Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Evidence-based medicine is the use of the highest-quality medical data currently available to assist in clinical decision making.
The Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence propose the hierarchy for levels of evidence shown in Fig. 10.32.1 .
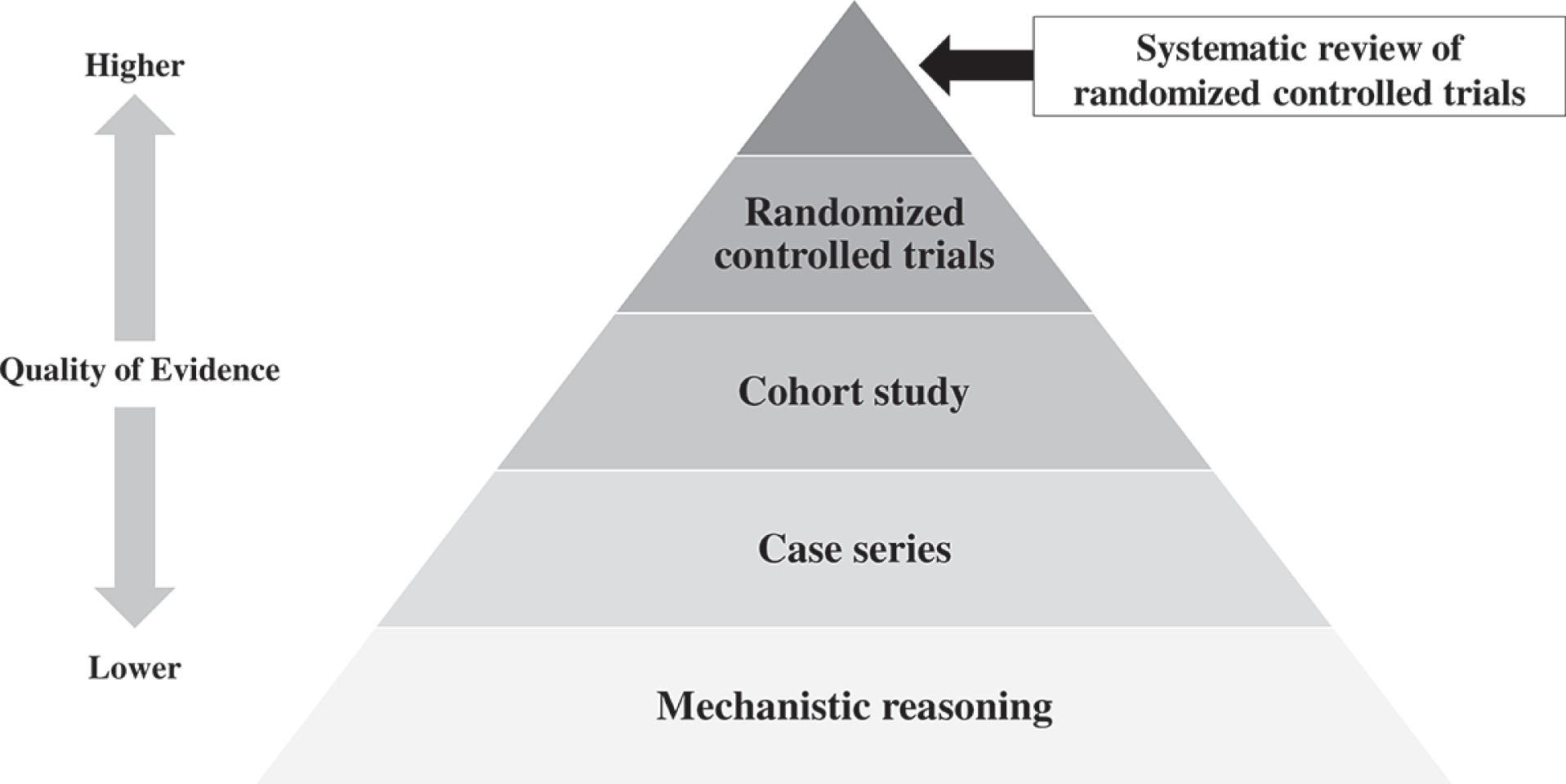
The practice of medicine will always be a combination of science and art. We learn the artistic aspects from our teachers and our own experience. The scientific parts of medicine involve using evidence that has been gathered through the rigorous observations of others in the course of clinical research.
Physicians have always attempted to use the best available medical knowledge to make decisions about their patients. During the early part of the 20th century, the best available knowledge mainly consisted of techniques and approaches that had been passed on from other physicians. These techniques and approaches were rarely scrutinized for effectiveness in a rigorous and systematic way. Most of medical practice was eminence-based (“driven by really charismatic and thoughtful, probably, to some degree, leaders in medicine”) rather than evidence-based. A movement toward improving the quality of medical practice with well-designed clinical trials began in the 1970s and 1980s.
The term evidence-based medicine gained popularity in the 1990s. Sackett defined evidence-based medicine as the “conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients.” The first step in practicing evidence-based medicine is to recognize a clinical problem or question that needs to be answered. For example, one might ask, “Does medical therapy work to prevent glaucoma in patients with elevated intraocular pressure (IOP)?” The next step is to find the best scientific evidence available addressing the question. When there are conflicting data, it can be difficult to draw an appropriate conclusion if all the data are weighted equally. To find the most reliable answer to a clinical question, it helps to focus on higher-quality sources of evidence, such as appropriately conducted and analyzed randomized controlled trials (RCTs) and case–control studies. However, in all instances, the reader must be aware of the inherent weaknesses and strengths of these studies.
Published reports of clinical experience are the foundation of evidence-based medicine. A common scenario for the development of increasingly stronger evidence is for a clinician to formulate an impression about a disease process or an intervention and then review and publish his or her experience as a retrospective case series. This then leads to a prospective study that can be observational or interventional. Finally, promising results from a nonrandomized prospective study can lead to an RCT.
An RCT is a study in which participants are randomly assigned to one or more groups. The process of randomization eliminates bias in the allocation of subjects to one treatment or another. The results of RCTs are often considered the highest-quality evidence and hence are the backbone of evidence-based medicine. For instance, the Cochrane Collaboration, a leading proponent of evidence-based medicine, largely limits its reviews to the analysis of RCTs.
A properly designed RCT has the potential to demonstrate a cause-and-effect relationship between a treatment and a specific patient response. In order for the results from an RCT to be useful, several conditions need to be met. The hypothesis of the trial has to be narrow in scope and clinically relevant. The appropriate number of patients has to be included in the study to ensure that a negative result is truly negative. Biases in the study have to be minimized.
Although considered a robust study design, RCTs vary widely in their quality, depending on their sample size, inclusion and exclusion criteria, rigor in randomization, masking of observers and subjects, loss to follow-up, and data presentation. Adherence to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting clinical trials ensures a minimum quality for an RCT, but even the best RCTs have some inherent weaknesses. For instance, inclusion and exclusion criteria associated with the studies may result in findings that are not applicable to a patient who would not have qualified for the study. For example, the Ocular Hypertension Treatment Study (OHTS), which set out to answer the question of whether medical therapy prevents the development of glaucoma in eyes with elevated IOP, did not specifically address the question of whether treatment was advantageous for patients with IOPs between 21 and 24 mm Hg. In addition, RCTs may not uncover all of the possible problems and adverse events associated with a particular therapy. An example of this can be found in the Collaborative Initial Glaucoma Treatment Study (CIGTS), a multicenter RCT in which individuals with newly diagnosed open-angle glaucoma (OAG) were randomized to initial medical or surgical treatment. The number of subjects who underwent trabeculectomy in the CIGTS, although sufficient to compare the success of initial medical versus initial surgical treatment of newly diagnosed OAG, precluded obtaining useful information about the rare, but serious, complication of bleb-related infection.
Another disadvantage of RCTs is that, depending on the endpoint, they may take a long time to reach completion. When the time frame of an RCT is several years or more, it is not unusual for the therapies that are being evaluated to have been replaced by seemingly better treatments by the time the data are published. This is especially problematic when studying glaucoma for several reasons. First, glaucoma has a lengthy, slow course, and patients have different rates of worsening. In addition, new treatments must be tested in patients already receiving IOP-lowering agents. Finally, treatment side effects must be minimal, considering the long asymptomatic course of the disease.
To counter these limitations in glaucoma research, Quigley and Wu et al. offer alternative suggestions that would promote more efficient glaucoma research. Quigley suggests using rates of visual field mean deviation (MD) as an outcome measure to study treatment effects, rather than evaluating the incidence of visual field progression by event-based methods. This would reduce the sample size needed to determine statistical significance in short-term clinical trials. Wu et al. assessed the validity of this suggestion by opting for linear mixed-effects models (LMMs) rather than survival analysis in order to discern rates of MD change between treatment and control groups. By using LMMs, this group detected a 30% treatment effect with 90% power with just 277 patients, in contrast to the 652 patients that would have been needed to elucidate the same conclusions using visual field pointwise progression.
When multiple RCTs for a given question exist, a meta-analysis can be performed. In a meta-analysis, statistical techniques are used to measure and compare the outcomes of multiple studies. By combining the outcomes of multiple studies, the overall sample size and statistical power are increased. Meta-analyses are particularly useful when there are small clinical trials that are inconclusive because of size and when different RCTs come to different conclusions. The averages of the study results are often weighted, placing more emphasis on larger studies. Forest plots are a convenient way of summarizing the statistical analyses performed on the studies included in a meta-analysis. Meta-analyses can be useful for reducing the biases and improving the precision that is associated with RCTs.
An example of a meta-analysis in glaucoma therapy is the study of Maier et al. addressing whether lowering IOP delays the progression of visual field defects in patients with OAG. This study is of particular note because it was published in the British Medical Journal and not an ophthalmology journal. It gives us insight into the limited conclusions that nonophthalmologists are willing to draw from our ophthalmological data. The authors are willing to conclude only that lowering IOP in eyes with elevated IOP lowers the risk of visual field loss and has unclear effects in eyes with unremarkable IOP, whereas we in the glaucoma community have generally accepted the conclusion that IOP-lowering treatment is beneficial even in eyes without elevated IOP.
Another meta-analysis compared the effectiveness and adverse effects of adding a second IOP-lowering agent to an eye already being treated with a topical prostaglandin. The authors appropriately followed established guidelines (QUORUM) for the performance of a meta-analysis, used predetermined inclusion and exclusion criteria for selecting trials, and reported on how well the included studies met quality criteria. They found that the effectiveness of the three classes of medications was similar but that they each had different side-effect profiles. A third study performed by Li et al. was a systematic review and network meta-analysis of 114 RCTs comparing the effectiveness of first-line IOP-lowering eyedrops in patients with ocular hypertension or primary open-angle glaucoma (POAG). The authors found that prostaglandin medications were the most efficacious class, with within-class differences being small and probably not clinically meaningful. Mean reductions in IOP (mm Hg) at 3 months compared with placebo were as follows: bimatoprost (5.61), latanoprost (4.85), travoprost (4.83), levobunolol (4.51), tafluprost (4.37), timolol (3.70), brimonidine (3.59), carteolol (3.44), levobetaxolol (2.56), apraclonidine (2.52), dorzolamide (2.49), brinzolamide (2.42), betaxolol (2.24), and unoprostone (1.91).
Although meta-analyses can put our clinical decision making on a firmer footing, they have their own limitations. First, bias and errors associated with the included studies may become magnified in the meta-analysis. Second, they often will not include negative studies because these studies may never have been published. When this is the case, the meta-analysis will yield a result that is more positive than the aggregate of all the data, published and unpublished. Thus when evaluating meta-analyses, it is important to ask what the authors have done to minimize potential bias.
For most clinical questions, multiple, high-quality RCTs do not exist, and a true meta-analysis cannot be carried out. In these instances, a systematic review of the literature can prove useful. Systematic reviews include a well-defined and carefully executed review of the literature, an algorithm for determining which types of studies to include in the review, and an assessment of the quality of the manuscripts reviewed. Because studies with different designs are included, the authors generally cannot make more than qualitative statements about the answers provided by the aggregate of studies. The Cochrane Collaboration has been at the forefront of performing meta-analyses of RCTs, reporting on laser peripheral iridoplasty for angle closure, the effectiveness of screening for OAG, medical versus surgical treatments for OAG, 5-fluorouracil (5-FU) for glaucoma surgery, fornix-based versus limbal-based conjunctival trabeculectomy flaps, and needling for encapsulated blebs.
One organization in the United States that provides funding for systematic reviews is the Agency for Health Research and Quality (AHRQ). Within ophthalmology, the AHRQ has funded systematic reviews in anesthesia for cataract surgery, treatments for OAG, surgical management of coexisting cataract and glaucoma, and the effects of omega-3 fatty acids on eye health. As part of the process of formulating its Preferred Practice Patterns, the panel of experts chosen by the American Academy of Ophthalmology (AAO) carefully reviews the literature and assigns each study a quality rating and an importance-to-clinical-care rating. The AAO, through its Ophthalmic Technology Assessments, has published systematic reviews in glaucoma, such as the effect of phacoemulsification on IOP in glaucoma patients. The Patient-Centered Outcomes Research Institute (PCORI), an organization dedicated to funding healthcare delivery and outcomes research at least until the year 2029, has analyzed multiple studies regarding laser trabeculoplasty in comparison with eyedrops to treat glaucoma. Importantly, the PCORI found that black and Hispanic patients are disproportionately affected. Their conclusions encourage future research to fill evidence gaps that may reduce disparities in treatment.
Clinicians who base their decisions solely on the results of RCTs, meta-analyses, and systematic reviews will be left with many situations for which they will have no guidance. As one can tell from opening any clinical ophthalmic journal, most articles rely on study designs generally considered less rigorous. These study designs include case reports, case series, cross-sectional studies, cohort studies, and case–control studies. These studies are still useful as long as the reader is aware of their limitations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here