Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver surgery techniques and liver imaging are constantly evolving, allowing for ever more detailed planning of surgical strategy and complex liver resections. The development of living-donor liver transplantation (LDLT; see Chapter 121 ) has led to a comfort and familiarity with a variety of techniques that are immediately applicable to complex, nontransplant liver surgery. The same techniques that are routinely used in LDLT, such as resection and reconstruction of vascular and biliary structures, are now more frequently being considered for the resection of hepatic tumors by surgeons experienced in both liver resection and transplantation techniques (see Chapters 122 and 125 ).
The principal difficulty of vascular reconstruction during hepatic resection is the necessary period of hepatic ischemia, although the liver can tolerate moderate periods of ischemia surprisingly well. During standard liver resections, the use of hepatic inflow occlusion is often used during the parenchymal transection to control blood loss (see Chapter 101 ). When combined with a low central venous pressure (CVP), inflow occlusion can result in a relatively bloodless transection of the liver. Ischemic preconditioning, meaning intermittent hepatic inflow occlusion, seems to protect the liver from subsequent ischemic injury , and is performed by applying a Pringle maneuver for 10 minutes, then reperfusing the liver for at least 10 minutes before reapplying inflow occlusion. The mechanisms by which ischemic preconditioning protects the liver from subsequent ischemia have not been fully elucidated, but even longer periods of ischemia are relatively well tolerated. The normal liver can tolerate 60 minutes or more of continuous inflow occlusion and warm ischemia, , but patients with cirrhosis or altered liver function from biliary obstruction or prolonged chemotherapy may tolerate significantly less ischemic insult before sustaining irreversible injury ; the same may be true of older patients. Even with normal liver function, the risk of irreversible liver damage after prolonged periods of continuous ischemia is significant.
Nevertheless, there are many cases where vascular reconstruction mandates that a bloodless field be maintained for longer time periods than the surgeon (or the liver) may find acceptable. For example, tumors that involve the retrohepatic inferior vena cava (IVC) or the hepatic veins (HVs) may require total vascular isolation for a significant period of time. Total vascular isolation may increase the degree of ischemic injury to the liver more than inflow occlusion alone because there is some evidence that backward diffusion of HV blood into the liver attenuates ischemic injury. In most cases, however, the procedure can be planned such that most of the hepatic parenchymal division is performed without total vascular exclusion, and caval clamping is reserved for the relatively short time that is required to deal with the IVC or HVs.
Most complex liver tumor resections can be done without the need for ex vivo liver resection or in situ cold perfusion, even when the tumor involves hepatic vascular structures (see Chapter 122 ). Tumors that involve hilar vessels usually can be approached by temporary occlusion of the hepatic artery (HA) or portal vein (PV) with relatively short ischemic times and without caval or HV isolation. When the HA and PV require reconstruction, it may be possible to do the reconstructions in sequence, maintaining oxygenated hepatic flow through one vessel or the other. Additionally, involvement of the IVC can often be approached with venous side-clamping, by exclusion of the cava while maintaining hepatic outflow (when the infrahepatic cava can be clamped below the insertion of the HVs), or by short-term total liver vascular isolation with or without veno-venous bypass. A minority of patients will have lesions that seem truly unresectable by any conventional technique. In general, these are cases where the vascular reconstruction is complex and is predicted to take a considerable length of time or where the transection of the liver is simply too dangerous to undertake in situ or without total vascular exclusion (as in an acute Budd-Chiari syndrome; see Chapter 86 ). The classic example is lesions that are centrally placed and involve all three main HVs, with or without involvement of the retrohepatic IVC. These few patients who require complex reconstruction of venous outflow may benefit from ex vivo or in situ hypothermic perfusion of the liver with hepatic resection and vascular reconstruction. The main advantages of this technique are better access to the tumor, minimizing ischemic injury to the future liver remnant using cold preservation, and higher likelihood of a complete R0 resection. At least theoretically, however, ex vivo liver resection surgery may increase vascular and biliary complications because of the need for re-anastomosis. There is a common concern that tumors requiring extensive liver resection are biologically advanced with an unfavorable prognosis, which may question the justification of a major surgery such as ex vivo liver resection. In our experience, however, good long-term survival rates have been achieved.
Experience with ex vivo and in situ cold perfusion liver resections is limited to a few high-volume liver surgery and transplantation centers throughout the world. In this chapter, we try to simplify the description of the techniques involved; we stress the importance of patient selection, preoperative evaluation and planning, intraoperative decision making, and postoperative care that can take place only in highly specialized and experienced centers. We have also collected a series of online educational videos that highlight many of the key points made in this chapter. These videos were created as part of the Toronto Video Atlas of Surgery ( www.TVASurg.ca ).
Fortner and colleagues (1974) were the first to describe the use of hypothermic perfusion during liver resection to protect the liver from ischemic injury in a series of 29 patients. Technical improvements in liver surgery over the next two decades, along with a growing understanding of the liver’s ability to tolerate normothermic ischemia, made the use of hypothermic perfusion unnecessary in most cases. Over the same period, liver transplantation (LT) had been applied to technically unresectable primary and secondary liver malignancy with dismal results. Although the procedure was technically feasible, transplantation for large, unresectable primary liver tumors, especially for metastatic lesions, resulted in the rapid recurrence of malignancy, either in the new liver or elsewhere, shortly after transplantation (see Chapter 108 ).
In response to patients with unresectable tumors who were considered inappropriate for LT, Pichlmayr and associates (1988) developed hypothermic perfusion with ex vivo liver resection. During ex vivo liver resection, the liver is removed completely from the body and perfused with cold preservation solution on the back table. The liver resection is performed on the back table in a completely bloodless field such that reconstruction of HV outflow can be performed under ideal conditions. Because morbidity and mortality rates from this procedure are relatively high, in situ and ante situm hypothermic perfusion techniques have been explored.
In this chapter, we describe the procedure and role of three techniques: in situ hypothermic liver perfusion, ante situm hypothermic liver perfusion, and ex vivo liver resection. These techniques overlap tremendously, and all mirror aspects of LDLT (see Chapters 109 and 121 ).
Both in situ and ante situm hypothermic perfusion do not necessarily require HA and/or biliary reconstruction. Hence the risk for vascular and biliary complications may be lower compared with ex vivo liver resection. However, the exposure with ex vivo liver resection is outstanding and this method can permit even parenchymal hypothermic perfusion (although not if the HV outflow is clotted, a real risk in the setting of a tumor that has led to an acute Budd-Chiari syndrome).
When applied, both in situ and ante situm hypothermic perfusion are performed using standard liver resection mobilization techniques (see Chapter 101 ). In these cases, the liver is placed in total vascular isolation and a cold perfusion is instituted through the PV or potentially through the HA. The hilar structures are left otherwise intact. The main difference between these two methods is that in ante situm hypothermic perfusion, the suprahepatic IVC (often with the cava below the HVs) is divided and the liver rotated anteriorly and counterclockwise to allow improved access to the area of the liver and IVC at the HV confluence.
The aim of in situ hypothermic perfusion is to provide a bloodless operative field together with hypothermic cellular protection, to permit prolonged and accurate dissection either for liver resections that necessitate vascular isolation of the liver or during the period of vascular reconstruction itself. Of the three surgical techniques outlined in this chapter, in situ hypothermic perfusion is the most straightforward from a theoretical point of view, but the exposure of the IVC and HVs can be very awkward and the vascular reconstructions, as a result, are often quite tricky.
Hannoun et al. (1993) presented their retrospective study of 34 patients who underwent major liver resection with a single period of vascular occlusion exceeding 1 hour. Importantly, all patients included in the study had a normal liver remnant. The authors concluded that continuous vascular occlusion (normothermic ischemia) during major liver resection is a useful maneuver that may be performed safely on normal hepatic parenchyma for up to 90 minutes. Liver cooling such as topical refrigeration or hypothermic perfusion was not used. However, the authors advocated for hepatic vascular exclusion with in situ hypothermic perfusion of University of Wisconsin (UW) preserving solution whenever the foreseen complexity of the hepatectomy suggested that vascular occlusion might exceed 120 minutes.
In 1996 the same group showed that these techniques can be successfully applied in patients with underlying liver disease (more sensitive to ischemic damage) that require liver resection. They showed that cooling of the hepatic parenchyma allows for major hepatic resection in patients with liver disease using vascular exclusion for longer than 1 hour without increased morbidity or mortality. Azoulay and colleagues (2005) compared the results of liver resection performed under in situ hypothermic perfusion with standard total vascular exclusion of the liver for less than 1 hour and for longer than 1 hour in terms of liver tolerance that was assessed by the peaks of aspartate transaminase (AST) and alanine transaminase (ALT) postoperatively, liver function (assessed by peak bilirubin and the prothrombin [PT] level), renal functions (assessed by peak of creatinine), postoperative morbidity, and mortality. In the patients that had total vascular exclusion without in-situ hypothermic perfusion, a veno-venous bypass was instituted for hemodynamic intolerance, occurring in 4 of 33 patients undergoing total vascular exclusion for less than 1 hour and 5 of 16 patients that had total vascular exclusion for longer than 1 hour. They demonstrated that hypothermic perfusion of the liver is associated with better tolerance to ischemia in the setting of total vascular isolation of any duration. Further, they showed that hypothermic perfusion of the liver is associated with better postoperative liver and renal function and lower morbidity compared with total vascular exclusion of more than an hour. They also showed that the size of the tumor, the need for PV embolization, and a planned vascular reconstruction were predictive factors for longer liver resection (>1 hour). These predictive factors should be taken into account when considering the need for hypothermic perfusion of the liver.
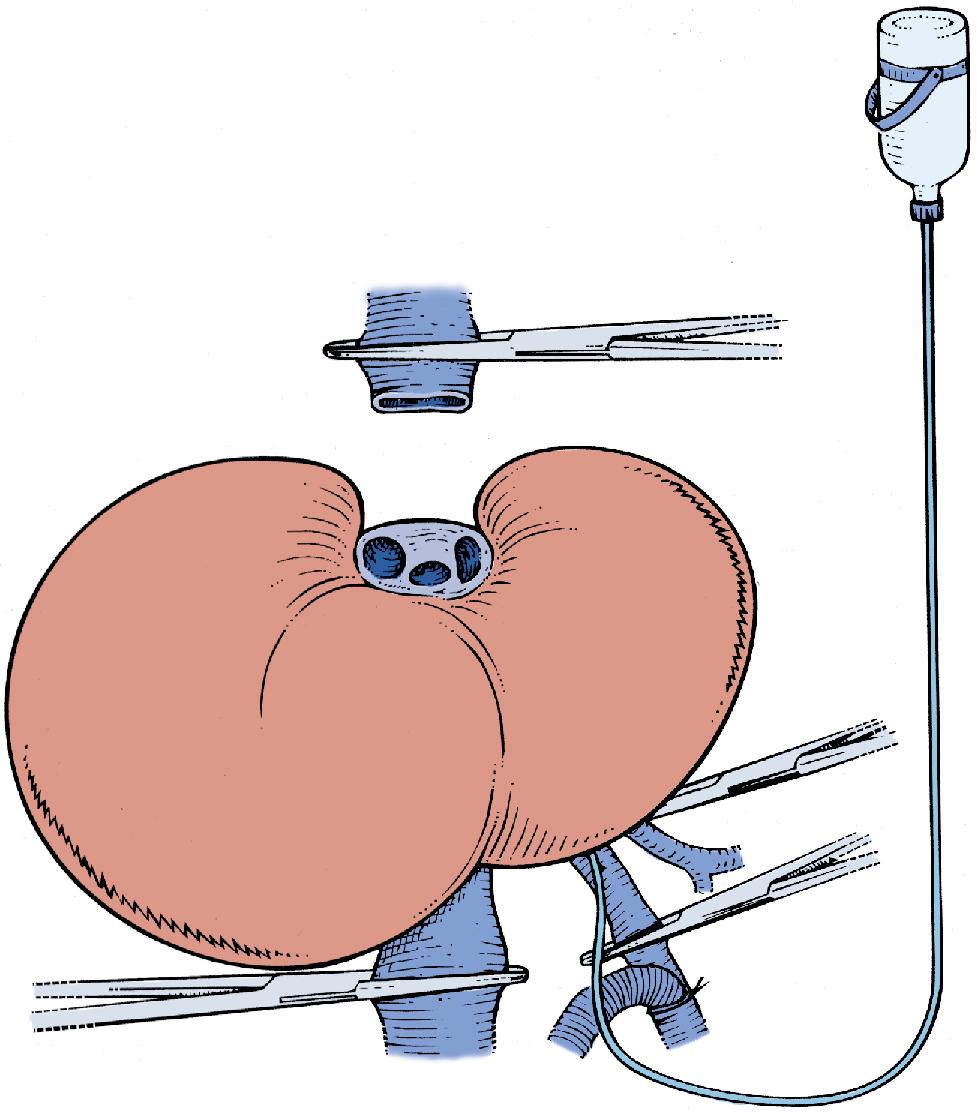
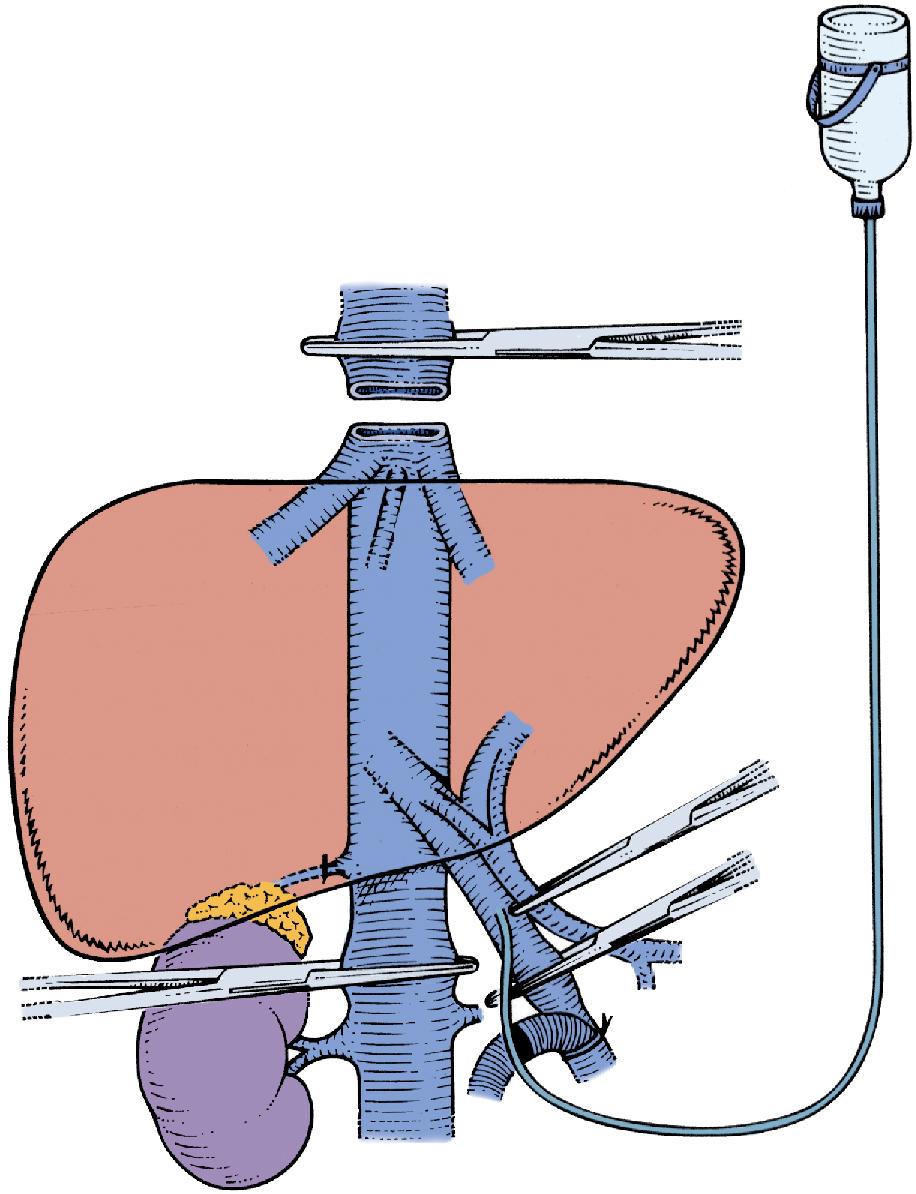
Total vascular exclusion of the liver, including the clamping of the portal triad, the IVC above and below the liver, is indicated for tumors involving or adjacent to the IVC and/or the confluence of the HVs into the IVC. To perform in situ cold perfusion as originally described by Fortner et al. in 1974, the liver is mobilized as for total vascular isolation with control of the suprahepatic IVC, infrahepatic IVC, and the portal structures. In their description of the procedure, the gastroduodenal artery is isolated and cannulated. The PV was cannulated either via a venotomy or via the branch of the lobe that was to be resected. These cannulae were kept patent with a slow infusion of Ringer’s lactate at room temperature. The common HA and PV are clamped proximal to the cannulae. The infrahepatic IVC and suprahepatic IVC are separately occluded by vascular clamps and a cavotomy is made immediately inferior to the liver for placement of a catheter to drain the perfusate. Ringer’s lactate solution, chilled to 4°C and with 5 mg/L heparin, is used for the cold perfusion. In this original description of in situ hypothermic perfusion, the authors universally used veno-venous bypass, and the entire hepatic parenchymal transection was performed after cold perfusion of the liver. If IVC or HV resection was required during resection of the tumor, the hepatic hypothermia was extended for a longer period of time, allowing for the safe reconstruction of the vascular structures.
The standard use of veno-venous bypass (cava and/or portal) reduces the time pressure involved in these cases and the gut edema associated with prolonged portal clamping. However, in our experience, most patients tolerate total vascular isolation without the need for veno-venous bypass. In some of the series described, a veno-venous bypass was performed routinely. If a veno-venous bypass is used, a sufficient length of the PV is dissected out (3–4 cm) for insertion of a perfusion catheter and the PV cannula for veno-venous bypass. Before clamping, the patient receives a bolus of at least 5000 U of heparin intravenously (IV). The infrahepatic IVC is clamped and the patient is placed on the caval portion of veno-venous bypass. It is generally advisable to isolate, ligate, and divide the right adrenal vein before clamping the infrahepatic IVC. A portal clamp is placed relatively superiorly on the PV with bypass instituted below this clamp. The portal cannula can be inserted, directed down toward the superior mesenteric vein (SMV) after complete PV division or by dividing just the anterior wall of the PV and sliding the cannula down the back wall. An alternative approach, although not part of the original description—and our preferred method—is to place a temporary biologic graft onto the anterior wall of the SMV and cannulate the graft.
Full veno-venous bypass is started. The liver side of the PV is cannulated with the perfusion solution tubing, and the HA is clamped. The suprahepatic IVC is clamped, and a transverse venotomy is created in the infrahepatic IVC just above the lower caval clamp. Cold perfusion of the liver is begun with preservation solution, and the effluent is suctioned from the venotomy in the infrahepatic IVC to prevent excessive body cooling ( Fig. 124.1 ).
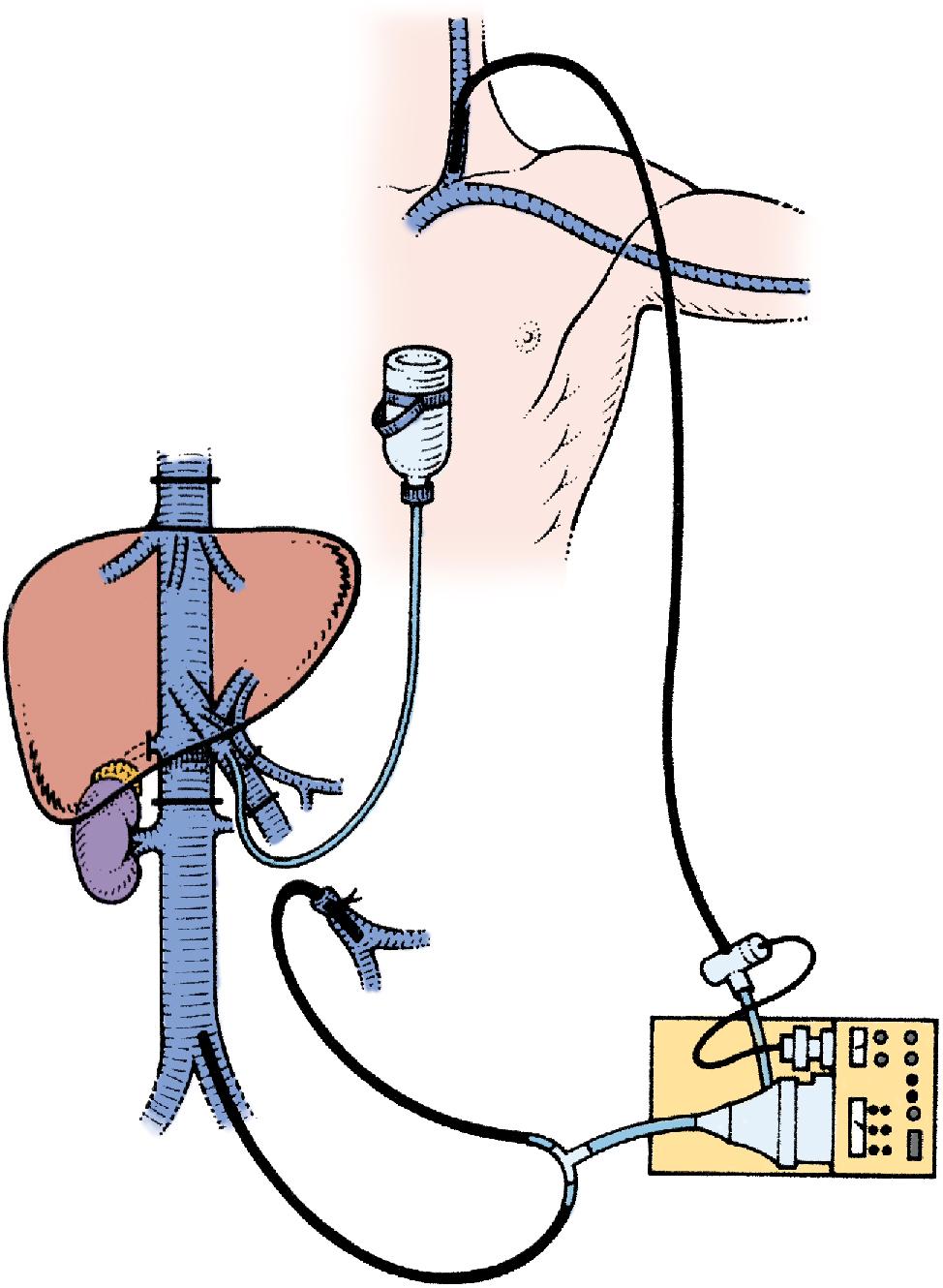
Possible cold preservation solutions include histidine-tryptophan-ketoglutarate (HTK) and UW solution. , The liver resection proceeds in a bloodless field with excellent visualization of intrahepatic structures. The liver can be cooled continuously throughout the parenchymal transection by slow infusion of cooling solution, or it can be cooled intermittently every 30 minutes by bolus infusion. At completion of the liver resection, the liver should be flushed of the cold preservation solution; this can be done by flushing the PV with cold 5% albumin before restoring flow to the liver or by allowing the initial 300 to 500 mL of venous effluent from the reperfused liver to be vented out the infrahepatic cava venotomy after reperfusion before removing the suprahepatic cava clamp. Next, the portal bypass cannula is removed, and the PV is repaired or reanastomosed if divided. If the liver has been flushed with 5% albumin, the infrahepatic venotomy is closed, and the suprahepatic cava clamp is removed to assess HV bleeding, which should be controlled if present. PV and HA inflow are reestablished.
If the liver is warm-flushed with the initial blood flow through the liver, the sequence is slightly altered to prevent flushing cold, high-potassium effusate into the cardiac return. If a warm-flush technique is used, portal flow is established with the suprahepatic cava clamp in place and the effluent vented though the cava below the suprahepatic clamp, whether through a venotomy or a caval anastomosis that has been deliberately left loose. The initial 300 to 500 mL of blood is suctioned, the venotomy or anastomosis is secured, and the suprahepatic cava clamp is removed. An alternative to this method described is to cold-flush the liver with 5% albumin, which allows for the sequential removal of clamps with separate assessment of HV and portal bleeding on reperfusion, but this method is somewhat time-consuming. The patient is decannulated from caval bypass if veno-veno bypass has been used.
The advent of LDLT, combined with the more frequent use of the anterior approach to liver resection and improved parenchymal transection techniques, have improved the ability to divide the liver parenchyma and dissect along the HVs without the need for extensive periods of inflow occlusion. Hence we try to do most of the parenchymal transection while avoiding inflow occlusion. Only rarely do we use in situ perfusion as previously described with total liver occlusion for the complete liver transection (the exception being cases where the HV outflow is severely compromised, and the parenchyma is very congested as a result). In patients in whom a single HV or the IVC requires reconstruction, most of the parenchymal transection can be performed without inflow occlusion under low-CVP conditions. As the parenchymal transection nears completion, total vascular isolation is applied to enable division and reconstruction of the vascular structures. This practice results in significantly shorter periods of hepatic ischemia and allows a different method of applying in situ cold perfusion that is simpler and generally does not require veno-veno bypass (see Videos: https://pie.med. utoronto.ca/TVASurg/project/extrighthep-insitu/ , https://pie.med.utoronto.ca/TVASurg/project/extlefthep-insitu/ , https://pie.med.utoronto.ca/TVASurg/project/extlefthep_cavalresection_cormatrix/ ).
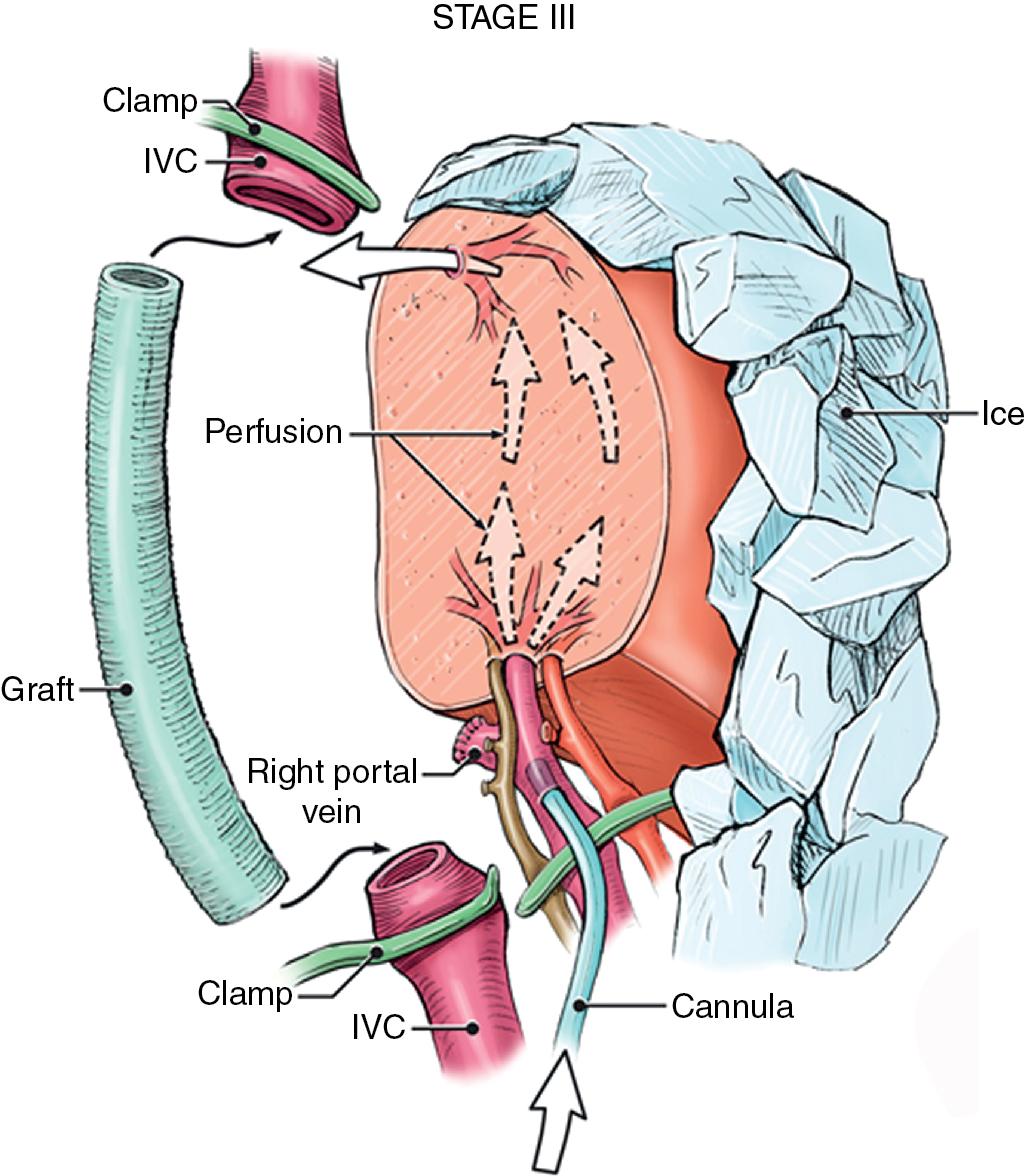
In this technique, the liver is mobilized as for total vascular isolation. The PV is dissected to the right and left branches, and perfusion tubing is placed into the main PV. One option is to cannulate the PV branch on the opposite side of the liver to be removed ( Fig. 124.2 ). The branch is divided, maintaining portal flow to the side of the liver to be left in but allowing access for cold perfusion. Alternatively, the anterior wall of the main PV can be used as a site of cannula insertion ( Fig. 124.3 ). As much of the hepatic parenchyma as possible is divided under low CVP conditions, maintaining hepatic perfusion until it becomes necessary to divide the vessels (HV and/or IVC) that require reconstruction.
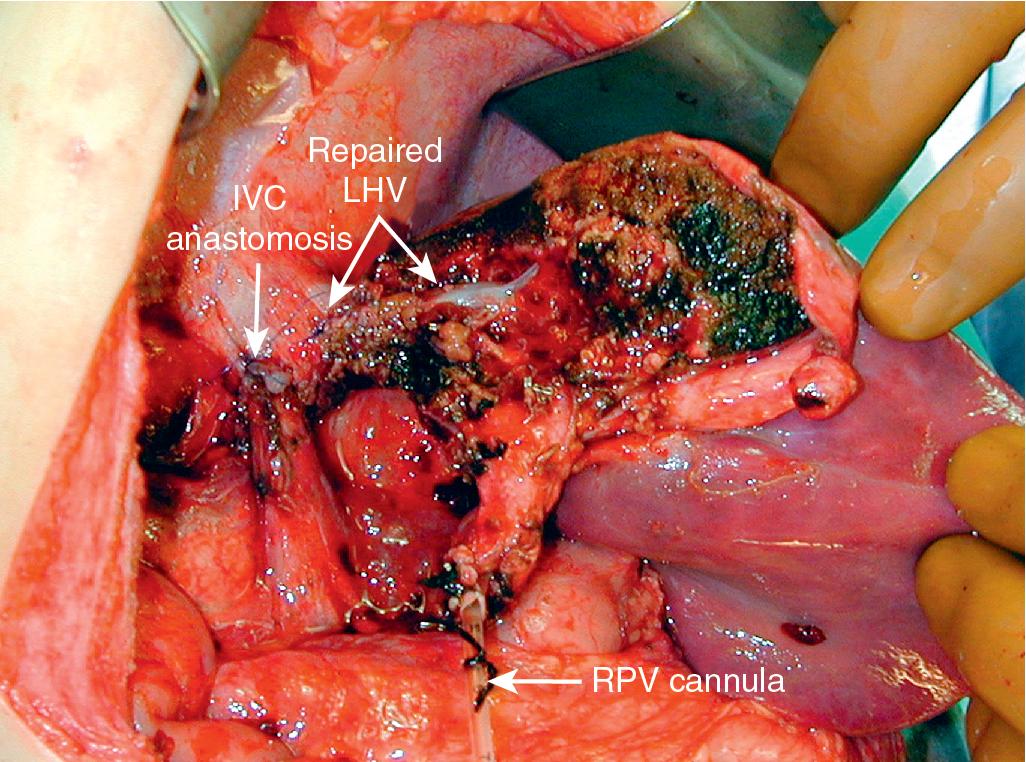
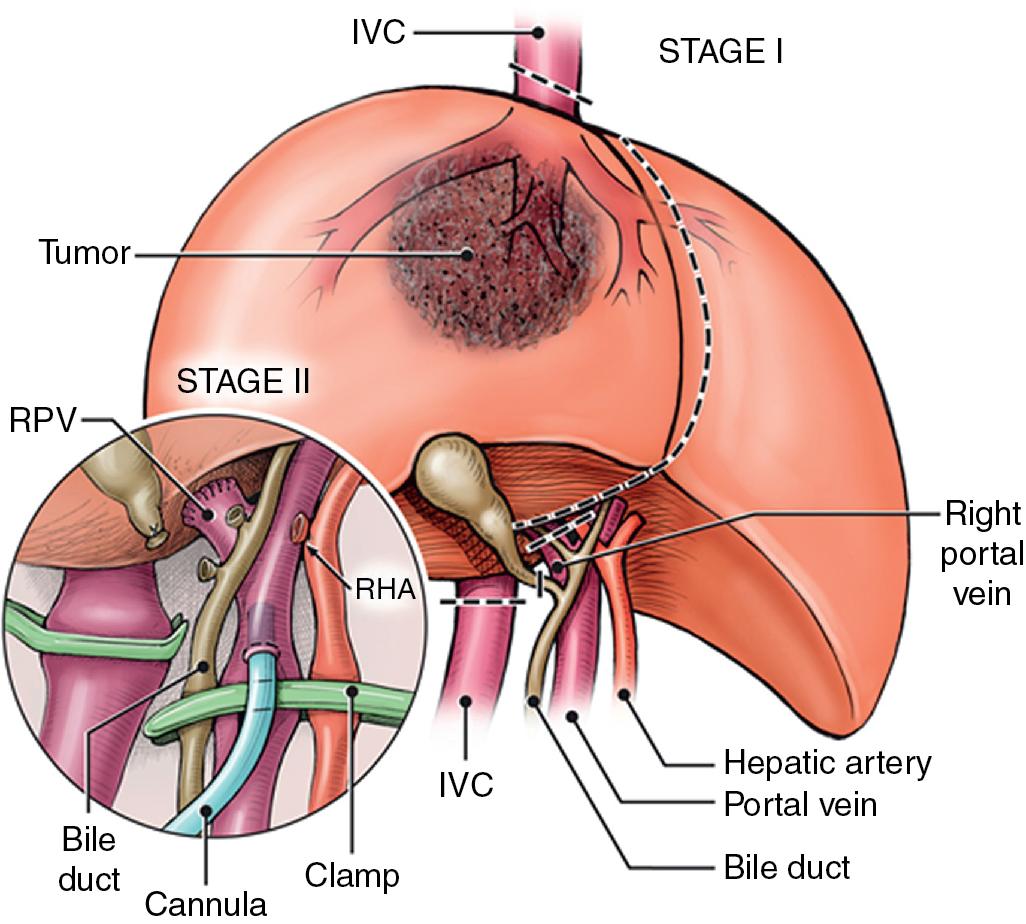
At this point, the patient is volume-loaded, inotropes are started as necessary, and clamps are placed sequentially on the infrahepatic IVC, PV, HA, and suprahepatic IVC. If only the HV requires reconstruction, caval flow can be maintained by placing a clamp tangentially down onto the IVC across the HV orifice, partially occluding the IVC ( Fig. 124.4 ). The anterior wall of the IVC or HV is incised, and cold perfusion of the liver is instituted through the portal cannula. Only the side of the liver remaining in place is perfused. The vessels are transected, and the specimen is removed. The vessels, HVs, or IVC can be reconstructed in a bloodless field without undue pressure of time ( Fig. 124.5 ). Before completing the anastomoses, the liver can be flushed with cold 5% albumin or flushed with 300 to 400 mL of PV blood with the vena caval clamps still in place, at which point the PV clamp is reapplied, the caval venotomy closed, and all clamps removed. Because of the shorter ischemic periods involved with this technique, we do not generally use veno-venous bypass; even longer periods of total vascular isolation do not necessarily require veno-venous bypass.
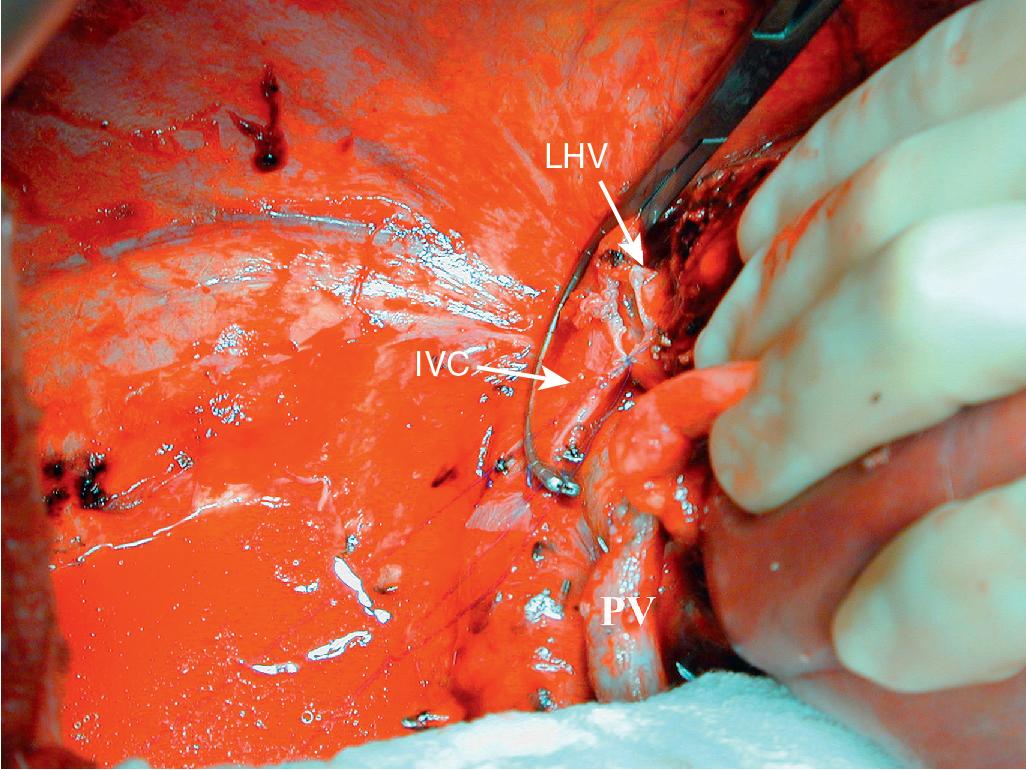
Of the three procedures described in this chapter, in situ hypothermic perfusion is the least complicated from a theoretical point of view. However, a real caution is that the IVC/HV reconstructions can be very awkward because of the angles involved. This surgical technique demands experience and should be limited to high-volume centers with LT experience. In 2015, Azoulay and colleagues published their experience with this procedure including 77 liver resections performed using standard total vascular exclusion with hypothermic portal perfusion and veno-venous bypass. Although only 33 cases required vascular reconstruction, the 90-day mortality rate was 19.5%. They also identified independent predictors of mortality, including age-adjusted Charlson Comorbidity Index (CCI) of 3 or more, tumor size 10 cm or more, and the presence of 50/50 criteria. The overall 5-year survival rate in their cohort was 30.4%. We strongly believe that the identification of preoperative predictors for mortality should be taken into account when selecting patients for this complex surgery.
Of the three techniques outlined in this chapter, the ante situm technique is by far the least frequently used. The ante situm technique of liver resection can be used when resection of the IVC and HVs is expected to be difficult and when improved access to the HVs and IVC is required. To improve the access to the posterior side of the liver avoiding the division of the portal triad, Hannoun et al (1991) designed this technique in which the suprahepatic (+/- infrahepatic) cava is divided, allowing rotation of the liver anteriorly to permit the liver to be essentially exteriorized while connected only by the hepatic pedicle. During the procedure the future remnant liver is perfused with a preservation solution via the PV or HA. The liver is then resected with the involved HVs, and the remnant HV(s) is/are reimplanted into the vena cava.
This technique combines in situ hypothermic perfusion with separation of the suprahepatic IVC. Dividing the suprahepatic cava—with the liver fully mobilized as for a bicaval LT—allows for better mobilization of the liver posteriorly by rotating the liver anteriorly towards the abdominal wall. We have used this technique when combined IVC–HV reconstruction is required. The ante situm technique uses the same technique as in situ cold perfusion, with several caveats. The suprahepatic IVC requires more extensive dissection to achieve enough length to clamp, divide, and subsequently reanastomose. Greater exposure of the suprahepatic cava can be obtained by dividing the phrenic veins and gently dissecting the IVC away from the diaphragm ( Fig. 124.6 ). When using this technique, we frequently also open the pericardium directly anterior to the IVC and mobilize the intrapericardial vena cava and lower atrium; alternatively, a sternotomy provides excellent exposure of the entire area ( Fig. 124.7 ). Nevertheless, we try to avoid sternotomy when possible because of the additional morbidity.
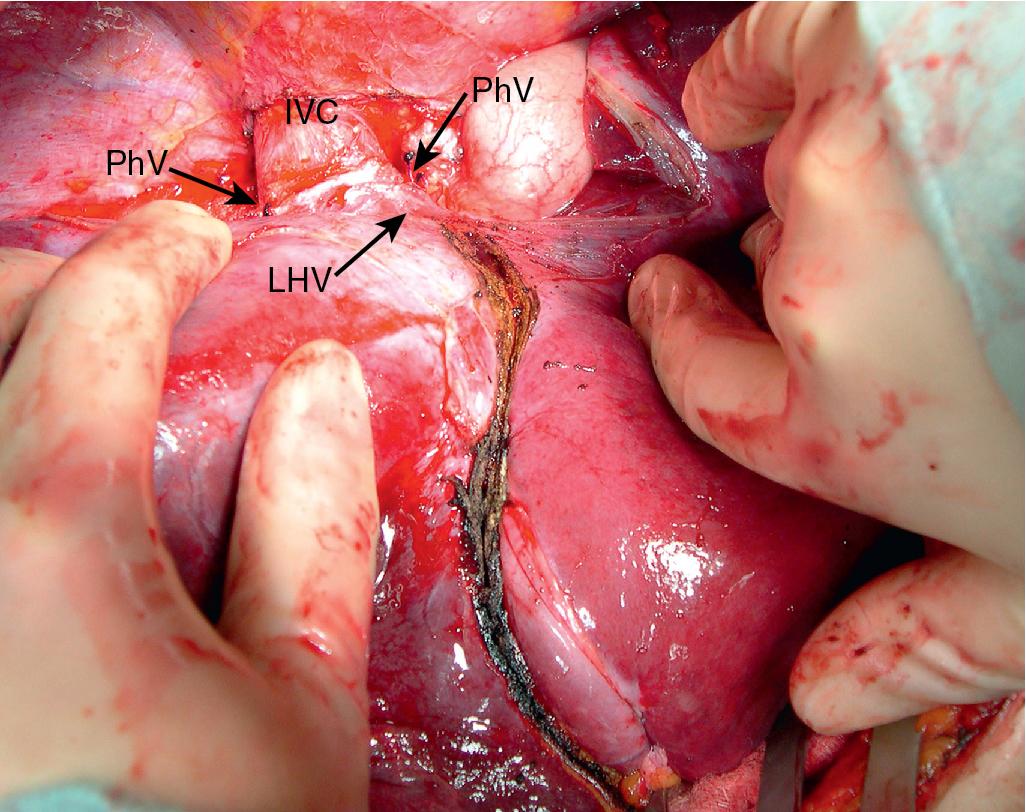
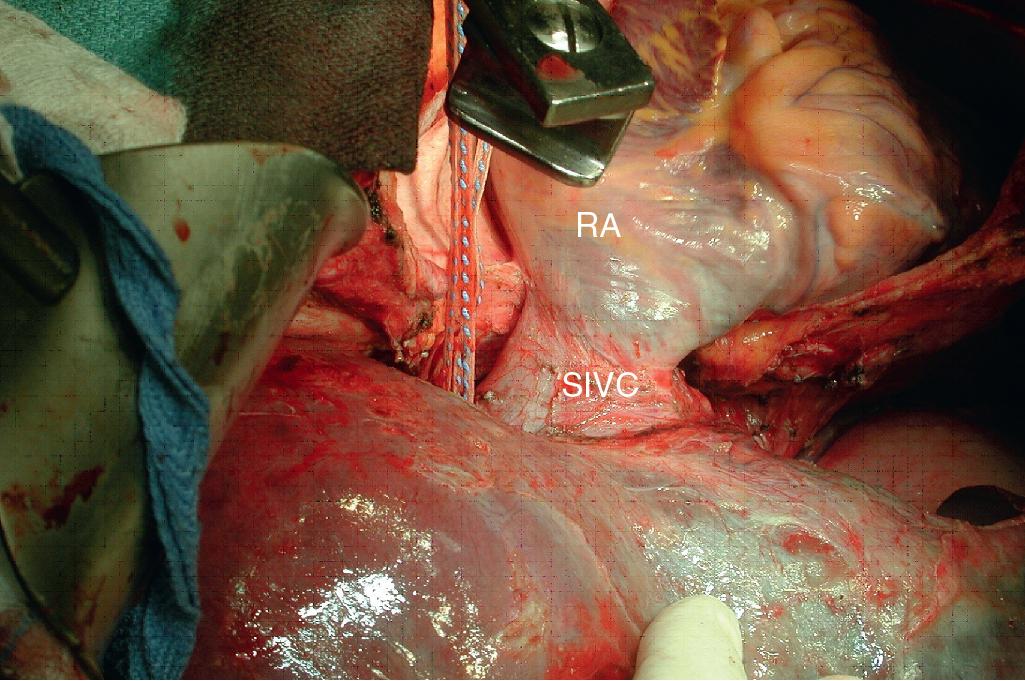
Control of the intrapericardial cava allows for placement of the upper clamp on the vena cava or caudal right atrium within the pericardium as a primary option or as a secondary option in case technical difficulties arise with placement of the original suprahepatic cava clamp. We perform as much of the liver transection as possible without inflow occlusion before cold perfusing the liver. Veno-venous bypass is generally recommended for this procedure; however, many patients tolerate IVC clamping without difficulty, particularly if the anesthesiologist volume loads the patient and institutes appropriate inotropic support (vasopressin is useful in this situation). Cold perfusion is instituted as originally described for in situ perfusion, although the perfusate is vented through the suprahepatic IVC once the suprahepatic cava is divided. Dividing the suprahepatic IVC allows the liver to be rotated forward toward the abdominal wall, enabling greater access to the area immediately around the IVC–HV junction (the hepatocaval confluence; Figs. 124.8 and 124.9 ).
If further access is required, the infrahepatic IVC can be divided, allowing the liver to be rotated completely up towards the abdominal wall, and the liver can be rotated around the portal axis counterclockwise so that the posterior aspect of the liver faces anteriorly and to the patient’s left (See Video: https://pie.med.utoronto.ca/TVASurg/project/antesitum/ ). With this technique, we have found that continuous, slow cold perfusion is required after the initial flush to prevent excessive warming of the liver. As a rule, the liver is first flushed with about 1 L of ice-cold preservation solution wide open, with the bag suspended at shoulder height. After the initial bolus, the infusion rate is slowed to about half of the original rate. Care is taken to suction the vented perfusate to avoid its systemic absorption.
The liver transection is completed, dividing the HV within the liver and resecting the origin of the IVC–HV junction en bloc with the tumor. If extension grafts are required, vascular reconstruction can be performed; the HV anastomoses, which are the most tenuous, are performed while the liver is rotated onto the abdominal wall. The liver is replaced, and the caval anastomosis is performed. The liver can be flushed with 5% albumin before reperfusion, or it can be flushed with warm portal blood, as previously described.
The ante situm approach gives better access to the IVC–HV junction than does simple in situ cold perfusion. The exposure is usually not as good as with a complete ex vivo approach. The advantage to the ante situm approach is that biliary and HA anastomoses are not required, reducing the ischemic time to the liver and reducing the potential for complications resulting from these additional anastomoses. Only rarely does this technique need to be applied, but it can be considered when the HV/IVC junction needs to be resected en bloc and all three HVs reconstructed to the side of a graft or cava ( Figs. 124.10–124.12 ; see Videos: https://pie.med.utoronto.ca/TVASurg/project/extlefthep-insitu/ , https://pie.med.utoronto.ca/TVASurg/project/antesitum/ ).
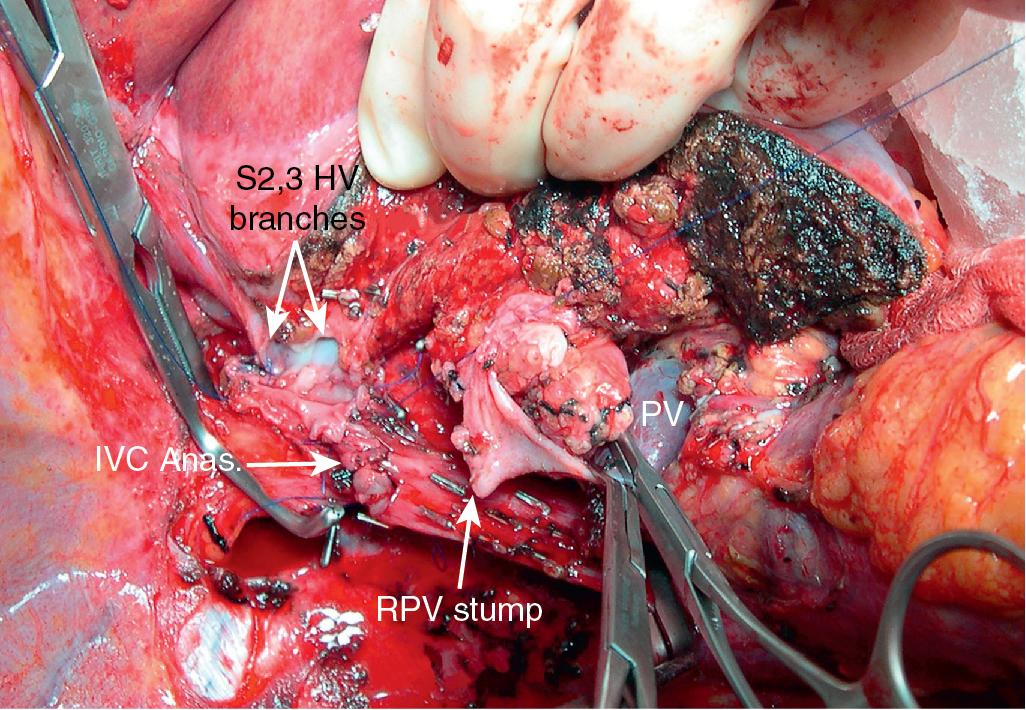
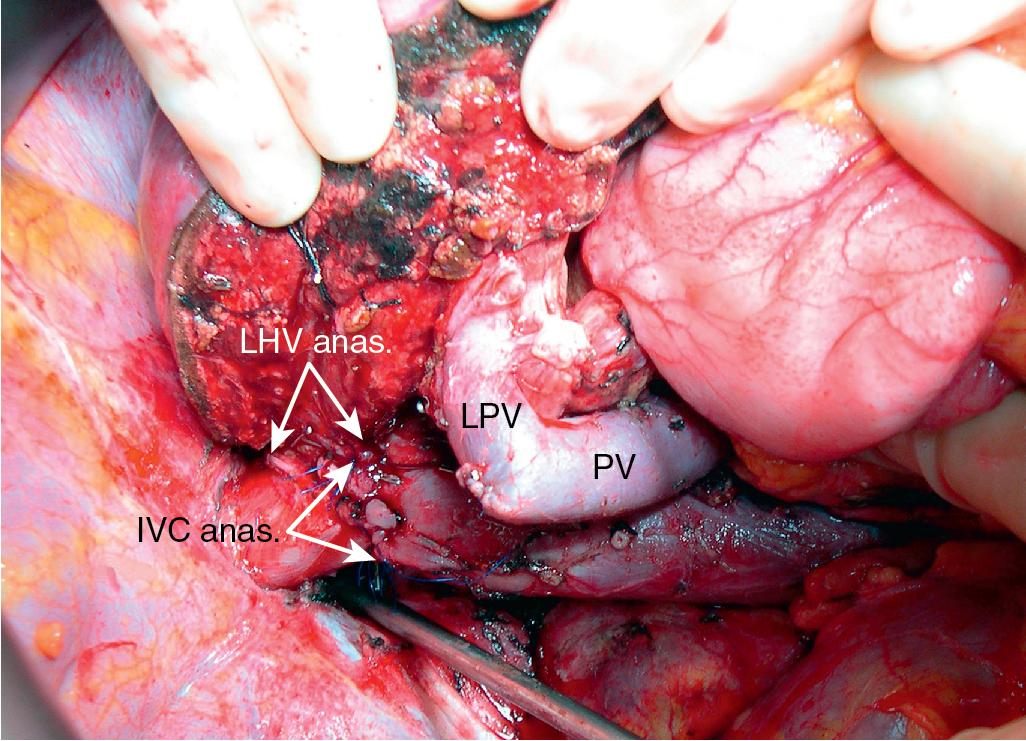
Although the techniques previously described can be applied to the majority of patients with difficult HV or vena caval reconstruction, patients who have tumors that involve the IVC and HVs that require complex venous repair; patients with combined, complex HV and hilar involvement; or patients in whom the HV outflow is severely compromised may be candidates for ex vivo resection. During ex vivo resection, the liver is completely removed from the patient and perfused with cold preservation solution on the back table. The hepatic resection is performed in a bloodless field, and vascular reconstructions are performed before reimplanting the remnant liver into the patient (see Videos: https://pie.med.utoronto.ca/TVASurg/project/extlefthep_exvivorppv/ , https://pie.med.utoronto.ca/TVASurg/project/exvivo_167resection/ ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here