Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Calcium, phosphorus, and magnesium are essential minerals that function in their physiologically active ionic forms in a wide range of cellular processes, and as key components of the bone mineral matrix. In utero, calcium, phosphorus, and magnesium are readily transferred from maternal to fetal circulations, and fetal mineral stores are predominantly accrued in the third trimester. In the postnatal period, the newborn must rapidly adapt to the cessation of the continuous transplacental supply of these nutrients by enabling homeostatic mechanisms that regulate intestinal absorption, bone deposition/resorption, and renal reabsorption of calcium, phosphate, and magnesium. Transcellular and paracellular transport pathways are regulated by a shared set of hormones including vitamin D, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and calcitonin. However, as detailed later, distinct mechanisms govern the homeostatic control of each of these three minerals in the fetus and newborn.
Calcium is a highly abundant and essential mineral in the human body. Although calcium is most widely recognized for its role as the major inorganic constituent of bone, it exerts countless other physiologic effects by serving as a cellular signal involved in gene expression, synaptic transmission, muscle contraction, and embryogenesis. Every cell in the human body relies on the specific decoding of calcium signals for its normal function and survival, and therefore is sensitive to minute variations in the calcium concentration within the cell and in its microenvironment. Because the intracellular calcium concentration is about 10,000-fold lower than the surrounding extracellular fluid (ECF), individual cells rely on active transport mechanisms to maintain a concentration gradient.
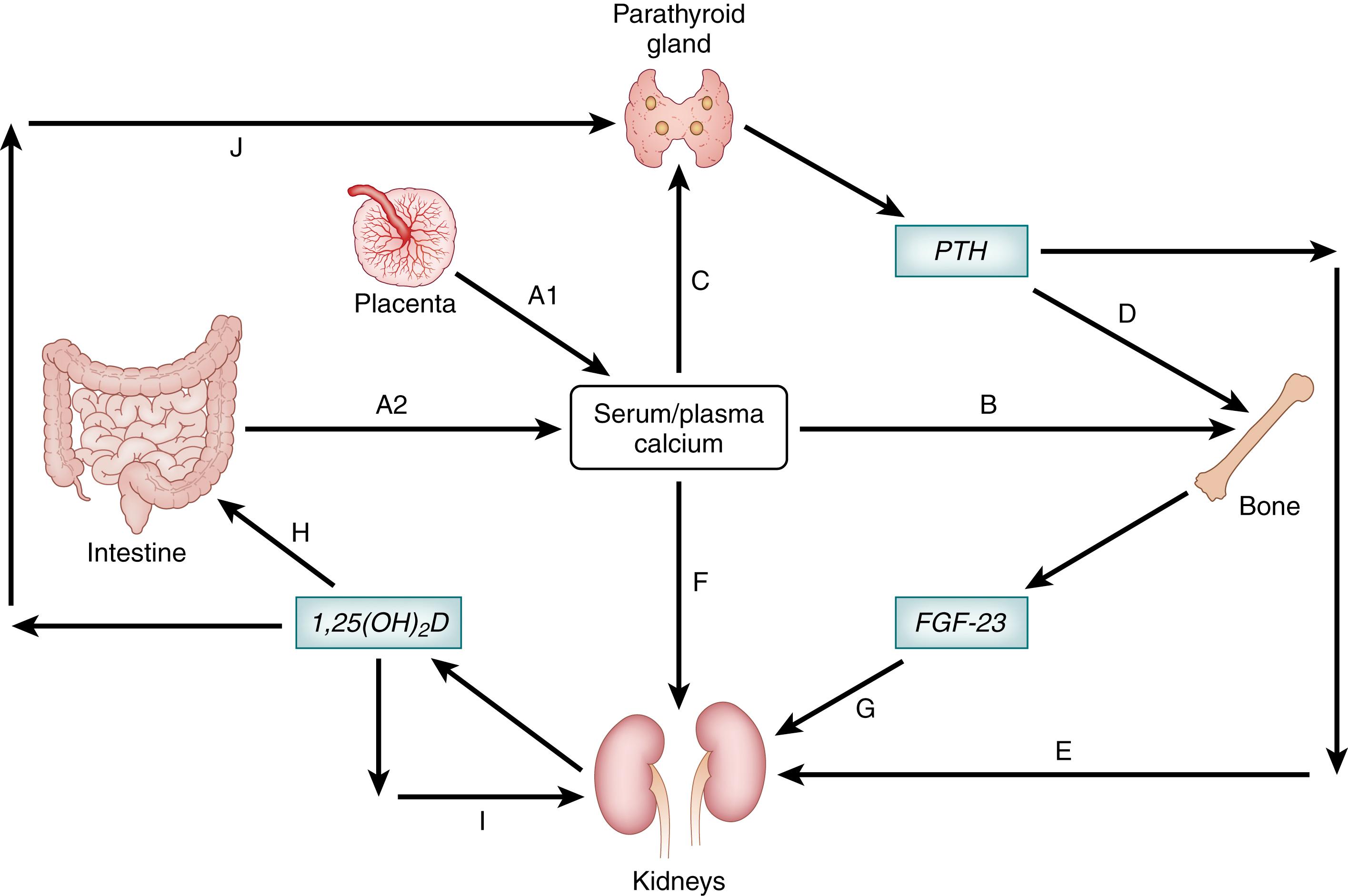
All tissues depend on the systemic regulation of calcium availability by a complex, interconnected series of feedback loops involving the bones, intestinal tract, and kidneys; in utero, the role of the intestines is essentially substituted by the placenta ( Fig. 26.1 ). Over 99% of total body calcium is in the skeleton. Bone contains a variety of calcium salts, of which hydroxyapatite is the least soluble and, thus, the most important structural component that integrates with collagen fibrils to form the bone matrix. However, other salts such as amorphous calcium phosphate are relatively abundant on the crystal surfaces of the bone matrix, where the calcium is in equilibrium with the ECF, constituting an exchangeable pool of calcium (about 1% of the calcium in bone) —a critical resource for maintenance of a physiologic serum calcium concentration. ,
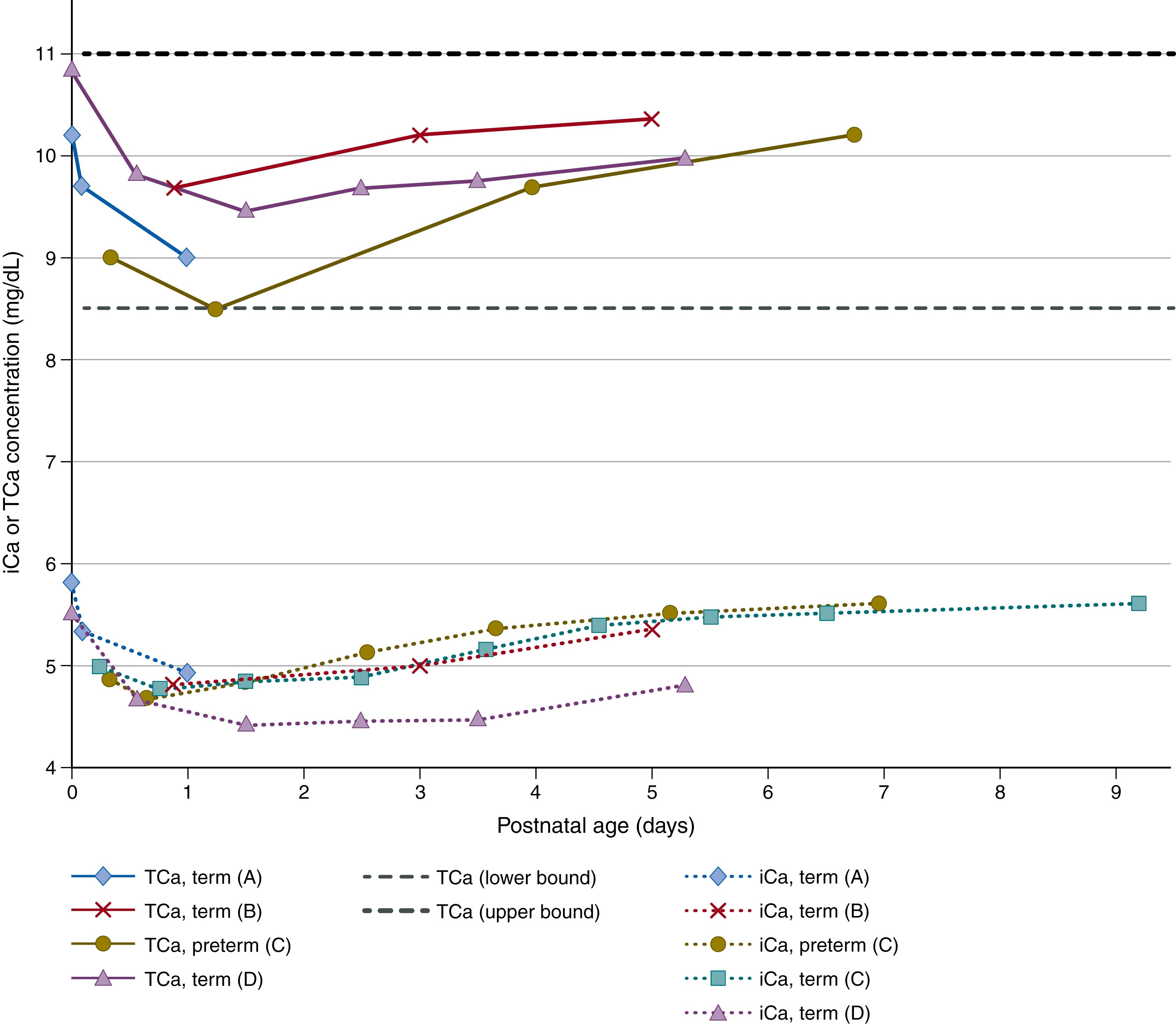
The remaining 1% of total body calcium is contained in all of the nonskeletal tissues, including soft tissues, blood (including serum/plasma), and other ECFs. Serum (or plasma) calcium is solubilized in three forms: 50% as the free ion, termed ionized calcium (iCa 2+ ) ; 40% bound to plasma protein (of which, 80% is bound to albumin and the remainder to globulins); and approximately 10% as diffusible complexes of calcium bound to anions such as bicarbonate, phosphate, or citrate. Ionized calcium is the only physiologically active fraction in blood; therefore tight regulation of the iCa 2+ concentration is a major focus of calcium homeostasis. Because of the substantial fraction of total plasma calcium that is bound to albumin, alterations in the plasma concentration of albumin can affect the measured total serum or plasma calcium concentration. Sudden changes in the concentration of albumin can change the iCa 2+ concentration (as observed upon rapid addition of albumin to neonatal serum in vitro ), but in vivo, this effect is transient and modest. Over the long term, iCa 2+ is relatively less affected by albumin concentrations compared to total calcium. Therefore the total calcium concentration (which includes all fractions) is often a poor predictor of the iCa 2+ concentration. The propensity for calcium to bind to proteins is reduced at acidic pH, such that iCa 2+ varies inversely with blood pH. Laboratory methods to quantify calcium concentrations in body fluids vary widely, and some studies have shown poor correlation of values generated by different automated chemistry platforms. Furthermore, published pediatric reference intervals may not reflect the expected pattern of decline and then recovery of serum/plasma calcium concentrations in the first week of life ( Fig. 26.2A ).
In the clinical context, measurement of an individual’s serum/plasma total calcium or iCa 2+ provides insights into the extent to which homeostasis is intact and operational. Since these values are expected to be maintained in narrow physiologic ranges, high or low values (hypercalcemia or hypocalcemia, respectively) indicate a perturbation or failure of homeostatic mechanisms. Therefore, it is essential to recognize that serum/plasma calcium measurements do not provide a reliable measure of calcium intake or calcium status (i.e., the extent to which an individual is in an appropriate calcium balance).
Calcium homeostasis is primarily controlled by two interacting hormones—vitamin D and PTH. The integrated effects of PTH on distal renal tubular calcium reabsorption (within minutes) and bone resorption (over hours), and the effects of the active metabolite of vitamin D on intestinal calcium absorption (over days), serve two primary goals in the fetus and newborn—to ensure appropriate accrual of calcium to enable skeletal growth and development, and to maintain the serum calcium concentration within a narrow physiologic range (see Fig. 26.1 ).
PTH is a peptide hormone released by the parathyroid glands in response to decreases in the serum ionized calcium concentration detected by calcium-sensing receptors (CaSR) on parathyroid cells. PTH mobilizes calcium by binding the type 1 PTH receptor (PTH1R) in its two primary target tissues—bone and kidney. PTH directly stimulates bone resorption through a variety of putative mechanisms, including the upregulation of the receptor activator of nuclear factor κB (NF-κB) ligand (RANKL), which leads to the differentiation of osteoclasts, and downregulation of osteoprotegerin, a RANKL inhibitor. In the kidney, PTH reduces urinary excretion of calcium by its actions in the distal nephron, and promotes the synthesis of 1,25-dihydroxyvitamin D [1,25(OH) 2 D] from 25-hydroxyvitamin D [25(OH)D] by upregulating the expression of the CYP27B1 1-alpha-hydroxylase in proximal renal tubular epithelial cells (see Fig. 26.1 ).
Vitamin D is a steroid hormone precursor that has an essential role in calcium and bone mineral metabolism at all life stages. Vitamin D is obtained from two sources—endogenous production from cutaneous 7-dehydrocholesterol in response to ultraviolet B (UVB) radiation exposure of the skin, or absorption from the intestinal tract following ingestion of foods (including breast milk) or dietary supplements that contain vitamin D. To attain its final biologically active state as 1,25(OH) 2 D, the parent vitamin D molecule (cholecalciferol or ergocalciferol) undergoes a series of two hydroxylation reactions: first, 25-hydroxylation is catalyzed primarily (though not exclusively) by the cytochrome P450 enzyme CYP2R1 in a relatively unconstrained reaction in the liver, yielding 25(OH)D; then, 25(OH)D circulates to the kidney where the mitochondrial enzyme CYP27B1 catalyzes the 1-alpha-hydroxylation reaction that generates 1,25(OH) 2 D. In contrast to 25-hydroxylation, the synthesis and activity of renal CYP27B1 are tightly controlled by the enhancing effect of PTH (as mentioned earlier) and the downregulatory effects of FGF23, a phosphaturic hormone described in more detail below, and by 1,25(OH) 2 D itself in a classical feedback loop (see Fig. 26.1 ). ,
Vitamin D activity in target tissues is primarily mediated by the high-affinity binding of 1,25(OH) 2 D to the vitamin D receptor (VDR), which is widely distributed. Numerous genes include VDR-binding sites and are responsive to 1,25(OH) 2 D-regulated gene transcription. Vitamin D metabolites are primarily catabolized and rendered relatively inactive by 24-hydroxylation catalyzed by CYP24A1 , which is downregulated by PTH and upregulated by FGF23. , The major circulating vitamin D metabolite is 25(OH)D, a well-established primary biomarker of vitamin D status at all life stages due to its relatively long serum half-life and its empirical responsiveness to recent vitamin D inputs, regardless of route. Although the bulk of the clinical and epidemiologic evidence supporting the use of serum/plasma 25(OH)D as a biomarker is based on studies of adults, there is nonetheless abundant evidence from prenatal and infant vitamin D trials to support its utility in the perinatal period, and in preterm and term infants. Although serum 25(OH)D reflects vitamin D status insofar as it is a measure of recent endogenous and exogenous vitamin D inputs, it is otherwise of limited value in providing information about the downstream effects of vitamin D, such as calcium absorption or bone mineral metabolism. There is insufficient evidence to establish serum 25(OH)D reference ranges specific for cord blood or early infancy; however, the lower limits of adequacy that are widely used in adults (25 or 30 nmol/L) are generally applied at all life stages, including the perinatal period. , Severe vitamin D deficiency, typically manifesting as serum 25(OH)D well below 25 nmol/L, causes vitamin D-deficient rickets (often referred to as nutritional rickets ), for which the primary clinical manifestations are due to defects in growth plate function and bone mineralization. To prevent infantile rickets, many organizations and national health agencies recommend routine oral vitamin D supplementation of breastfed term and preterm infants. ,
The primary biologically active vitamin D metabolite, 1,25(OH) 2 D, may also be readily measured in serum or plasma, but due to its short half-life and tightly regulated production, 1,25(OH) 2 D is not an appropriate clinical indicator of systemic vitamin D status or intake. In infancy, measurement of serum/plasma 1,25(OH) 2 D may be useful in the diagnosis of inborn errors of vitamin D metabolism, such as vitamin D 1-alpha-hydroxylase deficiency caused by mutations in CYP27B1 , or other rare disorders of calcium handling.
Vitamin D, 25(OH)D, and 1,25(OH) 2 D in circulation are bound by the vitamin D binding protein (DBP), or to albumin, or exist at very low concentrations as free steroids. DBP, which has much higher affinity for 25(OH)D than albumin, binds about 85% of 25(OH)D compared to 15% bound to albumin and less than 0.1% as the free form. DBP-bound 25(OH)D may be preferentially endocytosed by renal tubular epithelial cells, rendering it available as a substrate for 1-alpha-hydroxylation. Moreover, variability in DBP concentrations and genetic polymorphisms in the gene encoding DBP may contribute to population variability in 25(OH)D concentrations. However, the essential role of DBP in vitamin D metabolism has been questioned, and there is emerging interest in the functional significance of “free” (unbound) 25(OH)D.
Numerous tissues, including the placenta, express both the VDR and the CYP27B1 1-alpha-hydroxylase, suggesting local autocrine/paracrine vitamin D functions. , Placental expression of CYP2R1, CYP27B1 , VDR , and other genes related to vitamin D metabolism are believed to contribute to the regulation of placental immunomodulation and may influence systemic maternal prenatal vitamin D status , ; however, there is little evidence to suggest an important effect of vitamin D metabolites of placental origin on fetal-neonatal calcium homeostasis. Vitamin D (parent compound) itself does not readily cross the placenta, and 1,25(OH) 2 D in fetal circulation is assumed to be mainly of fetal origin. , Conversely, maternal 25(OH)D is readily transferred across the placenta, and maternal and cord blood concentrations are highly correlated, , although possibly to a lesser extent in preterm deliveries. Therefore 25(OH)D of maternal origin is considered to be the major contributor to fetal and early neonatal vitamin D status, outweighing genetic factors. Accordingly, cord blood 25(OH)D concentrations are affected by the same factors that determine maternal vitamin D status, including characteristics that influence the mother’s endogenous production of vitamin D (e.g., season, sunlight exposure, latitude, skin pigmentation) and maternal prenatal vitamin D intake from diet and supplement use.
Numerous prenatal vitamin D supplementation trials have proven that improvements in maternal vitamin D status are associated with concordant increases in neonatal vitamin D stores, as reflected by cord blood or early neonatal 25(OH)D concentrations. , , However, the effect of maternal-fetal 25(OH)D transfer is transient, as infant vitamin D status is largely dependent on postnatal inputs by about 2 months of age. In the postnatal period, cutaneous production and oral intake contribute to vitamin D status, similar to older children and adults. Breast milk was traditionally assumed to be a poor source of vitamin D, but it is now accepted that the breast milk vitamin D metabolite content (primarily the concentration of vitamin D itself) is a function of maternal vitamin D intake, such that relatively high maternal intakes of vitamin D can maintain physiologic 25(OH)D concentrations in breastfeeding infants. Newborns, including those born preterm, have full capacity to absorb vitamin D and carry out the serial hydroxylation reactions that convert vitamin D to 25(OH)D and 1,25(OH) 2 D, even in the first few days of postnatal life. Thus physiologic immaturity of the vitamin D metabolic pathway is not usually considered to be a factor that impairs early neonatal calcium handling.
There has been considerable interest in C3-epimers of 25(OH)D and 1,25(OH) 2 D (C3-epi-25(OH)D and C3-epi-1,25(OH) 2 D, respectively) based on the observation of relatively high concentrations of C3-epi-25(OH)D in infants compared to other life stages. Maternal prenatal C3-epi-25(OH)D increases through pregnancy, but does not appear to efficiently cross the placenta, such that fetal C3-epi-25(OH)D is generated primarily within the fetal–placental unit from maternally derived 25(OH)D; however, neither maternal nor fetal C3-epi-25(OH)D accounts for the relatively high concentrations of infant C3-epi-25(OH)D, suggesting neonatal generation of the epimer from infant 25(OH)D or epimerized vitamin D. C3-epimers of vitamin D can be metabolized analogously to their corresponding non-epimeric forms, but bind the VDR relatively weakly; therefore C3-epi-1,25(OH) 2 D may lack some of the conventional functional attributes of 1,25(OH) 2 D.
Calcitonin was historically portrayed as a major factor in bone mineral homeostasis, but this has not been supported by accumulated evidence in humans. In the early newborn period, calcitonin was found to be unresponsive to changes in serum calcium. Conversely, there has been substantial interest in the calcium-regulating role of FGF23, a phosphaturic factor produced by bone (described in detail later in relation to phosphate homeostasis). The secretion of FGF23 is upregulated by 1,25(OH) 2 D, and its primary target organ is the kidney, where FGF23 acts to increase urinary phosphate excretion and inhibit renal 1,25(OH) 2 D synthesis. Data suggest that FGF23 may also interfere with 1,25(OH) 2 D-mediated intestinal calcium absorption ; however, the physiologic relevance of this effect in humans is unknown.
Fetal calcium accrual occurs throughout gestation but is particularly important in the third trimester, not only because accrual is a direct function of total body weight, but also because total body calcium becomes a progressively greater fraction of fetal body weight at later gestational ages. , The rate of skeletal calcium deposition peaks at about 35 weeks of gestation, reaching approximately 130 mg/kg/day. About 26 to 30 g of calcium are accrued by the time of term delivery. ,
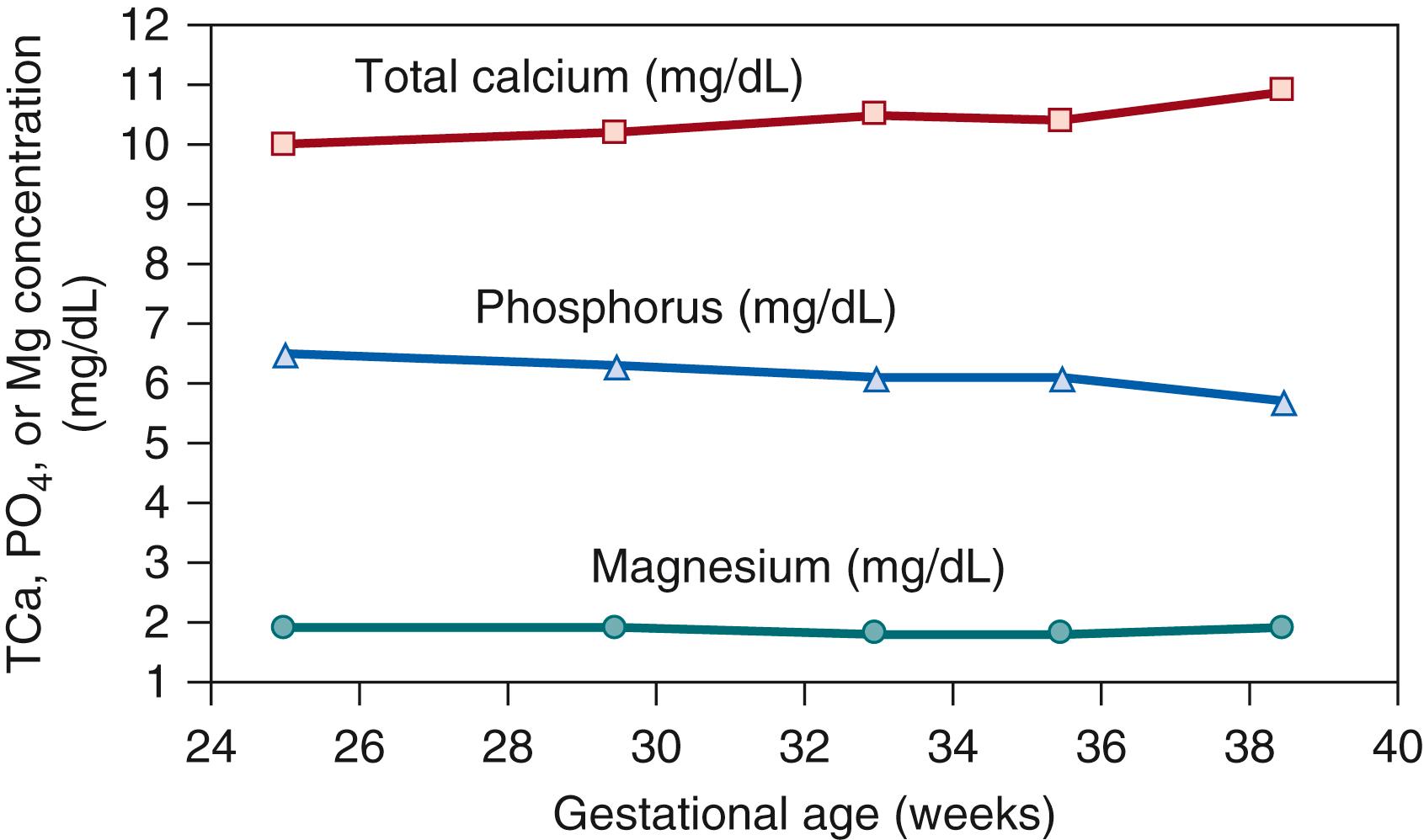
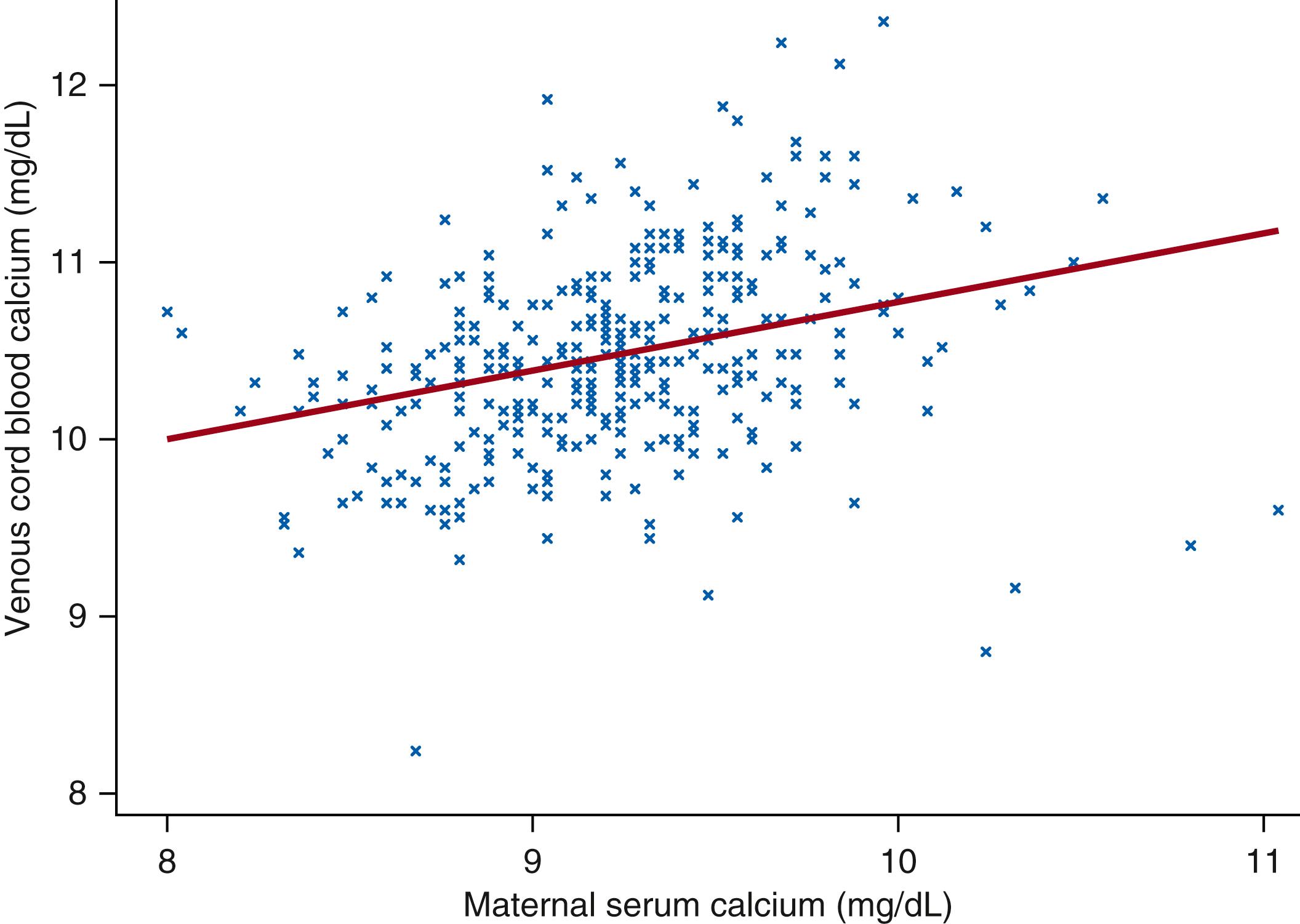
The increase in the rate of fetal calcium accrual during gestation is paralleled by a gradual increase in the calcium concentration in fetal circulation (as indicated by cord blood serum concentrations) ( Fig. 26.3 ). Fetal (umbilical cord blood) serum calcium concentrations are correlated with—but consistently higher than—their corresponding maternal concentrations in both preterm and term infants ( Fig. 26.4 ). The existence of a steep concentration gradient underscores the necessary presence of active transport mechanisms responsible for efficient maternal-fetal calcium transfer. The regulation of active transplacental calcium transport has been the subject of long-standing inquiry, particularly with respect to its responsiveness to maternal and fetal vitamin D status. Evidence from animal models suggests that both parathyroid-hormone related protein (PTHrP) and PTH regulate transplacental calcium flux via their binding to the PTH/PTHrP receptor. Accordingly, animals and human neonates with inactivating PTH/PTHrP receptor mutations develop severe chondrodysplasias in utero. Endogenous fetal PTH is capable of regulating fetal calcium concentrations, but its expression is normally suppressed by the relatively high fetal calcium concentration, mediated by the CaSR. However, the regulation of placental mineral transport by PTH or PTHrP has not been well characterized in humans, and newer evidence suggests that fetal serum calcium concentrations may be further directly influenced by other PTH-independent mechanisms of calcium influx into fetal bone.
The presumed mechanism of active transplacental calcium transport is remarkably similar to that involved in intestinal calcium absorption (described below), in that it is a three-step process involving uptake of calcium by the trophoblasts via the transient receptor potential vanilloid type 6 (TRPV6) cation channel, intracellular shuttling of calcium through the trophoblast by calbindin-D9k, and extrusion at the basolateral aspect into the ECF by a Ca 2+ -ATPase ( Fig. 26.5 ; Table 26.1 ). Skeletal abnormalities in fetuses with TRPV6 mutations suggest a dominant role for this calcium-selective channel in third trimester transplacental calcium transport in humans. The similarities between intestinal and transplacental calcium transport mechanisms raise the possibility that active transplacental transfer would be responsive to maternal-fetal vitamin D status, as in the intestine. However, rodent studies generally do not support the direct dependency of transplacental calcium transport, fetal calcium homeostasis, or fetal skeletal development on fetal VDR or 1,25(OH) 2 D expression. , Moreover, findings from animal models are consistent with observations of relatively normal skeletons and serum calcium concentrations in newborns with mutations in VDR or CYP27B1 .
| Mineral | Serum/Plasma Fractions (%) | Circulating Free Ions in Serum/Plasma | Active Transport | Passive Transport | ||||
|---|---|---|---|---|---|---|---|---|
| Protein-Bound | Salt Complexes | Free Ions | Cellular Uptake | Intracellular Transit | Basolateral Extrusion | |||
| Calcium | 40 | 10 | 50 | Ca 2+ | TRPV5 TRPV6 Cav1.3 |
Calbindin-D9k | PMCA1 NCX1 |
Claudin-2 Claudin-5 Claudin-15 |
| Phosphorus | 15–17 | 33–35 | 50 | HPO 4 2− H 2 PO 4 − |
NaPi-IIa NaPi-IIb NaPi-IIc PiT2 |
Undefined | Undefined | Unspecified tight junction proteins |
| Magnesium | 20–30 | 5–15 | 55–70 | Mg 2+ | TRPM6 TRPM7 |
Undefined | Undefined | Claudin-16 Claudin-19 |
Maternal vitamin D metabolites may affect fetal calcium balance by mechanisms that are independent of fetal VDR expression. VDR-independent effects of 1,25(OH) 2 D on fetal trophoblast expression of TRPV6 or Calbindin-D9k 30,58,59 remain to be fully characterized, but may occur via 1,25(OH) 2 D binding of protein disulfide isomerase family A member 3 (PDIA3), also known as the 1,25D3-membrane associated, rapid response steroid binding protein (1,25D3-MARRS) . In addition, vitamin D in the maternal circulation appears to influence the extent to which circulating maternal calcium is rendered available for transfer to the fetus. In humans, some randomized controlled trials of high-dose maternal vitamin D supplementation in pregnant women have demonstrated that vitamin D slightly increases cord blood calcium concentrations. , Consistent with animal models, this effect seems to be attributable to increases in maternal serum calcium availability, rather than a direct effect of vitamin D on the rate of transplacental calcium flux. Notably, even if vitamin D can directly or indirectly increase transplacental calcium flux, human fetuses appear to be protected against hypercalcemia, even in the context of maternal hyperparathyroidism and elevated fetal 25(OH)D.
At birth, the abrupt termination of the maternal-to-fetal calcium supply chain via the placental and umbilical cord necessitates an urgent transition to postnatal mechanisms that serve to maintain serum calcium in a physiologic range as well as promote bone growth and mineralization. Numerous longitudinal studies have described the typical pattern of changes in serum ionized and total calcium that occur in most preterm and term infants during the first several days of lifean initial decline in the first 48 hours, followed by a rebound and then stabilization over the rest of the first week of life (see Fig. 26.2A ). , Some otherwise healthy infants do not experience the classic postnatal nadir and therefore may appear to have unusually high serum calcium values by the end of the first week of life, but this usually normalizes by the second or third week. Most infants mount an effective PTH response to the natural first-week nadir in serum calcium, as demonstrated by a spiking increase in the PTH concentration in the immediate postnatal period, which attenuates as the calcium concentration stabilizes. Even in very low-birth-weight (VLBW) infants (<32 weeks; <1500 g), PTH increased as calcium declined in the first few days of life, and the pattern was reversed following a bolus infusion of calcium. Impaired parathyroid gland responsiveness may therefore account for the exaggerated postnatal hypocalcemia (which may be symptomatic) that occurs in some newborns. , , The reason for blunted PTH responses in these infants is unknown but has been related to severe maternal vitamin D deficiency. , In contrast to the uncertain role of vitamin D in calcium homeostasis in utero, there is strong evidence that vitamin D plays a critical role in regulating serum calcium homeostasis in the immediate postnatal period. In fact, randomized controlled trials of maternal prenatal vitamin D supplementation have consistently demonstrated that higher newborn vitamin D status attenuates the physiologic postnatal infant nadir in serum calcium in the first week of life, without an apparent increase in the risk of infantile hypercalcemia. , , It remains unclear whether this early postnatal effect of vitamin D is primarily a result of the rapid mobilization of calcium from bone or direct effects of vitamin D on intestinal calcium absorption.
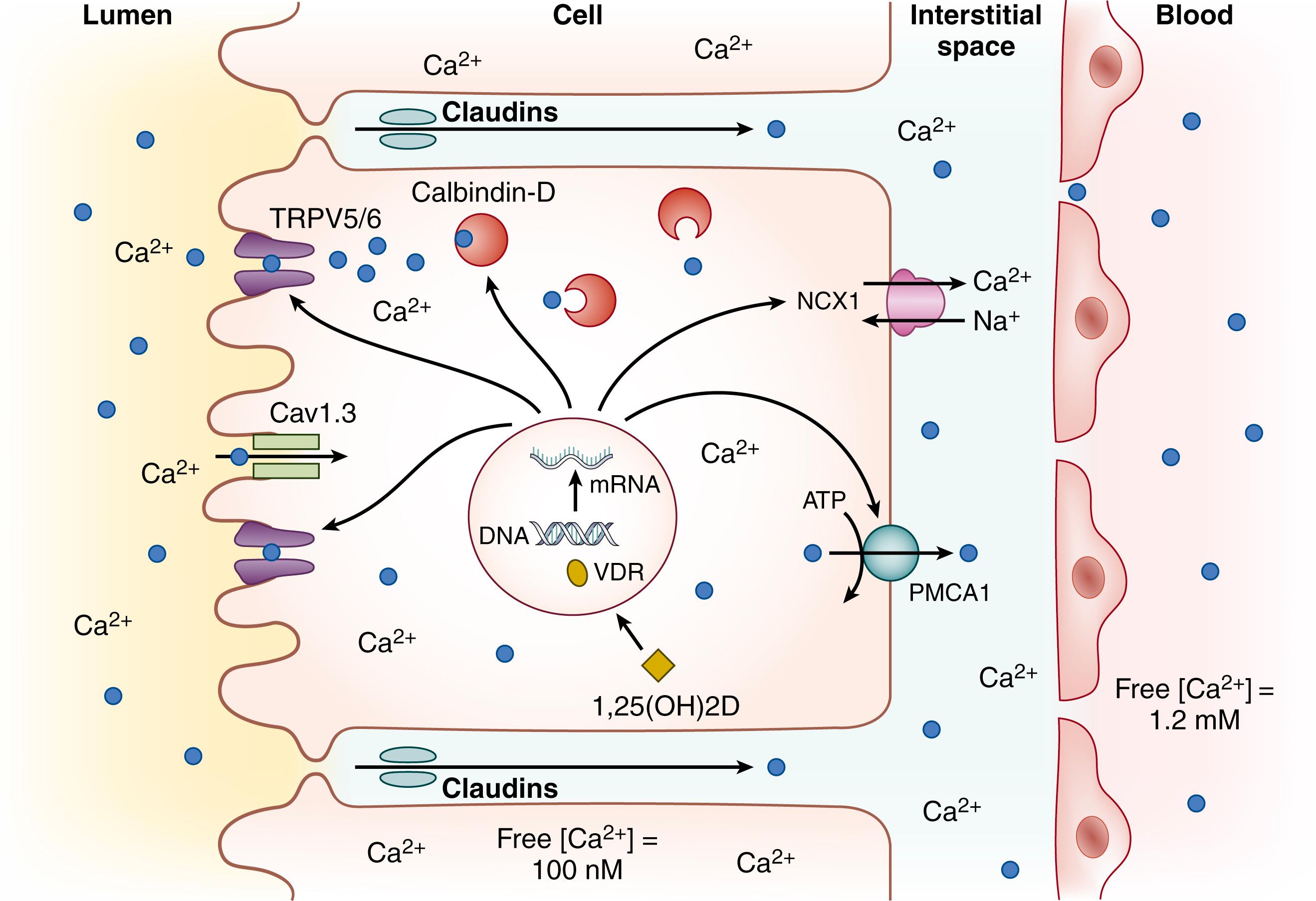
Beginning in the postnatal period, the gastrointestinal tract assumes its major importance in calcium homeostasis by providing the only physiologic route of calcium intake, as well as by regulating the relative balance between calcium absorption and excretion.The routes by which calcium crosses the intestinal mucosa into systemic circulation have a remarkably similar architecture as those that govern calcium transit across the placental trophoblast layer, as described earlier (see Fig. 26.5 ). Two major transport processes enable calcium absorption from the intestinal lumen in humans: active transcellular transport and passive paracellular transport (see Table 26.1 ). Active transcellular calcium absorption is a three-step process involving the apical entry of calcium into the cell by TRPV6, buffering and facilitated diffusion of calcium through the enterocyte by calbindin-D9k, and energy-dependent basolateral extrusion of calcium into the ECF of the lamina propria—against the electrochemical gradient—by the plasma membrane Ca 2+ -ATPase 1 (PMCA1). Although the TRPV6-calbindin-D9k-PMCA1 pathway is the most well-described active calcium transport mechanism, emerging evidence indicates that other proteins and channels are also likely involved. , These include Cav1.3, an L-type apical calcium channel that may be complementary to TRPV6, and the sodium-calcium exchanger 1 (NCX1), which functions similarly to PMCA1 to extrude calcium at the basal aspect of enterocytes (see Fig. 26.5 ). Paracellular calcium absorption, which predominates in the distal small bowel and colon, is a passive diffusional and nonsaturable process of calcium flow via tight junctions. , Numerous studies have indicated that paracellular calcium absorption may be regulated; for example, claudins are intercellular components of tight junctions that modulate paracellular permeability and calcium transport, and their expression may be modulated by vitamin D. ,
In older children and adults, the duodenum displays the highest rate of intestinal calcium absorption as it primarily expresses the cellular machinery for active transport of calcium from the lumen to the extracellular space; yet, due to its longer length, the distal small bowel (primarily the ileum) is likely where the majority of ingested calcium is absorbed. Nonetheless, there remains considerable uncertainty about the relative contributions of transcellular (active) versus paracellular (passive) routes, or proximal versus distal sites of calcium uptake, particularly in early infancy. Early evidence suggested that calcium absorption in preterm infants is primarily a nonsaturable function of calcium intake, and thus primarily dependent on paracellular mechanisms, whereas active transport mechanisms are underdeveloped at birth and mature in the early postnatal period. , However, studies of a piglet model suggested that active transport mechanisms are indeed present in the immediate postnatal period, but are vitamin D-independent. , More recent insights into the age-related dynamics of calcium absorption were revealed by a mouse study showing that transcellular transport mediated by TRPV6 and Cav1.3 was present in the distal small bowel rather than the duodenum prior to weaning; notably, the pattern was reversed after weaning age, when the duodenum acquired—and the distal small bowel lost—the capacity for active TRPV6-mediated transcellular transport. However, the extent to which these findings are applicable to humans remains unknown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here