Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors wish to acknowledge the previous contributions of Dr. Anthony Morise, which have laid the foundation for this chapter.
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Exercise electrocardiographic testing is among the most fundamental and widely used tests for the evaluation of patients with cardiovascular disease (CVD). It is easy to administer, perform, and interpret; it is flexible and adaptable; and it is reliable, inexpensive, and readily available in hospital or practice settings. The exercise test has been used by clinicians for more than half a century, and its durability can be attributed to its evolution over time. Primarily developed to detect the presence of myocardial ischemia secondary to coronary artery disease (CAD), the exercise electrocardiogram (ECG) is now recognized for its power in predicting prognosis. Exercise test variables beyond the ST segment yield important information, particularly when used in combination with clinical information, to predict outcomes and guide therapy in a broad range of individuals, from the healthy to those disabled by heart disease. Emerging applications of exercise electrocardiography have demonstrated its usefulness in the evaluation and management of patients with a wide variety of cardiovascular conditions, including valvular heart disease, congenital heart disease, genetic cardiovascular conditions, arrhythmias, and peripheral artery disease (PAD). When used appropriately with adjunctive modalities to measure gas exchange and ventilation or with imaging techniques such as echocardiography or nuclear perfusion imaging (see Chapter 16, Chapter 18 ), the power of the exercise ECG is greatly enhanced. The exercise ECG is the clinician’s beacon that can guide optimal care for a great majority of patients with known or suspected CVD. This chapter provides a detailed foundation of information on the physiology of exercise testing and the exercise ECG. Other chapters address adjunctive imaging techniques and further discuss the use of exercise testing in patients with specific cardiovascular conditions.
Exercising muscles require energy to contract and relax. Most of this energy is derived from oxidative metabolism to generate adenosine triphosphate; thus, energy requirements at rest and for any given amount of physical activity (work rate) can be estimated from measurements of total-body oxygen uptake (
). The Fick equation ( Fig. 15.1 ) demonstrates that
is determined by the product of cardiac output and oxygen extraction at the periphery (i.e., arteriovenous oxygen difference).
is easily expressed in multiples of resting oxygen requirements (metabolic equivalents [METs]), with 1 MET being resting energy expenditure and defined as approximately 3.5 mL O 2 /kg body weight/min. This convenient system indexes the amount of energy used during any given physical activity against that used at rest. Accordingly, 5-MET activity requires five times the energy expenditure at rest.
max is the peak oxygen uptake achieved during performance of the highest level of dynamic exercise involving large muscle groups and by definition cannot be exceeded despite increases in work rate. It is related to age, sex, heredity, exercise habits, and cardiovascular status. Cardiac output can increase as much as four to six times resting levels in the upright position. Maximum cardiac output is the result of a twofold to threefold increase in heart rate (HR) from resting levels and an increase in stroke volume. Stroke volume in healthy persons generally plateaus at 50% to 60% of
max. Oxygen extraction at the periphery can increase as much as threefold, and the maximum arteriovenous O 2 difference has a physiologic limit of 15 to 17 mL O 2 /100 mL blood. During clinical exercise testing, patients are prompted to exercise not until they attain
max but rather to the
that is attained during symptom-limited, maximum tolerated exercise; this level is termed the
peak.
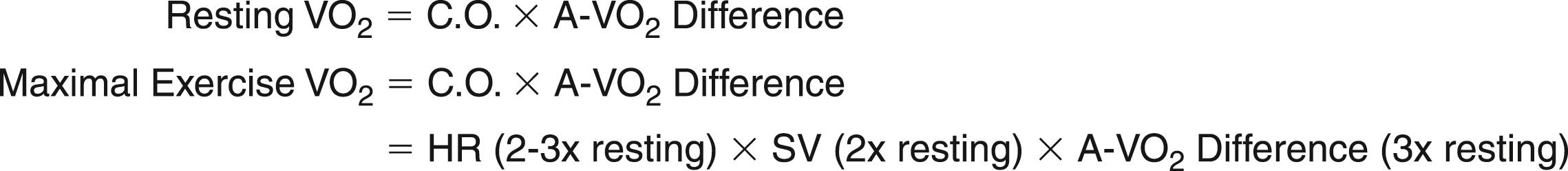
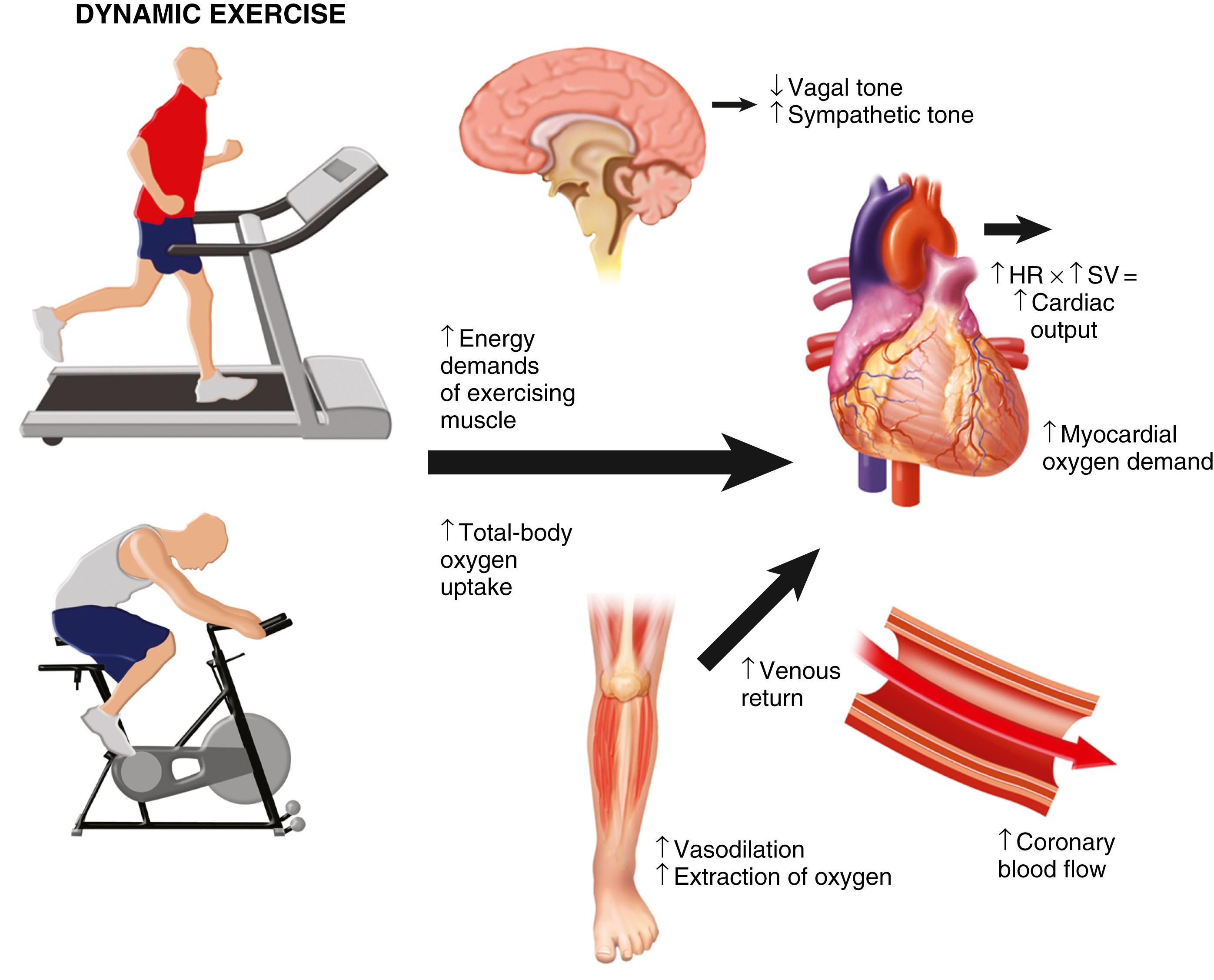
Myocardial ischemia occurs when the supply of oxygenated blood to myocardial cells is inadequate to meet demands. Many factors affect the delicate balance of supply and demand ( Fig. 15.2 ) . Exercise testing is performed to stress these relationships and observe the physiologic responses that ensue. This enables the clinician not only to assess for the development of myocardial ischemia, but also to evaluate at what level of myocardial oxygen demand and physical activity (work rate) ischemia occurs.
Myocardial oxygen demand is related to HR, blood pressure (BP), left ventricular (LV) contractility (myocardial shortening per beat), and LV wall stress. The latter is related to LV pressure, wall thickness, and cavity size. Changes in any of these interdependent factors can affect myocardial need for oxygenated blood. Of these parameters, HR and BP are the easiest to measure and monitor. The product of HR and systolic BP, termed the rate-pressure product , is a reliable index of myocardial oxygen demand and can be readily assessed clinically.
During acute endurance (high-repetition/low-resistance) exercise (e.g., walking or cycling), cardiac output rises in response to the metabolic needs of the exercising muscles (estimated by measured
). Diminution of vagal tone and a rise in sympathetic tone lead to an increase in HR and LV contractility. Stroke volume also rises because of increases in venous return of blood from exercising muscles, and blood flow is redistributed from the renal, splanchnic, and cutaneous circulation to the exercising muscles. Accumulation of metabolites in the actively contracting muscles causes vasodilation of muscle arterioles, which increases skeletal muscle blood flow up to four times that of resting levels and results in a reduction in aortic outflow impedance. This in turn allows more complete systolic ejection, thereby further increasing stroke volume. Systolic BP increases mostly because of the rise in cardiac output, whereas diastolic BP either remains constant or falls as a result of the reduction in vascular resistance. The size and location of the exercising muscle groups will have different effects on the hemodynamic response to exercise. Dynamic arm exercise elicits higher HR and BP responses at any given work rate than does dynamic leg exercise. Arm work yields differences in sympathetic output, peripheral vasodilation, venous return, and metabolic requirements, which are influenced not only by the exercising muscle mass but also by the stabilizing muscles recruited during arm exercise.
Resistance (low-repetition/high-load) exercise (e.g., weightlifting) is not generally used during graded exercise testing but may be used in work simulation testing or exercise training regimens. This type of exercise generates an increased sympathetic response, leading to an increase in HR; however, venous return, especially during straining, may decrease. Therefore, the rise in cardiac output is relatively small in comparison to that achieved with endurance exercise and is primarily caused by increases in HR. Muscle contraction during resistance exercise generates compressive force on muscle capillaries that leads to elevated peripheral resistance. This rise in vascular resistance coupled with an increase in cardiac output yields an increase in both systolic and diastolic BP. Elevations in systolic BP from rest to exercise are proportionally greater than the elevations in HR during resistance exercise than during endurance exercise. Therefore, both endurance exercise and resistance exercise increase myocardial oxygen demand because of variable increases in HR, BP, LV contractility, and LV wall stress (the latter caused by increases in LV pressure and/or volume during exercise).
Coronary blood flow increases during exercise in response to neurohumoral stimulation (primarily sympathetic beta receptor stimulation) and as a result of the release of endothelial substances, including nitric oxide. In healthy persons during acute exercise, coronary arteries dilate and coronary blood flow rises in response to the increases in myocardial oxygen demand. Most often, coronary flow is compromised as a result of atherosclerotic plaque within the lumen of the coronary artery (see Chapter 36 ). Plaque may cause minimal stenosis or complete occlusion of the artery. Several factors influence the significance of a given luminal stenosis, including the degree of luminal obstruction, the length of the obstruction, the number and size of functioning collateral vessels, the magnitude of the muscle mass supplied, the shape and dynamic properties of the stenosis, and the autoregulatory capacity of the vascular bed. In general, a 50% to 70% reduction in luminal diameter will impair peak reactive hyperemia, whereas 90% or greater stenosis will reduce resting flow. However, exercise stimulates local changes in vasomotor tone as a result of neuromodulation, endothelial dysfunction, and local factors, and these changes can further influence the supply of oxygenated blood to the myocardium. Atherosclerotic arteries often fail to dilate and may actually constrict with exercise, thus further reducing the supply of blood in the setting of increased demand.
It is important to clinically assess the patient before performing the exercise test to evaluate the indications for the test, the appropriateness of the specific test that has been ordered to answer the question posed, the ability of the patient to perform exercise, and whether the patient has any contraindications to exercise testing ( Table 15.1 ). Information from the medical history as provided by the patient, chart review, and the ordering provider and/or the patient’s primary care physician or cardiologist can be most useful in this pretest evaluation. A brief physical examination that addresses the components outlined in Table 15.2 can also be helpful. A current standard resting 12-lead ECG is useful in assessing HR, rhythm, conduction abnormalities, and evidence of previous myocardial infarction (MI) and should be compared with the most recent previous ECG, if available.
Absolute Contraindications
Relative Contraindications
|
History
Physical Examination
|
Diagnostic exercise testing in patients without known CAD is best performed by withholding cardioactive medications on the day of the test to better assess for an ischemic response. On the other hand, functional testing in patients with known CAD is best performed with patients taking their usual medications to evaluate the effects of the medications on HR, BP, symptoms, and ischemia during exercise (see “Pharmacologic Influences on Interpretation”).
In patients with permanent cardiac pacemakers, it is important to obtain information from the patient’s cardiologist regarding the type of pacemaker (single or dual chamber), programmed mode, rate responsiveness, and pacing HR limits before the test. Similarly, in patients with implantable cardioverter-defibrillators (ICDs), information regarding ICD rhythm detection and treatment algorithms should be obtained so that the peak HR during the exercise test is maintained at least 10 beats/min below the programmed HR threshold for anti-tachycardia pacing and defibrillation. Additional details of patient assessment are provided elsewhere.
Before exercising, patients should be made familiar with the symptom rating scales that might be used during testing. These are described further elsewhere and may include the Borg Scale of Perceived Exertion.
As the technology of exercise electrocardiographic testing has evolved, several different types of lead systems have been developed and used. Details regarding these lead systems, along with skin preparation techniques, are provided elsewhere. The importance of adequate skin preparation cannot be overstated; this is essential to optimize the quality of the exercise ECG. To obtain a high-quality 12-lead ECG during testing, electrode placement on the torso is standard for routine testing. Torso electrodes are placed under the lateral aspect of the clavicles for the arm leads and on the lower end of the rib cage or high under the rib cage for the leg leads. A standard 12-lead ECG should be performed before placement of the torso limb leads because such lead placement may alter the inferior lead complexes and result in previous Q waves being either mimicked or hidden. A standing ECG is then used as the basis for determining the presence of exercise-induced ECG changes.
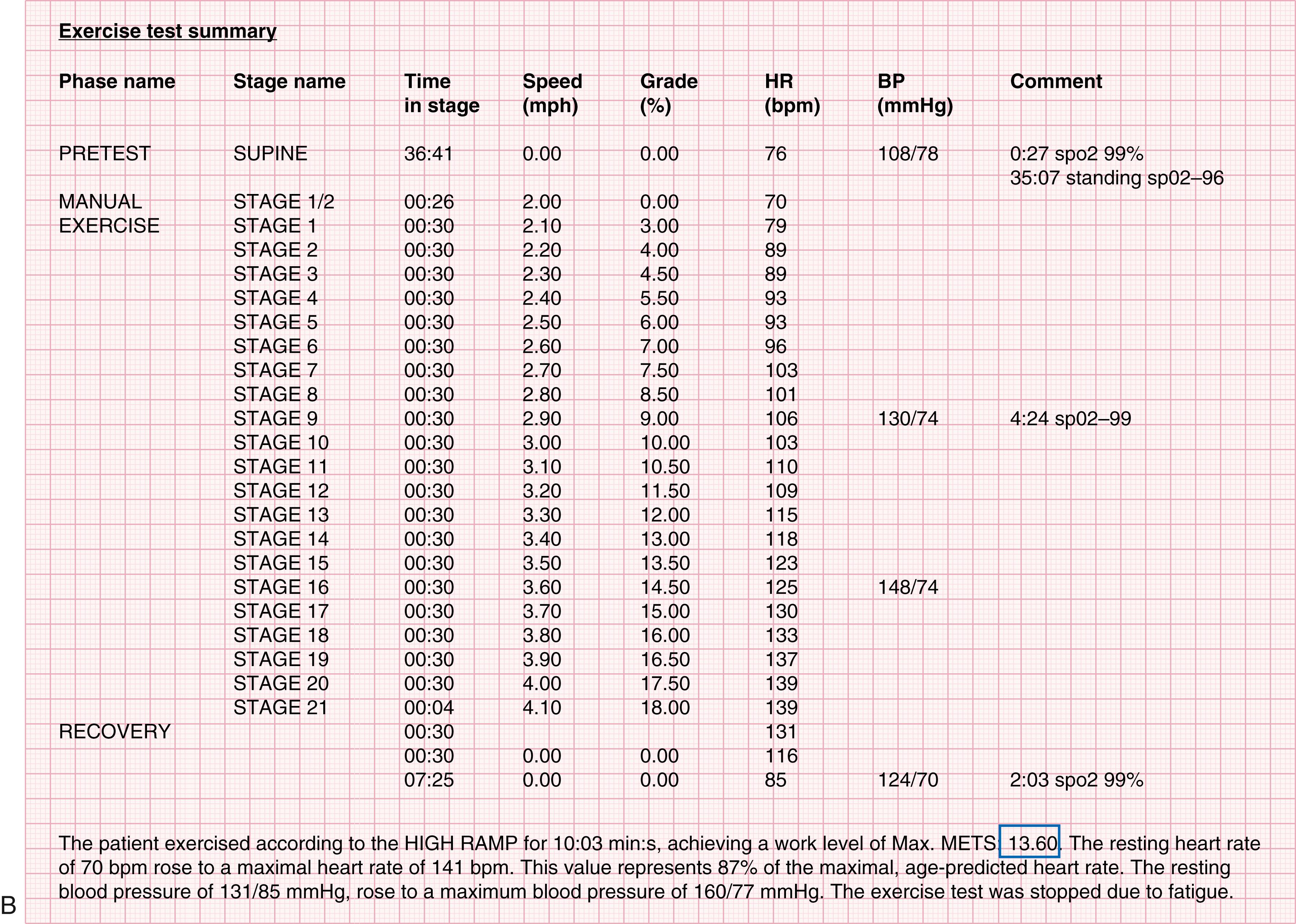
The testing modality and protocol should be selected in accordance with the patient’s estimated functional capacity based on age, estimated physical fitness from the patient’s history, and underlying disease. Several exercise test protocols are available for both treadmill and stationary cycle ergometers. Patients who have low estimated fitness levels or are deemed to be at higher risk because of underlying disease (e.g., recent MI, heart failure) should be tested with a less aggressive exercise protocol. Treadmill and cycle ergometers may use stepped or continuous ramp protocols. Work rate increments (stages) during stepped protocols can vary from 1 to 2.5 METs. Ramp protocols are designed with stages that are no longer than 1 minute and for the patient to attain peak effort within 8 to 12 minutes. Accordingly, ramp protocols must be individualized and selected to accommodate the patient’s estimated exercise capacity. Because there are no widely published or standard sets of ramp protocols, individual exercise testing laboratories usually develop their own customized protocols that accommodate a wide range of fitness levels. Table 15.3 provides examples of such protocols. The American College of Sports Medicine (ACSM) details a variety of treadmill and cycle ergometer testing protocols.
| Stage ∗ | Very low Ramp | low Ramp | Moderate Ramp | High Ramp | Athlete’s Ramp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mph | % Grade | Mets | mph | % Grade | Mets | mph | % Grade | Mets | mph | % Grade | Mets | mph | % Grade | Mets | |
| 1 | 1.0 | 0.0 | 1.8 | 1.0 | 0.0 | 1.8 | 1.5 | 1.5 | 2.5 | 2.1 | 3.0 | 3.5 | 1.8 | 0.0 | 2.4 |
| 2 | 1.1 | 0.2 | 1.9 | 1.1 | 0.5 | 1.9 | 1.6 | 2.0 | 2.7 | 2.2 | 4.0 | 3.9 | 2.1 | 0.5 | 2.7 |
| 3 | 1.2 | 0.4 | 2.0 | 1.2 | 1.0 | 2.1 | 1.7 | 2.5 | 2.9 | 2.3 | 4.5 | 4.2 | 2.4 | 1.0 | 3.2 |
| 4 | 1.3 | 0.6 | 2.1 | 1.3 | 1.5 | 2.3 | 1.8 | 3.0 | 3.1 | 2.4 | 5.5 | 4.6 | 2.7 | 1.5 | 3.6 |
| 5 | 1.4 | 0.8 | 2.2 | 1.4 | 2.0 | 2.5 | 1.9 | 3.5 | 3.4 | 2.5 | 6.0 | 5.0 | 3.3 | 2.0 | 4.1 |
| 6 | 1.5 | 1.0 | 2.3 | 1.5 | 2.5 | 2.7 | 2.0 | 4.0 | 3.6 | 2.6 | 7.0 | 5.5 | 3.3 | 2.5 | 4.6 |
| 7 | 1.6 | 1.2 | 2.5 | 1.6 | 3.0 | 2.9 | 2.1 | 4.5 | 3.9 | 2.7 | 7.5 | 5.8 | 3.6 | 3.0 | 5.2 |
| 8 | 1.7 | 1.4 | 2.6 | 1.7 | 3.5 | 3.1 | 2.2 | 5.0 | 4.2 | 2.8 | 8.5 | 6.4 | 3.9 | 3.5 | 6.1 |
| 9 | 1.8 | 1.6 | 2.8 | 1.8 | 4.0 | 3.4 | 2.3 | 5.5 | 4.5 | 2.9 | 9.0 | 6.8 | 4.2 | 4.0 | 7.3 |
| 10 | 1.9 | 1.8 | 2.9 | 1.9 | 4.5 | 3.6 | 2.4 | 6.0 | 4.8 | 3.0 | 10.0 | 7.4 | 4.5 | 4.5 | 8.4 |
| 11 | 2.0 | 2.0 | 3.1 | 2.0 | 5.0 | 3.9 | 2.5 | 6.5 | 5.1 | 3.1 | 10.5 | 7.8 | 4.8 | 5.0 | 9.5 |
| 12 | 2.1 | 2.2 | 3.2 | 2.1 | 5.5 | 4.2 | 2.6 | 7.0 | 5.5 | 3.2 | 11.5 | 8.5 | 5.1 | 5.5 | 10.6 |
| 13 | 2.2 | 2.4 | 3.4 | 2.2 | 6.0 | 4.5 | 2.7 | 7.5 | 5.8 | 3.3 | 12.0 | 8.9 | 5.4 | 6.0 | 11.5 |
| 14 | 2.3 | 2.6 | 3.6 | 2.3 | 6.5 | 4.8 | 2.8 | 8.0 | 6.2 | 3.4 | 13.0 | 9.7 | 5.7 | 6.5 | 12.2 |
| 15 | 2.4 | 2.8 | 3.8 | 2.4 | 7.0 | 5.1 | 2.9 | 8.5 | 6.6 | 3.5 | 13.5 | 10.1 | 6.0 | 7.0 | 13.0 |
| 16 | 2.5 | 3.0 | 3.9 | 2.5 | 7.5 | 5.5 | 3.0 | 9.0 | 7.0 | 3.6 | 14.5 | 10.9 | 6.3 | 7.5 | 13.8 |
| 17 | 2.6 | 3.2 | 4.1 | 2.6 | 8.0 | 5.8 | 3.1 | 9.5 | 7.4 | 3.7 | 15.0 | 11.4 | 6.6 | 8.0 | 14.7 |
| 18 | 2.7 | 3.4 | 4.3 | 2.7 | 8.5 | 6.2 | 3.2 | 10.0 | 7.8 | 3.8 | 16.0 | 12.2 | 6.9 | 8.5 | 15.5 |
| 19 | 2.8 | 3.6 | 4.5 | 2.8 | 9.0 | 6.6 | 3.3 | 10.5 | 8.3 | 3.9 | 16.5 | 12.6 | 7.2 | 9.0 | 16.4 |
| 20 | 2.9 | 3.8 | 4.7 | 2.9 | 9.5 | 7.0 | 3.4 | 11.0 | 8.7 | 4.0 | 17.5 | 13.3 | 7.5 | 9.5 | 17.3 |
Exercise tests may be submaximal or maximal relative to the patient’s effort. In addition to common indications for stopping the exercise test ( Table 15.4 ) , submaximal exercise testing has a predetermined endpoint, often defined as a peak HR (e.g., 120 beats/min or 70% of predicted maximum HR) or an arbitrary MET level (e.g., 5 METs). Submaximal tests are used in patients early after MI before discharge from the hospital because they can provide prognostic information to guide management. They can also be useful in the evaluation of a patient’s ability to engage in daily activities after discharge and can serve as a baseline for cardiac rehabilitative exercise therapy (see “ Physical Activity and Exercise Prescription”). Symptom-limited tests are designed to continue until the patient demonstrates signs and/or symptoms necessitating termination of exercise (see Table 15.4 ) . Whatever modality or protocol is used, standard patient monitoring and measurements are made during and early after exercise ( Table 15.5 ).
Treadmill testing provides a more common form of physiologic stress (i.e., walking) in which patients are more likely to attain a higher oxygen uptake and peak HR than during stationary cycling. Cycling may be preferable when orthopedic or other specific patient characteristics limit treadmill testing or during exercise echocardiographic testing to facilitate acquisition of images at peak exercise. The most frequently used stepped treadmill protocols are the Naughton, Bruce, and modified Bruce ( Table 15.6 ).
Data from American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia: Wolters Kluwer; 2018.
| Stage | Time | Speed (mph) | Grade (%) | Mets |
|---|---|---|---|---|
| REST | 00.00 | 0.0 | 0.0 | 1.0 |
| 1 | 03.00 | 1.7 | 10.0 | 4.6 |
| 2 | 03.00 | 2.5 | 12.0 | 7.0 |
| 3 | 03.00 | 3.4 | 14.0 | 10.1 |
| 4 | 03.00 | 4.2 | 16.0 | 12.9 |
| 5 | 03.00 | 5.0 | 18.0 | 15.1 |
| 6 | 03.00 | 5.5 | 20.0 | 16.9 |
| 7 | 03.00 | 6.0 | 22.0 | 19.2 |
During treadmill exercise, patients should be encouraged to walk freely and use the handrails for balance only when necessary. Excessive handrail gripping and support alter the BP response and decrease the oxygen requirement (METs) per given workload, thereby resulting in an overestimation of exercise capacity ( eFig. 15.1 ) and an inaccurate HR-and BP-to-workload relationship. Exercise capacity (peak METs) can be estimated for treadmill exercise by using data provided by ACSM, as long as the equipment is calibrated regularly. When precise determination of oxygen uptake is necessary, such as assessment of patients for heart transplantation (see Chapter 60 ), evaluation by expired gas analysis is preferred over estimation (see “ Cardiopulmonary Exercise Testing”). Normal values for exercise capacity in healthy adults at different ages are available and may serve as a useful reference in the evaluation of a patient’s exercise capacity.
A cycle ergometer is smaller, quieter, and less expensive than a treadmill. Because a cycle ergometer requires less movement of the arms and thorax, quality electrocardiographic recordings and BP measurements are easier to obtain. However, stationary cycling may be unfamiliar to many patients, and its success as a testing tool is highly dependent on patient skill and motivation. Thus, the test may end before the patient reaches a true cardiopulmonary endpoint. Unlike treadmill testing, in which the work being performed involves movement of the patient’s body weight at a given pace, stationary cycle work involves cycling at a given pace against an external force and is generally independent of the patient’s body weight, which is supported by the seat. As shown in Table 15.7 , the MET level attained at a given work rate varies with the patient’s body weight. Accordingly, at the same given cycle ergometer work rate, a lighter person will attain higher METs than will a heavier person. Mechanically braked ergometers require that the patient’s cycling speed be kept constant. Electronically braked cycle ergometers automatically adjust external resistance to the cycling speed to maintain a constant work rate at a given stage. Electronically braked cycle ergometers allow simple programming of ramp protocols. As with treadmill ramp protocols, customized cycle ergometer ramp protocols that accommodate a wide range of fitness levels need to be established by individual exercise testing laboratories.
| Body Weight | Exercise Rate (KP • m • min −1 and Watts) | |||||||
|---|---|---|---|---|---|---|---|---|
| KP | Lb | Kpms 300 WATTS 50 | 450 75 | 600 100 | 750 125 | 900 150 | 1050 175 | 1200 200 |
| 50 | 110 | 5.1 | 6.9 | 8.6 | 10.3 | 12.0 | 13.7 | 15.4 |
| 60 | 132 | 4.3 | 5.7 | 7.1 | 8.6 | 10.0 | 11.4 | 12.9 |
| 70 | 154 | 3.7 | 4.9 | 6.1 | 7.3 | 8.6 | 9.8 | 11.0 |
| 80 | 176 | 3.2 | 4.3 | 5.4 | 6.4 | 7.5 | 8.6 | 9.6 |
| 90 | 198 | 2.9 | 3.8 | 4.8 | 5.7 | 6.7 | 7.6 | 8.6 |
| 100 | 220 | 2.6 | 3.4 | 4.3 | 5.1 | 6.0 | 6.9 | 7.7 |
Arm ergometry is an alternative method of exercise testing for patients who cannot perform leg exercise. Although this test has diagnostic usefulness, it has been largely replaced by nonexercise pharmacologic stress techniques.
The 6-minute walk test can be used as a surrogate measure of exercise capacity when standard treadmill or cycle testing is not available. Distance walked is the primary outcome of the test. It is not useful in the objective determination of myocardial ischemia and is best used in a serial manner to evaluate changes in exercise capacity and the response to interventions that may affect exercise capacity over time. The 6-minute walk test protocol is discussed in detail elsewhere ( Table 15.8 ) .
| Testing Site The Six Minute Walk Test Protocol should be performed indoors, along a long, flat, straight, enclosed corridor with a hard surface that is seldom traveled. The walking course must be 30 m in length. A 100-ft (30.4 m) hallway is required and its length should be marked every 3 m. The turnaround points should be marked with a cone (such as an orange traffic cone). A starting line, which marks the beginning and end of each 60-m lap, should be marked on the floor using brightly colored tape. Measurements Assemble all necessary equipment (lap counter, timer, clipboard, worksheet) and move to the starting point. Set the lap counter to zero and the timer to 6 min. Position the patient at the starting line. You should also stand near the starting line during the test. Do not walk with the patient. As soon as the patient starts to walk, start the timer. Do not talk to anyone during the walk. Use an even tone of voice when using the standard phrases of encouragement. Each time the patient returns to the starting line, click the lap counter once (or mark the lap on the worksheet). At the end of 6 min, tell the patient to stop walking, and measure the total distance traveled (meters). Heart rate, blood pressure and oxygen saturation should be measured at rest and at the end of exercise as well. The main outcome of this test is total distance traveled. Patient Instructions Standardized scripted patient instructions should be used, and are provided elsewhere |
Because of the inaccuracies associated with estimating oxygen uptake (
) and METs from work rate with the treadmill or cycle ergometer, many laboratories perform cardiopulmonary exercise testing (CPX), which uses ventilatory gas exchange analysis during exercise to provide a more reliable and reproducible measure of
. Peak
is the most accurate measure of exercise capacity and is a useful reflection of overall cardiopulmonary health. Measurement of expired gases is not necessary for all clinical exercise testing, but the additional information can provide important physiologic data that can be useful in both clinical and research applications. Measures of gas exchange primarily include
, carbon dioxide output (V̇ co 2 ), and minute ventilation. Use of these variables in graphic form provides further information on the ventilatory threshold and ventilatory efficiency.
CPX is well established as useful in the following situations:
Evaluation of exercise capacity in selected patients with heart failure, to assist in estimation of prognosis, evaluate the response to medications and other interventions, and assess the need for cardiac transplantation.
Evaluation of exertional dyspnea. Such testing can provide useful information for differentiating cardiac from pulmonary limitations as a cause of exercise-induced dyspnea or impaired exercise capacity when the cause is uncertain.
Evaluation of the patient’s response to specific therapeutic interventions (e.g., medications; programmed pacing; cardiac rehabilitation) in which improvement in exercise tolerance is an important goal or endpoint.
Emerging evidence demonstrates that CPX can provide valuable clinical information in patients with hypertrophic cardiomyopathy (HCM), suspected or confirmed pulmonary hypertension, suspected myocardial ischemia, suspected mitochondrial myopathy, and confirmed chronic obstructive pulmonary disease or interstitial lung disease. More recently, utility of CPX has been demonstrated in the assessment of perioperative risk and valvular heart disease.
The technical aspects of CPX have become simplified with contemporary metabolic carts, but meticulous maintenance and calibration of these systems are required for optimal use. The personnel involved in administering and interpreting the test must be trained and proficient in this technique. The test also requires additional time, as well as patient cooperation. CPX used in combination with Doppler echocardiography can provide complementary information regarding cardiac output, myocardial contractile function, and valvular function.
Absolute Indications
Relative Indications
|
During the Exercise Period
During the Recovery Period
|
Over the past 30 years since the American Heart Association (AHA) published its first set of Standards for Adult Exercise Testing Laboratories , the role of the physician in ensuring that the exercise laboratory is properly equipped and appropriately staffed with qualified personnel who adhere to a written set of policies and procedures specific to that laboratory has not changed. In subsequent iterations of their respective guidelines, the AHA, ACSM, American College of Cardiology (ACC), and American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) have consistently addressed this issue. In 2000 the ACC/AHA/American College of Physicians/American College of Sports Medicine Competency Task Force focused its efforts on outlining the specific cognitive and training requirements for personnel involved in supervising and interpreting exercise ECGs and was the first to look beyond the specific professional type (e.g., physician, nurse, exercise physiologist) and focus on specific competencies of the individual staff member (see “Classic References”). In 2014 these recommendations were updated to define further the roles of each staff member involved with exercise testing. This statement clearly defined different levels of supervision as follows: (1) “personal supervision” requires a physician’s presence in the room; (2) “direct supervision” requires a physician to be in the immediate vicinity, on the premises or the floor, and available for emergencies; and (3) “general supervision” requires the physician to be available by phone or by page. Common to every guideline is the recommendation that patients be screened before exercise testing to assess their risk for an exercise-related adverse event, so that the most appropriate personnel to supervise the test can be provided. Exercise testing may be supervised by nonphysician staff members who are deemed competent according to the criteria outlined in the ACC/AHA statement. In all such cases the physician should be immediately available to assist as needed (i.e., provide direct supervision). In high-risk patients the physician should personally supervise the test (i.e., provide personal supervision).
Exercise is associated with increased risk for an adverse cardiovascular event, and details regarding the safety of exercise testing and emergency preparedness in exercise laboratories are addressed in depth in guidelines from the AHA , and the ACSM. Nonetheless, the safety of exercise testing is well documented, and the overall risk for adverse events is quite low. In several large series of individuals with and without known CVD, the rate of major complications (including MI and other events requiring hospitalization) was less than 1 to as high as 5 per 10,000 tests, and the rate of death was less than 0.5 per 10,000 tests. The incidence of adverse events depends on the study population. Patients with recent MI, reduced LV systolic function, exertion-induced myocardial ischemia, and serious ventricular arrhythmias are at highest risk. , A report of 5060 CPX studies performed in patients with severe functional impairment and a variety of high-risk cardiac diseases, including heart failure, HCM, pulmonary hypertension, and aortic stenosis, further supports the safety of exercise testing. The adverse event rate was 0.16%, and the most common adverse event was sustained ventricular tachycardia (VT). No fatal events were reported.
Maintenance of appropriate emergency equipment, establishment of an emergency plan, and regular practice in carrying out the plan are fundamental to ensuring safety in an exercise testing laboratory (see “Classic References”).
Any chest pain produced during the exercise test needs to be factored into the exercise test conclusion and report.
First, are the symptoms reported during the test the same or similar to the reported historical symptoms that prompted the exercise test? If the answer is yes, the provider can assess the objective test responses and discern whether they support the presence of CAD. If the answer is no, differences between the produced and historical symptoms need to be clarified. In addition, the symptoms produced need to be categorized according to whether they are consistent with angina. Distinguishing anginal from nonanginal chest pain is important at the time of occurrence of the chest pain. Angina is not well localized, pleuritic, or associated with palpable tenderness (see Chapter 13, Chapter 35 ), and the only opportunity to define these qualities may be at the exercise test.
Second, exercise-induced angina is an important clinical predictor of the presence and severity of CAD, equal to or greater than ST-segment depression. Consideration of limiting versus nonlimiting chest pain, in addition to any induced angina, has been incorporated into the Duke Treadmill Score, as well as into other treadmill scores (see later) . These factors will have an impact on the prognostic and diagnostic assessment of the test results, and ultimately the next step in the clinical evaluation.
Third, exercise-induced typical angina predicts an adverse prognosis and is worthy of further evaluation regardless of the ST-segment response or the exercise capacity. In a series of 3270 patients without known coronary disease referred for exercise testing, Christman and colleagues found that typical angina defined by physicians and exercise physiologists at the exercise test was a predictor of adverse events, including death, nonfatal MI, and revascularization. This was found irrespective of the presence or absence of a positive ST-segment response or good exercise capacity.
Lastly, if the patient stops exercise earlier than anticipated because of dyspnea, careful consideration should be given as to whether an anginal equivalent is present. If the presenting symptom was dyspnea with exertion, this becomes even more relevant.
Functional capacity is a strong predictor of mortality and nonfatal cardiovascular outcomes in both men and women with and without CAD. Even though exercise capacity is most accurately measured by CPX, a reasonable estimate can be obtained from treadmill testing alone. The best methods for estimating predicted METs are the following simple regression equations.
The reported exercise time can be translated into METs or METs based on the exercise test protocol. The reported METs can then be expressed as a percentage of the predicted METs. Table 15.9 provides an alternative qualitative classification of functional capacity that adjusts for age and sex.
| Estimated Functional Capacity (Mets) | |||||
|---|---|---|---|---|---|
| age (YR) | Poor | Fair | Average | Good | High |
| Women | |||||
| ≤29 | <7.5 | 8–10 | 10–13 | 13–16 | >16 |
| 30–39 | <7 | 7–9 | 9–11 | 11–15 | >15 |
| 40–49 | <6 | 6–8 | 8–10 | 10–14 | >14 |
| 50–59 | <5 | 5–7 | 7–9 | 9–13 | >13 |
| ≥60 | <4.5 | 4.5–6 | 6–8 | 8–11.5 | >11.5 |
| Men | |||||
| ≤29 | <8 | 8–11 | 11–14 | 14–17 | >17 |
| 30–39 | <7.5 | 7.5–10 | 10–12.5 | 12.5–16 | >16 |
| 40–49 | <7 | 7–8.5 | 8.5–11.5 | 11.5–15 | >15 |
| 50–59 | <6 | 6–8 | 8–11 | 11–14 | >14 |
| ≥60 | <5.5 | 5.5–7 | 7–9.5 | 9.5–13 | >13 |
In addition to clinical factors, functional capacity can be related to familiarity with the exercise equipment, level of training, and environmental conditions in the exercise laboratory. Patients who cannot adequately perform an exercise test or who undergo a pharmacologic stress test have a worse prognosis than do those who can perform an exercise test.
Functional capacity should always be incorporated into the results, conclusions, and/or recommendations of the exercise test report. Functional capacity can be incorporated into available multivariable scores such as the Duke Treadmill Score or the Cleveland Clinic Prognostic Score to classify the prognosis as low, intermediate, or high risk (see Prognostic Value ).
The maximum HR with exercise is a fundamental physiologic parameter that provides the clinician relevant information concerning the intensity of exercise, the adequacy of the exercise test, the effect of medications that influence HR, the potential contribution to exercise intolerance, and the patient’s prognosis. The maximum achievable HR (HRmax) is unique for each patient but can be estimated by using regression equations that adjust for the patient’s age. The most familiar equation, which was developed principally in middle-aged men, is:
Although easy to apply and calculate, there is considerable variability with this equation, especially in patients with CAD who are taking beta blockers. Newer equations have been proposed to more accurately replace the “220 − age” rule to generate the maximum age-predicted HR (MPHR):
The inability of the heart to increase its rate to meet the demand placed on it is termed chronotropic incompetence. It is considered an independent predictor of cardiac or all-cause mortality, as well as other adverse cardiovascular outcomes.
A submaximal study is assigned when the peak HR achieved is below the MPHR. An inadequate study is defined by failure to achieve a predefined goal, such as 85% of MPHR. If a patient without known CAD has an inadequate study, the term nondiagnostic study is often applied. As usual, this “nondiagnostic” status is relative. In the presence of any other diagnostic endpoints, such as 2-mm or greater ST-segment depression, exercise-induced hypotension, or exercise-induced anginal chest pain, the HR adequacy question becomes irrelevant.
Chronotropic incompetence typically has been defined by the adjusted HR reserve, incorporates both resting and peak HRs, as well as the age-adjusted HRmax. However, before “chronotropic incompetence” is applied, consideration should be given to the effort exerted in performing exercise, present medications, in particular beta blockers, and the reason for termination of the exercise test. Effort applied to the exercise is often defined by the symptoms produced or by indices of perceived exertion (e.g., Borg scale). , These work well in most settings but can also be defined quantitatively by using CPX parameters such as the respiratory exchange ratio. For the usual non-CPX application, the following formula defines the chronotropic index:
Failure to achieve a chronotropic index higher than 80% defines the presence of chronotropic incompetence and this predicts a poor prognosis. Criteria for assessing chronotropic incompetence in patients with atrial fibrillation (AF) have not been established.
The HR increases during exercise because of an increase in sympathetic tone and a decrease in vagal tone. At the cessation of exercise, under normal circumstances, the reverse process occurs. In athletes and normal persons, there is a biexponential response, with an initial steep 30-second fall in HR followed by a shallower decline thereafter. This biexponential response disappears with the administration of atropine and becomes similar to the response in patients with heart failure. Thus, the initial steep phase is due to parasympathetic activation. Abnormal HR recovery (HRR) has been defined by many methods, but the most commonly accepted include less than 12 beats/min decrement after 1 minute with post-exercise slow walking cool-down, less than 18 beats/min after 1 minute with immediate cessation of movement into either the supine or sitting position, and less than 22 beats/min after 2 minutes. In healthy individuals, short-term reproducibility has been demonstrated (see “Classic References”).
Abnormal HRR is associated with an increase in all-cause mortality in both asymptomatic individuals and patients with established heart disease. This association is independent of the chronotropic index, beta blockade, CAD severity, LV function, Duke Treadmill Score, and ST-segment depression. HRR adds to the prognostic ability of peak
. When considered in a multivariable format assessing prognosis, HRR has been found to be an independent predictor of adverse outcomes even when combined with nuclear variables.
Exercise BP responses, as with those for HR, reflect the balance between sympathetic and parasympathetic influences. Systolic BP, pulse pressure (difference between systolic and diastolic BP), HR-BP product (also called the double product ), and double-product reserve (change in double product from peak to rest) all increase steadily as work rate increases. Diastolic BP increases only minimally or may fall. In most normal individuals, systolic BP will increase to well above 140 mm Hg and the double product to higher than 20,000.
This response is usually defined as greater than 210 mm Hg in men and greater than 190 mm Hg in women. Even though these exercise responses are considered abnormal, they are not generally reasons to terminate exercise. Such responses may be indicative of the future development of hypertension or adverse cardiac events.
This has been variably defined but most frequently as systolic pressure during exercise falling below resting systolic pressure. Another definition is a 20 mm Hg fall after an initial rise. Either of these definitions would be an absolute reason to terminate the exercise test. The former definition is more predictive of a poor prognosis and is often related to severe multivessel CAD with LV dysfunction, especially when noted with other signs of ischemia, such as ST depression or angina at a low workload. Its positive predictive value (PPV) is higher in men than in women. Its presence usually warrants consideration of prompt invasive evaluation. Exercise-associated hypotension may also be seen in patients with cardiomyopathy, LV outflow tract obstruction, enhanced vagal tone, hypovolemia, antihypertensive medications, and arrhythmias. In addition, one study of 57,442 patients suggests that exercise-induced hypotension may be a predictor of future AF.
One systolic BP response that needs to be appreciated might be called “pseudo–exercise-induced hypotension.” This response occurs in patients who are anxious about the exercise study and begin exercise with a somewhat elevated systolic pressure. As exercise proceeds in the first stage, this elevated BP usually settles down or “falls” toward its customary resting level and the patient looks well. As exercise continues, continued observation reveals a gradual upward trend in BP. Considerable judgment needs to be used when interpreting this response.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here