Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In classifying non-Hodgkin's lymphomas, considerable attention has been paid to architectural, cytologic, and functional similarities among the various lymphomas and normal lymphoid tissue, exemplified by the peripheral lymph node. However, studies of extranodal lymphomas, particularly gastrointestinal lymphomas (accounting for the majority), suggest that their clinicopathologic features are related not to lymph nodes but to the structure and function of mucosa-associated lymphoid tissue (MALT).
The anatomic distribution and structure of lymph nodes are adapted to deal with antigens carried to the node in afferent lymphatics, which drain sites at various distances from the node. Permeable mucosal sites, such as the gastrointestinal tract, are particularly vulnerable to pathogens and antigens because they are in direct contact with the external environment, and specialized lymphoid tissue—MALT—has evolved to protect them. MALT includes gut-associated lymphoid tissue, nasopharyngeal lymphoid tissue (tonsils), and other less well-characterized aggregates of lymphoid tissue related to other mucosae. Gut-associated lymphoid tissue serves as the paradigm for MALT.
The mucosal immune system in the gastrointestinal tract comprises three lymphoid compartments: intraepithelial lymphocytes; lamina propria lymphocytes, plasma cells, and accessory cells; and organized collections of B cells and T cells found throughout the small intestine, appendix, and colorectum. When concentrated in the terminal ileum, these organized lymphoid collections are termed Peyer's patches. MALT lymphomas essentially recapitulate the features of Peyer's patches.
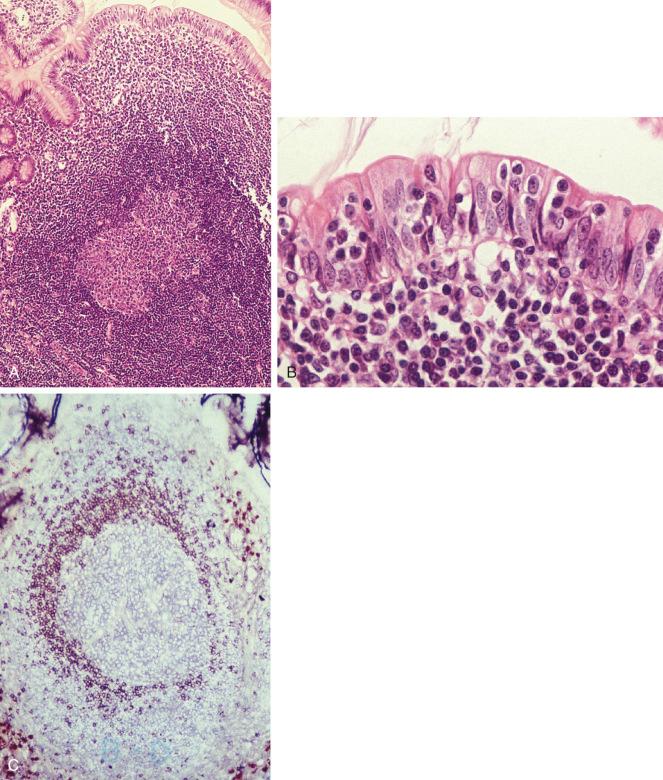
Peyer's patches are unencapsulated aggregates of lymphoid cells that bear a certain resemblance to lymph nodes ( Fig. 19-1, A ). Each Peyer's patch nodule consists of B-cell and T-cell areas and associated accessory cells. The B-cell area comprises a germinal center surrounded by a mantle zone of small B lymphocytes, which is broadest at the mucosal aspect of the follicle. Surrounding the mantle zone is a broad marginal zone in which most of the cells are small to intermediate-sized B lymphocytes with moderately abundant, pale-staining cytoplasm and nuclei with a slightly irregular outline, leading to a resemblance to centrocytes. The marginal zone extends toward the mucosal surface, and some marginal-zone B cells enter the overlying dome epithelium, where they form the lymphoepithelium, which is a defining feature of MALT (see Fig. 19-1, B ). Immunohistochemical studies of Peyer's patches have shown that the B-cell follicles are identical to those of lymph nodes. In contrast to the immunoglobulin (Ig)M-positive and IgD-positive mantle zone, the peripheral marginal-zone cells are IgM positive but IgD negative (see Fig. 19-1, C ). Lateral to the deep aspect of the B-cell follicle is a T-cell zone in which high endothelial venules are prominent, equivalent to the paracortical T-zone of the lymph node.
MALT lymphoma is an extranodal lymphoma comprising morphologically heterogeneous small B cells, including marginal-zone (centrocyte-like) cells, cells resembling monocytoid cells, small lymphocytes, and scattered immunoblast and centroblast-like cells. There is plasma cell differentiation in a proportion of cases. The infiltrate is in the marginal zone of reactive B-cell follicles and extends into the interfollicular region. In epithelial tissues, the neoplastic cells typically infiltrate the epithelium, forming lymphoepithelial lesions.
MALT lymphomas arise in a wide variety of extranodal sites ( Box 19-1 ), but curiously, most of these are not sites where MALT is normally present, such as the terminal ileum or the tonsils. One could question whether the term MALT is appropriate for lymphomas, such as those of the orbit, that do not arise in mucosal or epithelial tissues; however, their close association with mucosal tissue, together with their histology, immunophenotype, and genetic and clinical properties, tend to support their classification as MALT lymphomas.
Gastrointestinal tract
Stomach
Intestine (including immunoproliferative small intestinal disease)
Salivary glands
Respiratory tract
Lung, pharynx, trachea
Ocular adnexa
Conjunctiva, lacrimal gland, orbit *
* Not mucosal.
Skin
Thyroid gland
Liver
Genitourinary tract
Bladder, prostate gland, kidney
Breast
Thymus
Rare sites
MALT lymphomas account for 7% to 8% of all B-cell lymphomas and at least 50% of primary gastric lymphomas. Most cases occur in adults, with a median age of 61 years. Overall, males and females show a largely similar incidence, although gender disparities are found at specific anatomic sites. For example, there is a female predominance in salivary gland and thyroid MALT lymphomas, whereas primary cutaneous marginal zone lymphoma shows a male predominance. There is a higher incidence of gastric MALT lymphoma in northeastern Italy, probably related to a high prevalence of Helicobacter pylori– associated gastritis in that region. A special subtype of small-intestinal MALT lymphoma known as immunoproliferative small intestinal disease (IPSID) occurs in the Middle East, parts of the Indian subcontinent, and the Cape region of South Africa.
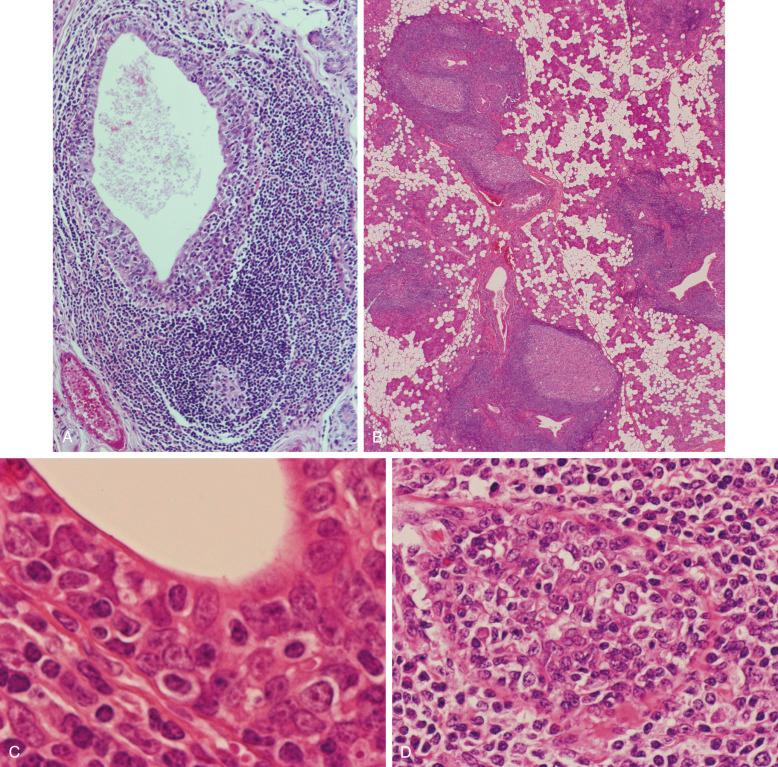
MALT lymphomas only rarely arise from native MALT; they usually arise from MALT that has been acquired as a result of a chronic inflammatory disorder (see later) at sites normally devoid of MALT, such as the stomach, salivary gland, lung, thyroid gland, and ocular adnexa. MALT lymphomas of the salivary gland and thyroid gland, organs normally containing no lymphoid tissue, are always preceded by lymphoepithelial (myoepithelial) sialadenitis (LESA), usually associated with Sjögren's syndrome and Hashimoto's thyroiditis, respectively. Histologic and immunohistochemical studies of the heavy lymphoid infiltrate that characterizes these two conditions have shown a remarkable resemblance to Peyer's patches. This is most graphically illustrated with reference to LESA. In this condition, lymphoid tissue accumulates around dilated salivary gland ducts and forms, in effect, small Peyer's patches, complete with a germinal center, mantle, small marginal zone, and, significantly, lymphoepithelium comprising collections of intraepithelial B cells ( Fig. 19-2, A ). This lymphoid tissue, known as acquired MALT, is also a feature of Hashimoto's thyroiditis, and it has been identified in fetal and neonatal lung from infants with pulmonary infections of an undetermined nature. It is also seen in a condition termed follicular bronchiolitis, which is associated with various autoimmune disorders, including Sjögren's syndrome. It is worth emphasizing that native MALT, in the form of bronchus-associated lymphoid tissue, is not present in normal lung. Likewise, lymphoid tissue is not present in normal stomach, the most common site of MALT lymphoma; here too, MALT is commonly acquired, almost always subsequent to infection with H. pylori, which precedes the development of most cases of gastric MALT lymphoma. Other infectious organisms have been implicated as etiologic agents of MALT lymphoma (see later).
Certain common factors relating to the acquisition of MALT may be relevant to the development of lymphoma at these sites. In most instances, autoimmunity seems to play an important role in the underlying disease. MALT accumulates in relation to columnar epithelium and appears to receive antigenic stimuli either from the epithelium itself or, like physiologic MALT, from antigens that enter the lymphoid tissue across the epithelium, rather than from antigens carried in afferent lymphatics. The functional characteristics of this acquired MALT and the degree to which it resembles normal MALT have not been investigated. Likewise, the factors that, in a small number of cases, result in the transformation of reactive MALT into lymphoma that recapitulates many of its normal morphologic and functional properties remain speculative.
Several lines of evidence suggest that gastric MALT lymphoma arises from MALT acquired as a consequence of H. pylori infection. H. pylori can be demonstrated in the gastric mucosa of the majority of cases of gastric MALT lymphoma. The first study in which this association was examined showed that the organism was present in more than 90% of cases. Subsequent studies have shown a lower incidence, but also that the density and detectability of H. pylori decrease as lymphoma evolves from chronic gastritis. A subsequent case-control study showed an association between previous H. pylori infection and the development of primary gastric lymphoma. More compelling evidence confirming the role of H. pylori in the pathogenesis of gastric lymphoma has been obtained from studies that detected the lymphoma B-cell clone in the chronic gastritis that preceded the lymphoma, as well as from a series of in vitro studies showing that lymphoma growth could be stimulated in culture by H. pylori strain-specific T cells when crude lymphoma cultures were exposed to the organism. Finally, following the initial study by Wotherspoon and colleagues, several groups have confirmed that eradication of H. pylori with antibiotics results in regression of gastric MALT lymphoma in 75% of cases (see later). Recent studies have shown that in the era of widespread use of antibiotic therapy, the incidence of gastric MALT lymphoma is declining, and the percentage of gastric MALT lymphoma containing H. pylori is also declining.
Immunoproliferative small intestinal disease (IPSID) is a variant of MALT lymphoma involving the small intestine that is often associated with secretion of an abnormally truncated immunoglobulin alpha heavy chain without accompanying light chains (alpha heavy chain disease). In 2004, Lecuit and associates reported detection of Campylobacter jejuni DNA in five of seven patients with IPSID and resolution of symptoms in one index patient following antibiotic therapy against Campylobacter . These findings suggested that C. jejuni may play the same role in IPSID as H. pylori does in gastric MALT lymphoma. A subsequent case report also described resolution following antibiotic therapy. In a polymerase chain reaction (PCR) study published in abstract format, Isaacson and colleagues confirmed an association between C. jejuni and IPSID but also detected the organism in other small intestinal lymphomas. Recently, Campylobacter coli, not C. jejuni, has been isolated from the stool of a patient with IPSID. These studies further suggest a relationship between Campylobacter and IPSID, but relatively few cases of IPSID (which is rare, in any case) have definitively been shown to respond to broad-spectrum antibiotics. Additional studies are required to further clarify the role of Campylobacter in the development of IPSID.
In 1991, Garbe and colleagues first described four cases of cutaneous lymphoma, later characterized as MALT lymphomas, associated with Borrelia burgdorferi infection. In 1997, Kutting and associates reported clinical cures of two cases of cutaneous MALT lymphoma following eradication of B. burgdorferi with the antibiotic cefotaxime. Several additional reports have followed from Europe, but there appears to be substantial geographic variability, and similar success has not been reported in the United States.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here