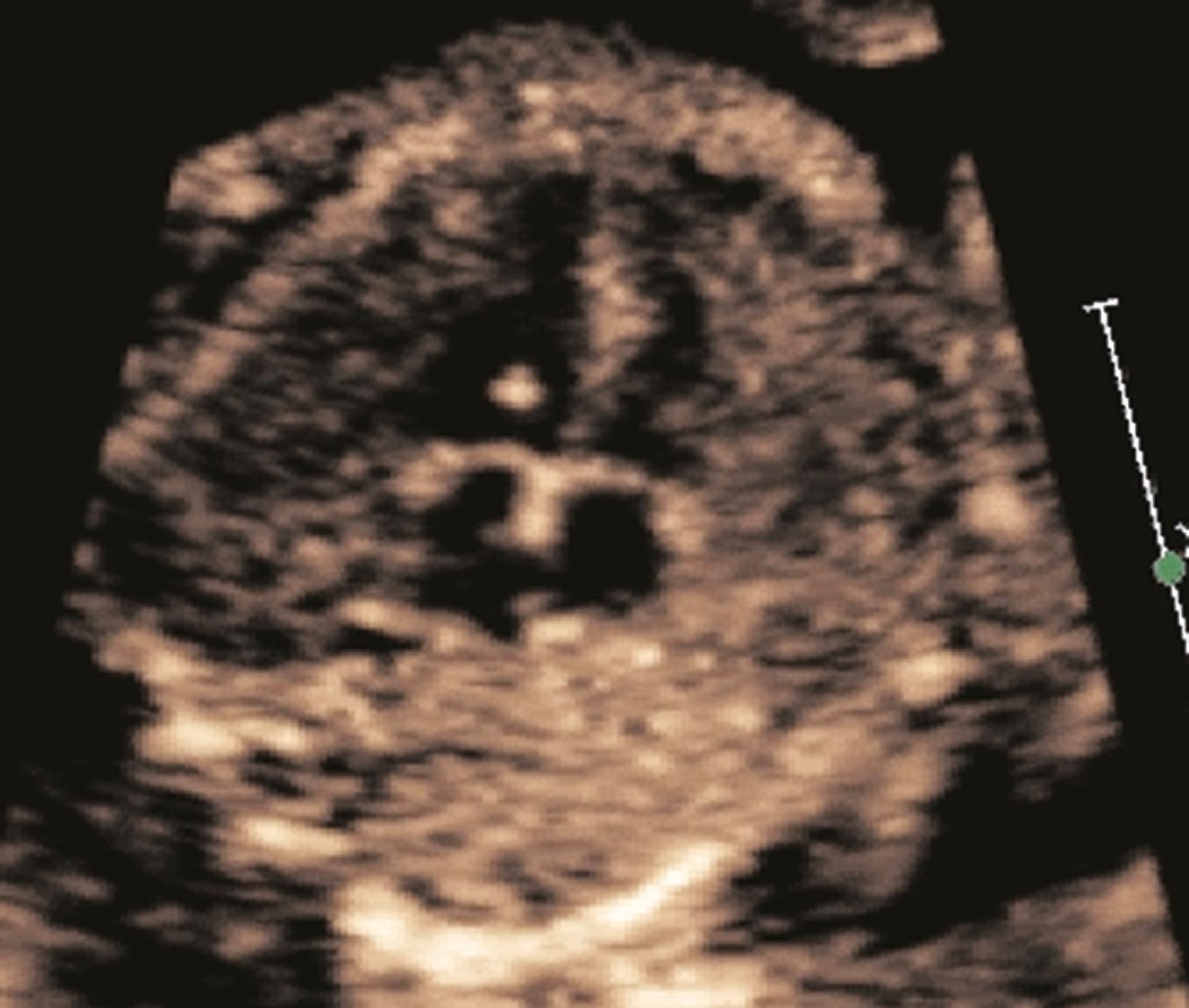
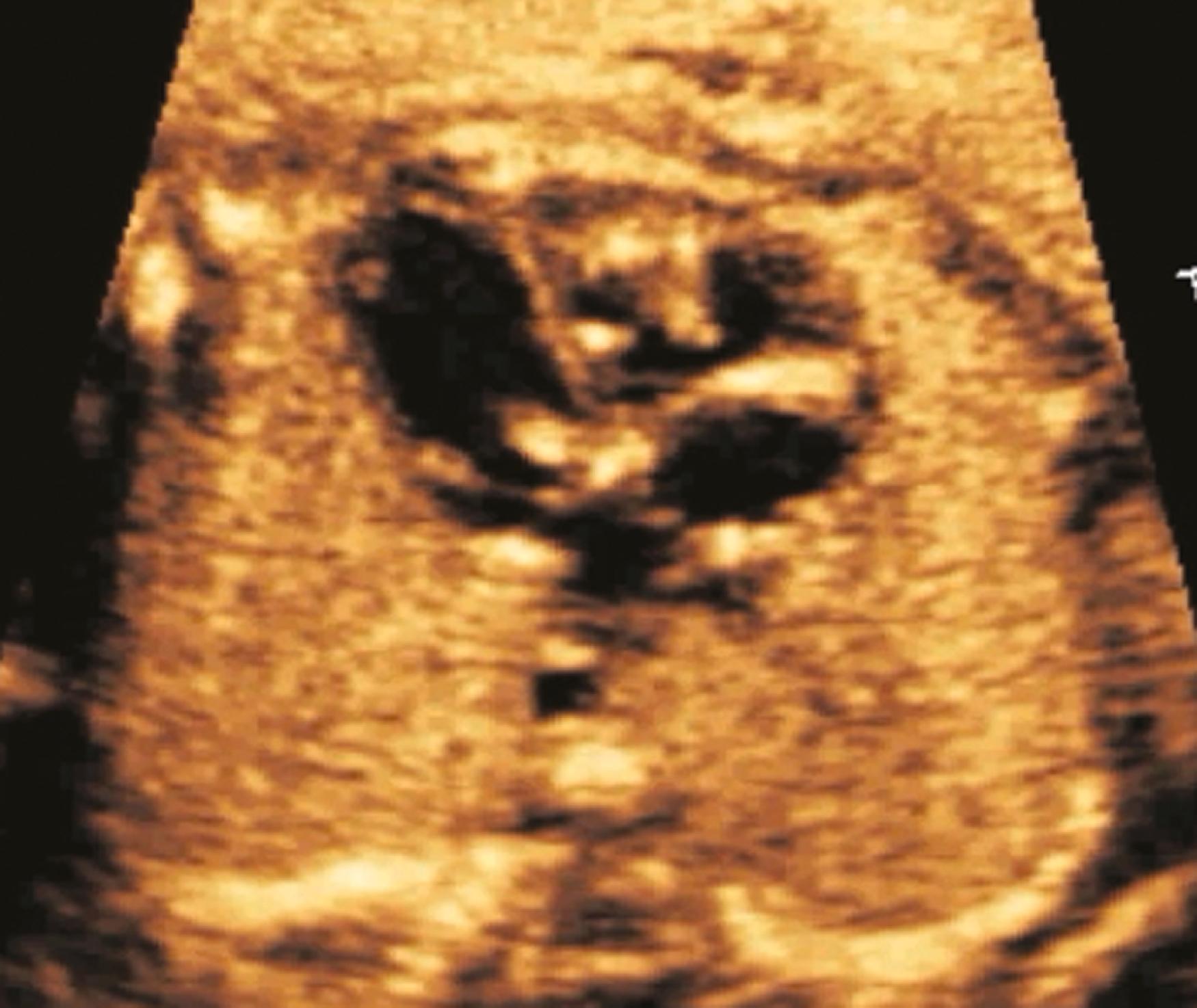
The ventricular septum should be evaluated for thickness, motion, and the presence of a VSD. The ventricular septum should be roughly the same thickness as the left ventricular posterior free wall, and it should contract symmetrically with the left ventricular free wall. Ventricular septal thickness may be evaluated from the 4CV and the basal short-axis view. In cases of suspected septal hypertrophy the septal thickness should be measured, typically during both diastole and systole. The entirety of the ventricular septum then needs to be evaluated for VSDs. Using multiple views and multiple sweeps, 2D imaging should be performed to identify any defect in any portion of the ventricular septum. To avoid false-positive dropout in the inlet and membranous or outlet portions of the ventricular septum, the 2D imaging should be performed relatively perpendicular to the ventricular septum and with the transducer angled slowly from the 4CV into the left ventricular long-axis view (see Figs. 23.4 and 23.5 and ). Color flow imaging should also always be performed in search of VSDs ( Fig. 23.23 ), using the same perpendicular orientations and sweeps; some defects may not be visualized with 2D imaging alone. On the other hand, some defects, particularly before 18 to 20 weeks’ gestation, may be evident with 2D imaging but have no visible shunting with color flow evaluation.
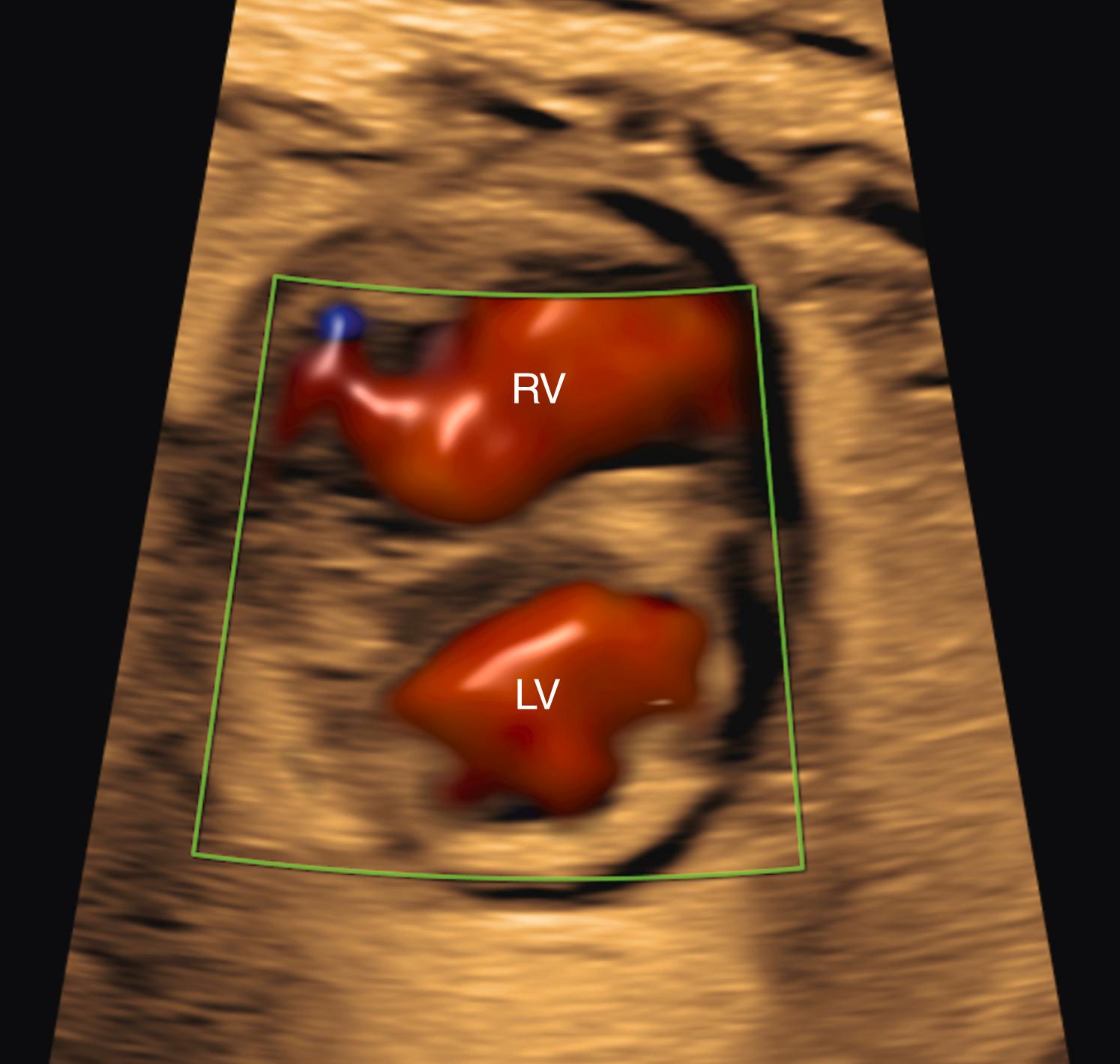
The semilunar (aortic and pulmonary) valves should be evaluated with at least as much attention to anatomy and function as is given to the evaluation of the atrioventricular valves. Consistent with the right-sided predominance seen at the levels of the atrium, atrioventricular valve, and ventricle, the pulmonary valve annulus should be equal in size to or, more commonly, slightly larger than the aortic valve annulus. The aortic valve can be best visualized with a left ventricular long-axis view (see Fig. 23.5 ), which is obtained with slight anterior/superior angulation from the 4CV. In addition, ideally the probe can be rotated slightly from a transverse cut toward a partially sagittal plane. The aortic valve may be evaluated further with a basal short-axis view, which is obtained from a sagittal orientation of the fetus. The pulmonary valve (RVOT crossing view; see Fig. 23.7 ) may be evaluated with slight anterior/superior angulation from the left ventricular long-axis view. In addition, the pulmonary valve may be evaluated further with a basal short-axis view (see ). The aortic valve should arise from the anterior aspect of the left ventricle and the pulmonary valve from the anterior aspect of the right ventricle. Both aortic and pulmonary valves should be thin and delicate, with unrestricted excursion during systole, resulting in the valve practically disappearing when fully open (see Fig. 23.5 ). During diastole the aortic and pulmonary valves should appear as central points or symmetric, thin plates. The aortic valve during diastole should appear on the left ventricular long-axis view as a thin, central plate, commonly parallel to the axis of the aortic root. The pulmonary valve during diastole, in contrast, should appear on the basal short-axis view as a thin plate but perpendicular to the axis of the main pulmonary artery. A subtle abnormality of either valve ( Figs. 23.24 and 23.25 and ![]() ; see ) during the early second trimester may progress to a significant valve abnormality, or rarely even to HLHS or HRHS, at term. Measurement of aortic or pulmonary valve annulus size and evaluation of the systolic Doppler flow profile are called for routinely in the AIUM practice parameter. Evaluation of aortic and pulmonary valves should also include color flow imaging to assess for aortic or pulmonary ( Fig. 23.26 ) regurgitation (always abnormal) or systolic turbulence ( Fig. 23.27 ) suggestive of increased flow volume or anatomic obstruction.
; see ) during the early second trimester may progress to a significant valve abnormality, or rarely even to HLHS or HRHS, at term. Measurement of aortic or pulmonary valve annulus size and evaluation of the systolic Doppler flow profile are called for routinely in the AIUM practice parameter. Evaluation of aortic and pulmonary valves should also include color flow imaging to assess for aortic or pulmonary ( Fig. 23.26 ) regurgitation (always abnormal) or systolic turbulence ( Fig. 23.27 ) suggestive of increased flow volume or anatomic obstruction.
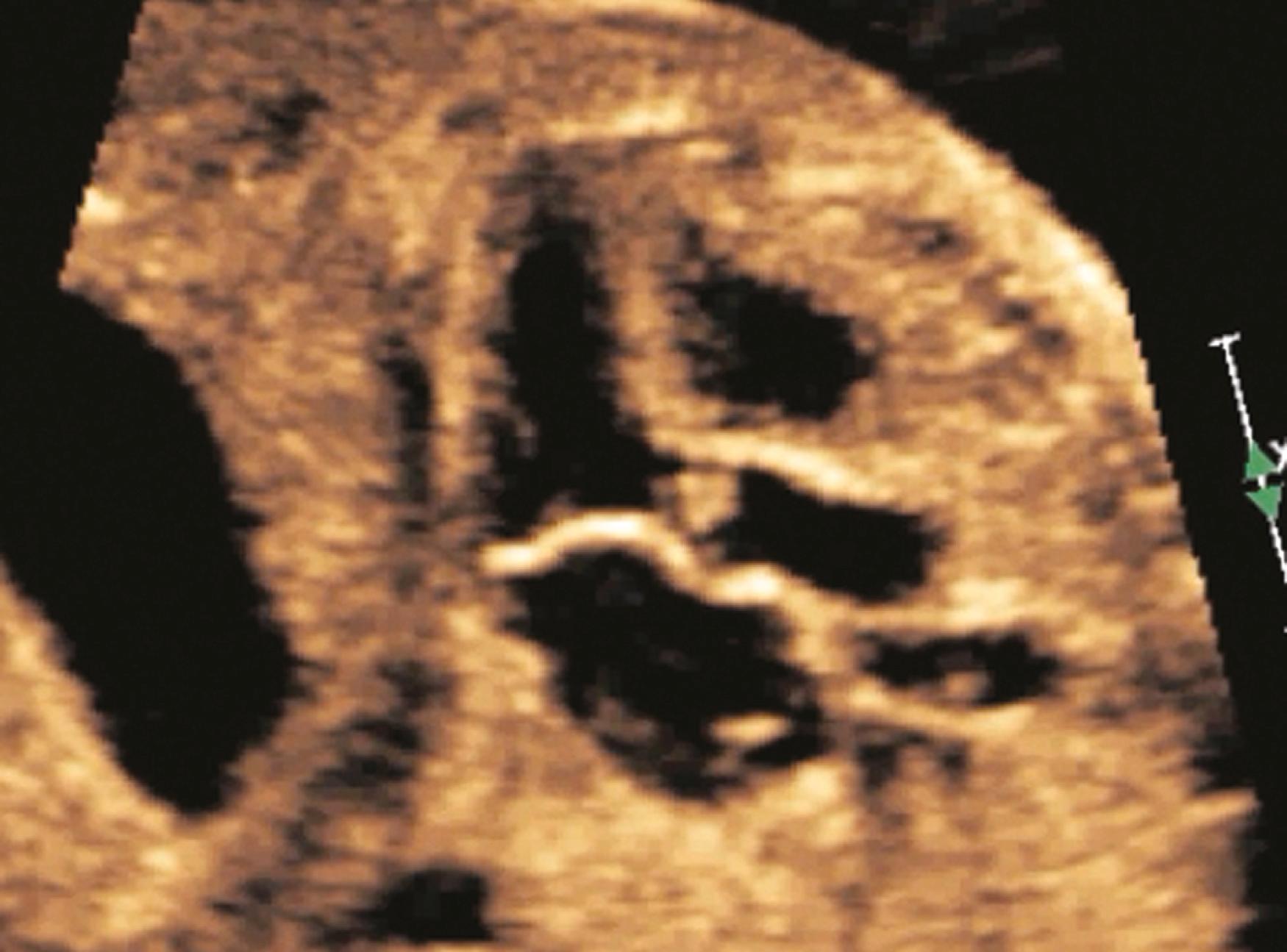
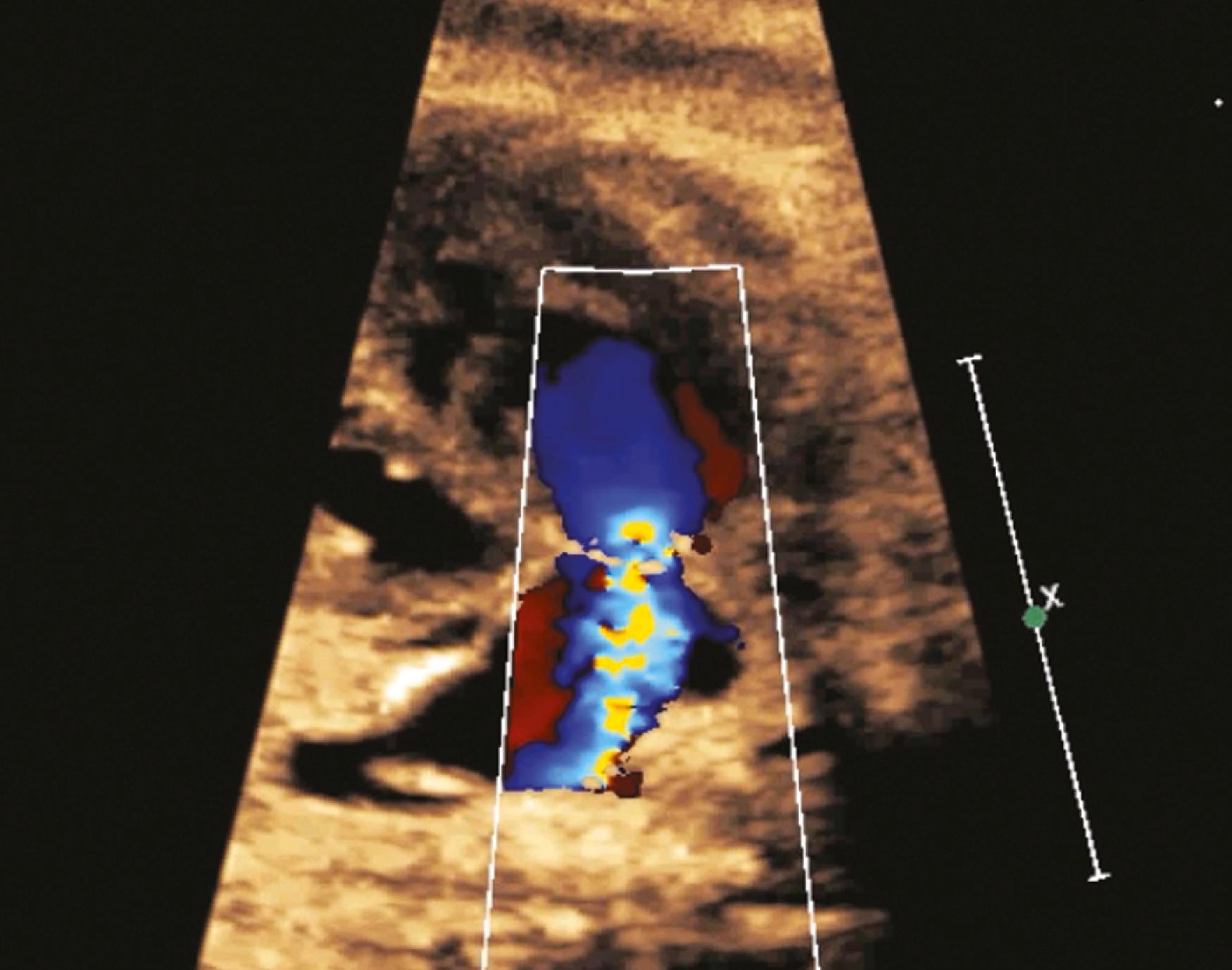
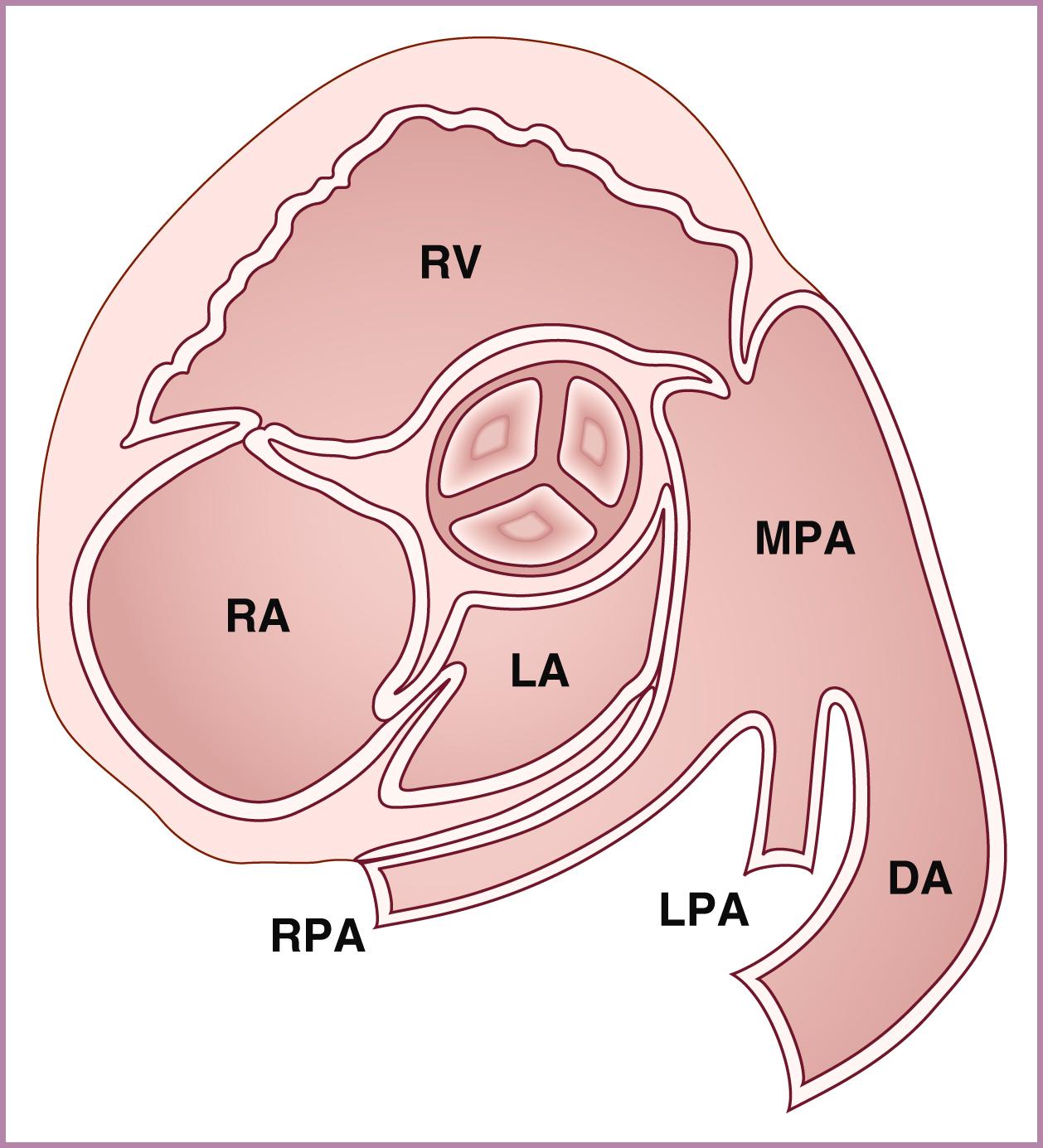
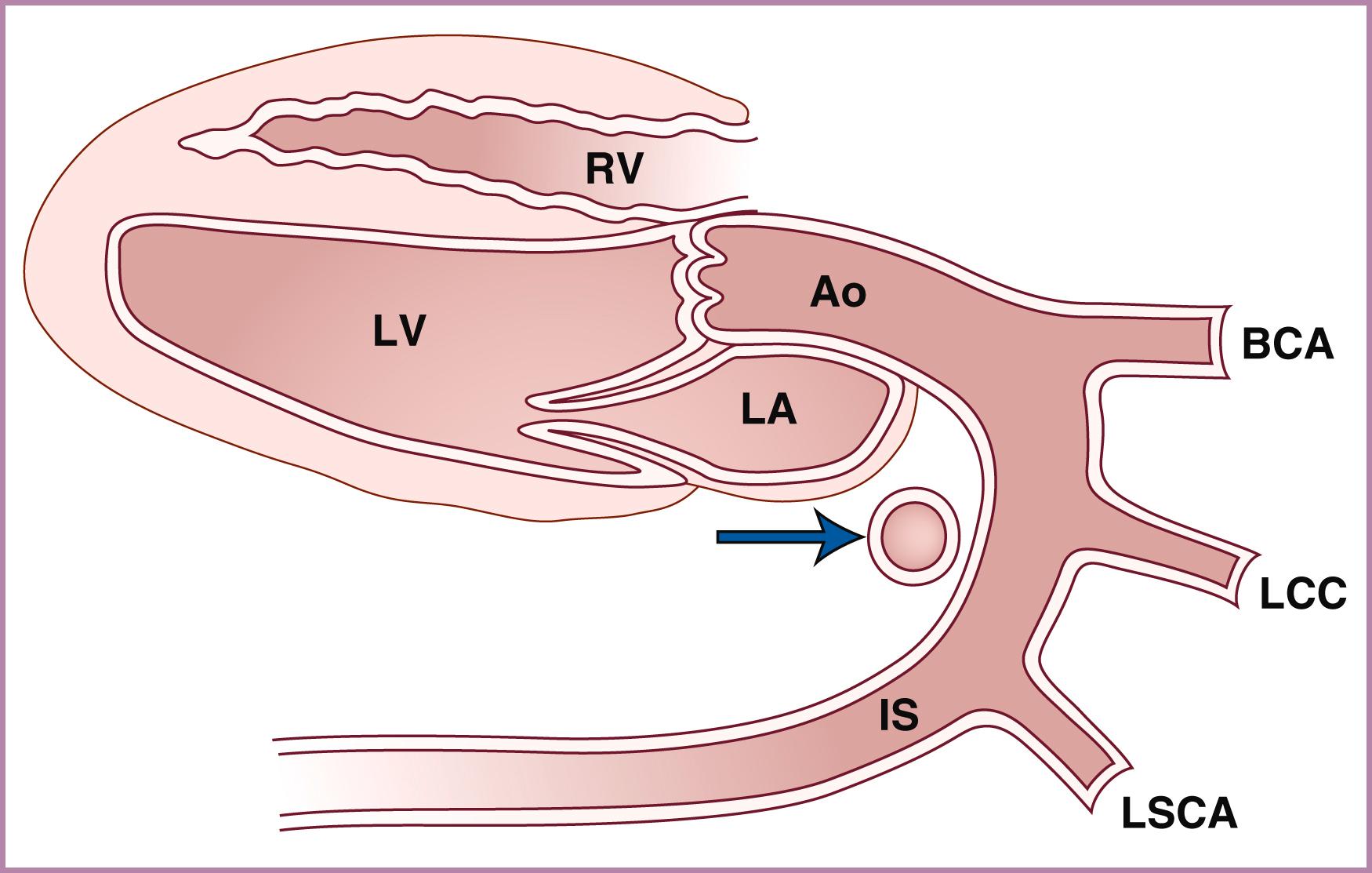
The ascending aorta (aortic root) and main pulmonary artery should arise from the left and right ventricles, respectively, and then cross at an angle of roughly 45 to 90 degrees. Demonstration of great artery crossing represents an important finding on the normal fetal echocardiogram. This crossing can be seen either with transverse imaging (slight anterior/superior angulation from the 4CV to left ventricular long-axis view to RVOT crossing view; see ) or with sagittal imaging (slight leftward angulation from sagittal aortic arch view to ductal arch view; ![]() ). In general the aortic root should point to the right of the spine (see Fig. 23.5 ), and the main pulmonary artery should point to the left of the spine (see Fig. 23.7 ). The use of a sweep, from the 4CV to the three-vessel tracheal view, can be a powerful way to rule out TGA. As with other right-left ratios in the fetal heart, the main pulmonary artery should be equal in size to or, more commonly, slightly larger than the aortic root. The ductal arch and basal short-axis views ( Figs. 23.28–23.30 ) should demonstrate the trifurcation of the main pulmonary artery into the right pulmonary artery (which wraps around the aorta in the basal short-axis view), the left pulmonary artery (which extends more posteriorly than the right pulmonary artery), and the ductus arteriosus (the largest of the three branches). The left pulmonary artery may be difficult to demonstrate, given its unusual course and easy overlapping/confusion with the ductal arch. Demonstration of the main pulmonary artery bifurcating is not adequate; both pulmonary artery branches along with the ductus must be clearly delineated. In some cases, bifurcation of the pulmonary artery into right and left branches may be best seen at the level of the three-vessel view; in other cases, the branch pulmonary arteries may be best visualized with sagittal imaging. Measurements of the pulmonary valve diameter and peak velocities in the vessels are called for routinely in the AIUM practice parameter.
). In general the aortic root should point to the right of the spine (see Fig. 23.5 ), and the main pulmonary artery should point to the left of the spine (see Fig. 23.7 ). The use of a sweep, from the 4CV to the three-vessel tracheal view, can be a powerful way to rule out TGA. As with other right-left ratios in the fetal heart, the main pulmonary artery should be equal in size to or, more commonly, slightly larger than the aortic root. The ductal arch and basal short-axis views ( Figs. 23.28–23.30 ) should demonstrate the trifurcation of the main pulmonary artery into the right pulmonary artery (which wraps around the aorta in the basal short-axis view), the left pulmonary artery (which extends more posteriorly than the right pulmonary artery), and the ductus arteriosus (the largest of the three branches). The left pulmonary artery may be difficult to demonstrate, given its unusual course and easy overlapping/confusion with the ductal arch. Demonstration of the main pulmonary artery bifurcating is not adequate; both pulmonary artery branches along with the ductus must be clearly delineated. In some cases, bifurcation of the pulmonary artery into right and left branches may be best seen at the level of the three-vessel view; in other cases, the branch pulmonary arteries may be best visualized with sagittal imaging. Measurements of the pulmonary valve diameter and peak velocities in the vessels are called for routinely in the AIUM practice parameter.
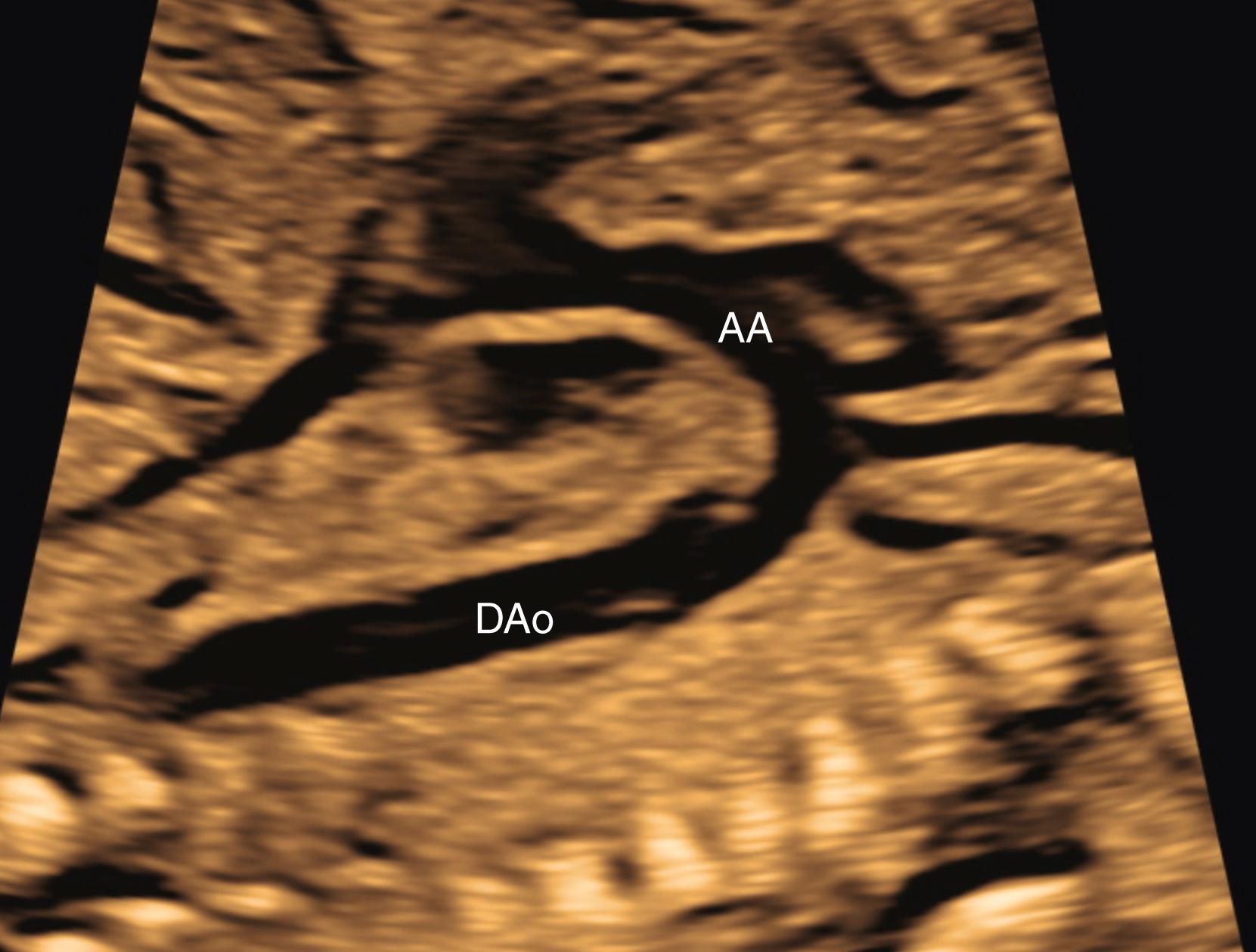
In cases of suspected pathology, the branch pulmonary arteries should be measured and their Doppler flow profiles obtained. For example, fetal pulmonary artery diameter may correlate with outcome in cases of congenital diaphragmatic hernia. In cases of suspected ductal constriction (i.e., tricuspid and pulmonary regurgitation across normal-appearing valves or fetal exposure to indomethacin ), the flow profile of the ductus arteriosus should be obtained parallel to flow on the sagittal ductal arch view. A pulsatility index of less than 1.9 suggests ductal constriction, whereas a pulsatility index greater than 3 suggests increased right ventricular output and left heart obstruction. The sagittal aortic arch view ( Figs. 23.31 and 23.32 ), obtained with rightward angulation from the ductal arch view, demonstrates the aortic arch in its entirety, including aortic root, transverse aortic arch, aortic isthmus, and descending aorta. Color flow imaging should always be performed of the entire aortic ( Fig. 23.33 ) and ductal arches. Measurements of aortic dimensions , are called for routinely in the AIUM practice parameter.
The three-vessel tracheal view has been demonstrated to be exceptionally powerful, both as a screening tool and as a component of the detailed fetal echocardiogram. , (See Figs. 23.33.1 and 23.33.2 ). As with other elements of the fetal echocardiogram, imaging should be performed with and without color Doppler and always with the real-time imaging, rather than only still-frame images. The three-vessel tracheal view should address vessel number, size, location, and direction of flow, along with the relationship of the transverse aorta to the trachea. Although in theory this view can be demonstrated simply with cephalad migration of the transverse views through the 4CV and outflow tracts, typically subtle changes in transducer pressure and angulation will be necessary to obtain optimal image quality.
Fetal CHF may be related to extracardiac disease (e.g., anemia, cerebral arteriovenous fistula, twin-twin transfusion syndrome) or to fetal heart disease ( Box 23.4 ). Some of the most severe forms of CHD (e.g., HLHS, TOF with pulmonary atresia and ductal-dependent pulmonary blood flow) do not typically generate fetal CHF. Rather, fetal heart disease that causes CHF almost invariably includes one or more of the following , , : sustained tachycardia or bradycardia (e.g., supraventricular tachycardia [SVT], complete heart block), myocardial dysfunction (e.g., dilated cardiomyopathy), valvar regurgitation (e.g., Ebstein anomaly of the tricuspid valve, TOF with absent pulmonary valve), or restrictive flow across the foramen ovale or ductus arteriosus. The fetus with absence of a valve (e.g., tricuspid atresia) or absence of a ventricle (e.g., HLHS) generally does better in utero than one with a poorly functioning valve or ventricle.
Anemia
Twin-twin transfusion syndrome
Arteriovenous malformation
Diaphragmatic hernia
Pericardial effusion
Cardiac causes
Hypertrophic cardiomyopathy
Dilated cardiomyopathy
Sustained bradycardia/tachycardia
Valvar regurgitation
Restrictive foramen ovale
Ductal constriction
Unlike the postnatal diagnosis of CHF, which relies more on clinical signs (tachycardia, tachypnea, hepatomegaly, rales, and peripheral edema) than on radiographic or echocardiographic findings, the prenatal diagnosis of CHF relies exclusively on sonographic findings. , , The sonographic findings associated with fetal CHF from any cause include (1) dilated, poorly squeezing ventricle with systolic and diastolic dysfunction; (2) cardiomegaly; (3) pericardial effusion; (4) tricuspid regurgitation; and (5) increased atrial flow reversal in the systemic veins ( Box 23.5 ). More detailed cardiac and peripheral Doppler evaluation may also be performed. , Severe forms of CHF lead to hydrops (see Fig. 23.1 ) and cardiac pulsations in the free-floating umbilical vein. The diagnosis of fetal CHF or hydrops should prompt further evaluation for clues to the etiology, as well as serial follow-up studies, because of the potential for progression of disease and even fetal demise.
Cardiomegaly
Tricuspid regurgitation
Pericardial effusion
Diminished ventricular systolic function
Increased atrial flow reversal in systemic veins
Despite great initial excitement and continued academic attention, 3D/4D fetal cardiac imaging , remains predominantly an investigational tool for improving the prenatal detection and diagnosis of CHD. Because of persistent challenges in image resolution, substantial learning curves, vulnerability to artifact, cumbersome algorithms, and expensive equipment, 3D fetal cardiac imaging has not yet become a viable alternative to conventional 2D imaging. The primary current advantage of 3D or 4D imaging over conventional 2D fetal cardiac imaging is its ability to help teach fetal cardiac anatomy and improve sonographer expertise with conventional 2D fetal cardiac imaging.
Three-dimensional imaging carries important theoretical advantages over 2D imaging that could ultimately revolutionize the clinical approach to fetal cardiac imaging. First, 3D imaging, by acquiring a comprehensive volume data set within a matter of a few seconds, makes fetal cardiac imaging less operator dependent and less time consuming to perform, at least in theory. Unfortunately, obtaining high-quality volumes requires expertise and, frequently, extended periods of time. Second, 3D volume data sets may be evaluated offline, enabling virtual examinations of the entire fetal heart, with reconstruction of any plane or sweep, after the initial short acquisition. Third, volume-rendering algorithms theoretically may display data with a “surgeon’s-eye” view, enabling visualization of the internal architecture and anatomy of the fetal heart or great arteries ( Figs. 23.34–23.36 ) from any perspective. Such displays improve comprehension of complex forms of CHD, leading to improved patient counseling and patient selection for fetal interventions. Moreover, evolving technology allows the 3D visualization of color flow jets in volume-rendered displays or as stand-alone “angiograms.” , Finally, 3D volume data sets theoretically may enable more accurate quantitative measurements of ventricular size and function, , , , which can translate into improved ability to predict the prenatal and postnatal courses of CHD from early in the second trimester (or even earlier). Unfortunately, virtual examinations remain cumbersome and challenging and uncommon outside of academic centers with particular expertise with 3D fetal cardiac imaging.
Three-dimensional fetal cardiac imaging may be performed with either a reconstructive , , , , or a real-time , , , , , approach. Reconstructive approaches, first described in 1996, , begin by acquiring a rapid sequence of parallel 2D images, with subsequent reconstruction of a volume or volumes, referred to as spatiotemporal image correlation (STIC). , Reconstructive approaches have a disadvantage in that they require the fetus and mother to be relatively motionless throughout the acquisition to minimize distortion of the reconstructed volume data set. In contrast, real-time approaches use specialized transducers to image the fetal heart as a volume, acquiring the entire fetal heart (or a portion thereof) virtually instantaneously. The volume data do not require reconstruction, and they do not require gating algorithms (as do reconstructive techniques) to view cardiac motion. Real-time approaches theoretically make the most sense for imaging the fetal heart, given their quick acquisition time, relative resistance to artifact derived from fetal motion, and lack of a need for cardiac gating. , , , , However, real-time approaches have poorer image quality than those obtained by STIC.
After volume data have been acquired, volume data sets may be reviewed interactively, displaying any plane, , rendered volume, , , or sweep of interest and theoretically allowing precise quantitative measurements of ventricular size and function. Investigators continue to develop new ways to display volume data , , , in an effort to maximally exploit the potential clinical usefulness of 3D sonographic data.
Within the next 10 years, additional approaches to 3D/4D imaging of the fetal heart will likely evolve. However, without improvements in resolution, speed, and display algorithms, 3D (or 4D) fetal cardiac imaging is unlikely to become the standard of care for screening or for detailed evaluation of complex CHD. Ultimately, 3D or 4D technology may enable automated display of important planes and sweeps, representing a novel approach to computer-aided prenatal detection and diagnosis of CHD.
The aim of this section is to help the obstetrician, perinatologist, radiologist, cardiologist, and sonographer recognize and diagnose the most common and important forms of fetal cardiac malformations ( Box 23.6 ) and understand their management and prognosis. The embryology of certain defects is described briefly; more detailed descriptions may be found elsewhere. Likewise, more thorough descriptions of individual defects, as well as additional lesion-specific management and prognosis, may be found elsewhere in standard texts. ,
Systemic venous abnormalities
Persistent right umbilical vein
Persistent left-sided superior vena cava
Interrupted inferior vena cava
Pulmonary venous abnormalities
Partial anomalous pulmonary venous return
Total anomalous pulmonary venous return
Septal defects
Atrial septal defect
Ventricular septal defect
Atrioventricular canal defect
Complete
Partial
Transitional
Ebstein anomaly of the tricuspid valve and tricuspid valve dysplasia
Right heart obstructive lesions
Tricuspid atresia
Pulmonary atresia with intact ventricular septum
Left heart obstructive lesions
Hypoplastic left heart syndrome (mitral stenosis/atresia)
Valvar aortic stenosis/atresia
Coarctation of the aorta
Outflow tract abnormalities
Tetralogy of Fallot
Double-outlet right ventricle
Transposition of the great arteries
Truncus arteriosus
Tumors
Rhabdomyoma
Intrapericardial teratoma
Ectopia cordis
Any single heart defect may occur in conjunction with other cardiac defects, so often the most difficult fetal cardiac defect to detect is the second one. Because of the complexity of various forms of fetal structural heart defects, as well as their common association with extracardiac fetal anomalies, a multidisciplinary team (e.g., obstetrician, perinatologist, radiologist, pediatric cardiologist, neonatologist, social worker, genetics counselor, nurse, and cardiothoracic surgeon) should jointly manage pregnancies complicated by complex fetal heart disease. With few exceptions, a coordinated attempt should be made to deliver the majority of fetuses with major CHD at or beyond 39 weeks’ gestation.
Normally, during embryonic development, bilateral umbilical veins course along each side of the fetal liver, carrying oxygenated blood from the placenta to the fetal heart. The right umbilical vein involutes, along with the left umbilical vein’s connection to the heart. The persistent portion of the left umbilical vein becomes the fetal umbilical vein, which carries oxygenated blood to the IVC via the ductus venosus. Rarely the right umbilical vein fails to involute and appears later in gestation as a persistent umbilical vein.
The fetus is typically found to have a large vein arising from the abdominal cord insertion site and draining anomalously either into the liver or, in an unobstructed fashion, directly into the right atrium ( Fig. 23.37 ).
A persistent right umbilical vein may be associated with structural cardiac defects, right heart disproportion, extracardiac defects, or aneuploidy, or it may simply be an isolated anomaly, usually well tolerated but occasionally associated with CHF. Commonly, a persistent right umbilical vein is associated with absence of the ductus venosus. The finding of a persistent right umbilical vein should prompt a detailed evaluation for other structural defects, cardiac and extracardiac, as well as an evaluation of cardiac function. Also, patients should be advised of the potential for aneuploidy.
All cases deserve neonatal clinical and echocardiographic follow-up, although if the anomaly is isolated the infant typically does well because the umbilical vein involutes after occlusion of the cord at delivery.
Normally, during embryogenesis the right-sided superior vena cava forms from the right anterior cardinal vein and the right common cardinal vein. Abnormal persistence of the left anterior cardinal vein, which normally involutes during embryogenesis, leads to the presence of a persistent LSVC in the fetus and newborn. A persistent LSVC typically drains through the coronary sinus into the right atrium ( Fig. 23.38 ; see Figs. 23.14 and 23.19 ). A right-sided superior vena cava (normal) may or may not be present ( Fig. 23.39 ). A persistent LSVC may be found as an incidental finding in approximately 0.5% of the adult population.
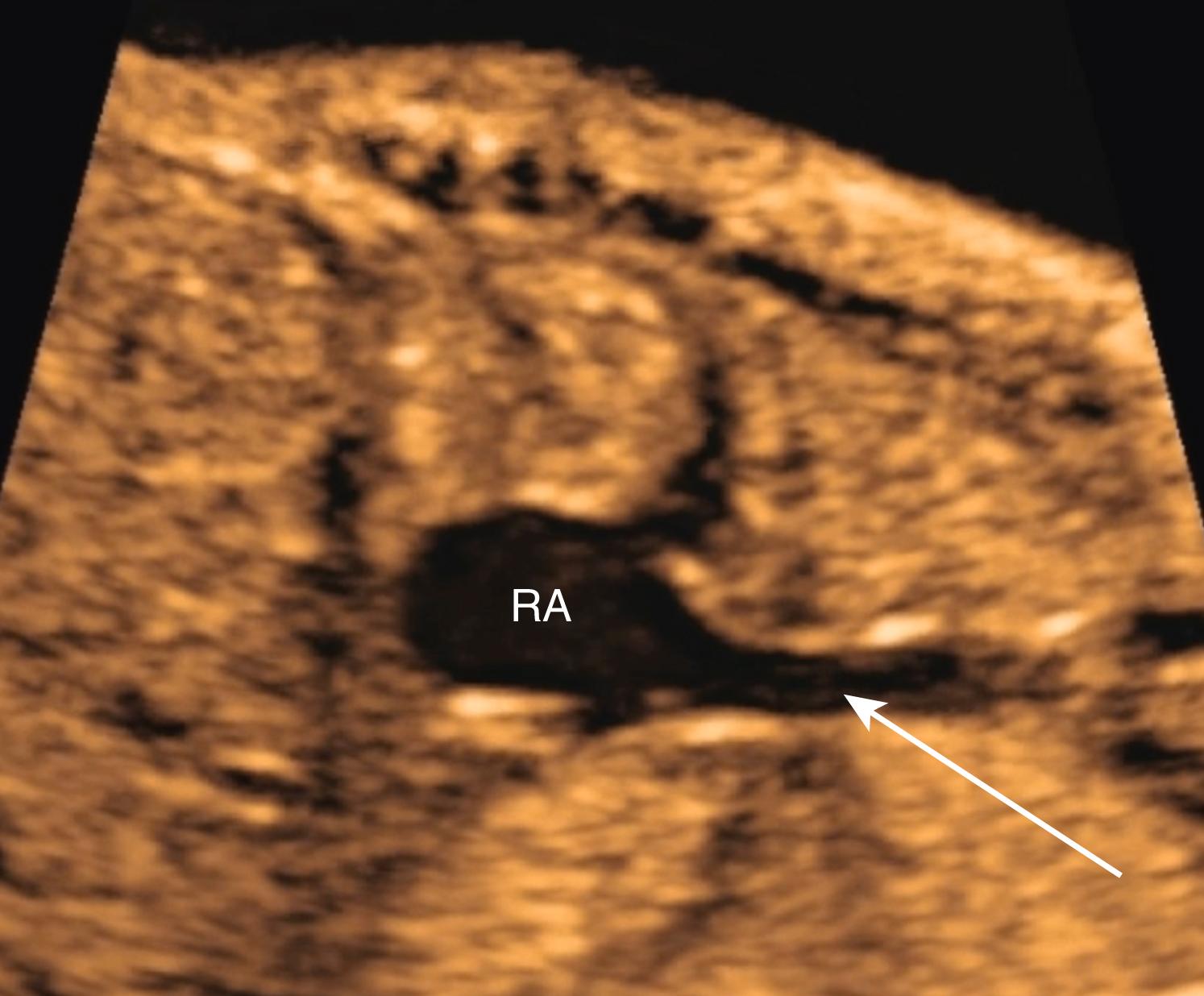
Persistent LSVC may be detected in the fetus by visualization of an enlarged coronary sinus (see Figs. 23.14 and 23.15 ) in the 4CV , or by direct visualization of the LSVC (see Figs. 23.16 and 23.38 ). The finding of a persistent LSVC may be isolated, resulting in physiologic return of deoxygenated blood to the right atrium, albeit via an abnormal pathway. Such cases represent variations of normal and do not require any intervention, either prenatally or after birth.
A persistent LSVC may be associated with left-sided obstructive lesions (e.g., coarctation of the aorta, valvar aortic stenosis, HLHS), so the finding should prompt a detailed fetal echocardiographic evaluation and neonatal echocardiographic follow-up because coarctation cannot definitively be ruled out with fetal scanning. In some cases, persistent LSVC is associated with extracardiac abnormalities or heterotaxy. , ,
The prognosis is excellent, with no need for medical or surgical intervention, unless the LSVC is associated with other cardiac or extracardiac abnormalities.
Normally, during embryogenesis the IVC forms from the right supracardinal vein, subcardinal-supracardinal anastomosis, right subcardinal vein, hepatic vein, and hepatic sinusoids. Occasionally the hepatic portion of the IVC fails to form, resulting in an interruption of the IVC. In such cases, venous return from the lower half of the fetus drains via a so-called azygos continuation of the IVC. The azygos vein returns deoxygenated blood to the right atrium via the superior vena cava.
Interrupted IVC with azygos continuation may be diagnosed prenatally from a transverse view of the abdomen. The cross-sectional image will show, in place of the IVC (in the liver), a cross-sectional image of an azygos vein located posterior to the descending aorta (see Fig. 23.13 ). Sagittal imaging confirms the absence of a direct connection from the IVC to the right atrium; only the hepatic veins are seen to connect directly. Using both 2D and low-velocity color flow sagittal imaging, the azygos vein can be seen longitudinally, located posterior to the aorta and coursing superiorly and anteriorly ( Fig. 23.40 ) to its termination in the superior vena cava.
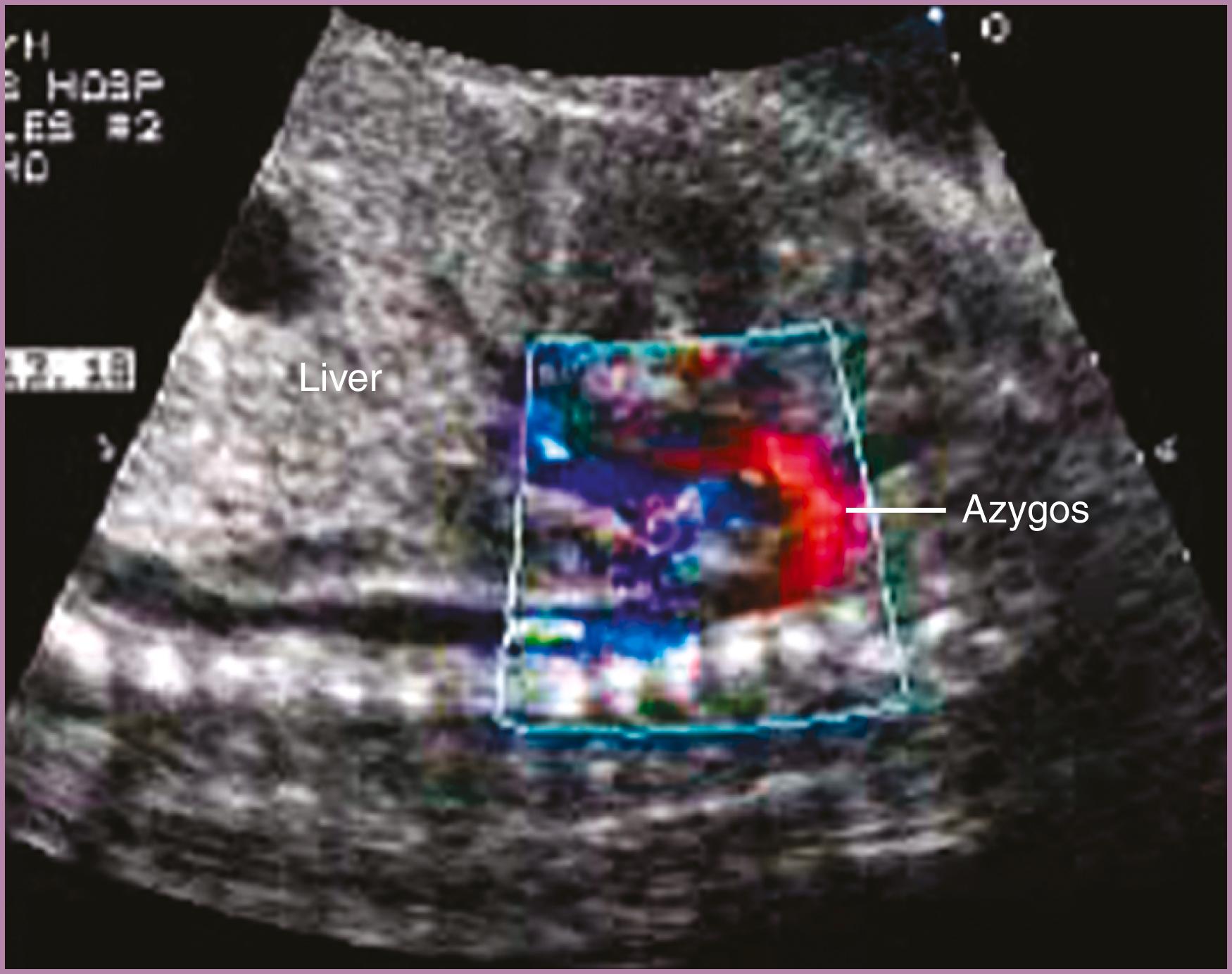
The finding of an interrupted IVC should prompt detailed fetal and neonatal echocardiographic evaluations because of the association of interrupted IVC with heterotaxy syndromes (typically polysplenia , , ) with or without structural heart disease. The diagnosis of heterotaxy may be further suggested by the presence of a midline liver or fetal bradycardia (absent sinus node).
The presence of an IVC with azygos continuation does not, in itself, require any treatment, either prenatally or postnatally. However, given the strong association with polysplenia-related abnormalities, all cases deserve neonatal echocardiographic follow-up.
Normally, during embryonic development the common pulmonary vein evolves to form both four separate pulmonary veins and much of the left atrium itself. Cases of partial anomalous pulmonary venous return (PAPVR), involving one to three veins, or total anomalous pulmonary venous return (TAPVR), involving all four veins, result from abnormalities of this portion of embryogenesis.
As with abnormalities of systemic venous return, both 2D and color flow imaging play important roles in the diagnosis of anomalous pulmonary venous return. Color flow imaging can facilitate visualization of normal (see Figs. 23.17 and 23.18 ) or anomalous ( Figs. 23.41 and 23.42 ) pulmonary venous return when the scale has been adjusted for low-velocity flows.
Anomalous pulmonary venous return frequently occurs in association with other structural cardiac defects, and is particularly common with heterotaxy. , ,
Because even detailed fetal echocardiography may not routinely demonstrate all four pulmonary veins, many cases of PAPVR are overlooked prenatally unless they are associated with other cardiac abnormalities. This section discusses two types of PAPVR that may be detectable before birth because of associated findings. In general, however, PAPVR does not usually require neonatal intervention and carries an excellent long-term prognosis.
Scimitar syndrome represents an unusual form of PAPVR in which the right-sided pulmonary vein (or veins) returns to a vein that descends through the diaphragm before joining the IVC just proximal to the right atrium. Postnatally, this vein may appear radiographically like a sword, or scimitar; hence the name of the syndrome.
Typically, a descending vein may be found draining a portion of the right lung to the IVC. Color flow imaging may demonstrate such anomalously draining right-sided pulmonary venous return. Commonly, a collateral vessel arising from the descending aorta supplies a separate part of the right lung. The fetus with scimitar syndrome may have minimal or no right heart disproportion but frequently presents with dextrocardia or mesocardia. Commonly the syndrome occurs in association with a secundum ASD, which similarly presents a diagnostic challenge prenatally.
Typically, surgery for scimitar syndrome can be postponed beyond the neonatal period or even beyond infancy. Other than having the potential for development of pulmonary hypoplasia, arrhythmias, and infection, most affected infants do well.
Sinus venosus ASDs (discussed in more detail later) typically occur in association with anomalous pulmonary venous return of one or both right-sided pulmonary veins to the superior vena cava.
Although affected fetuses may not present with right heart disproportion, the ASD high in the atrial septum may be detectable prenatally, with an absent superior rim of the atrial septum. Color flow imaging may demonstrate anomalously draining right-sided pulmonary veins.
Sinus venosus defects do not generally require intervention during infancy; ultimately, however, closure of the defect is required, with baffling of the right-sided pulmonary venous drainage to the left atrium. Prognosis is usually excellent, but some patients have residual pulmonary venous obstruction or atrial arrhythmias.
TAPVR involves abnormal drainage (and usually abnormal connection) of all four pulmonary veins to anywhere in the heart other than the left atrium.
TAPVR can be diagnosed prenatally , but is often missed. Right heart disproportion is present in many cases, but in others it is minimal or absent. Affected fetuses lack the normal infolding of tissue between the left atrial appendage and the left pulmonary vein, and color flow imaging will demonstrate absence of pulmonary venous return to the left atrium. TAPVR may present with an increased distance between the descending aorta and the left atrium. Infradiaphragmatic TAPVR may manifest as a dilated IVC, because the pulmonary veins descend below the diaphragm as a common vein before draining into the IVC via the ductus venosus. The descending vein, which is typically partially obstructed in the liver, should be evaluated with color flow imaging (see Fig. 23.41 ). Many cases of TAPVR can present with a prominent venous confluence, or twig sign, posterior to the left atrium at the level of the 4CV. With intracardiac TAPVR, the pulmonary veins drain to the coronary sinus, causing the coronary sinus to be markedly dilated (see Fig. 23.42 ). Finally, in cases of supracardiac TAPVR, 2D and color flow imaging may demonstrate a large common vein draining cephalad toward a left-sided vertical vein or toward a right-sided superior vena cava.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here