Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
THE LIVER AND BILIARY TREE are derived from the endoderm of the dorsal foregut during the late third to the early fourth week of gestation. By the sixth week, the fetal liver primarily serves as a hematopoietic organ, while critical biologic functions such as glycolysis, bile acid synthesis, and metabolic waste processing are managed by the maternal liver through fetoplacental circulation. Oxygenated blood is shunted from the placenta to the right atrium through the ductus venosus. Functional closure of the ductus begins immediately after birth, with complete functional closure occurring in up to 95% of infants by 2 weeks of age. Anatomic closure takes place shortly thereafter.
The functional development of the liver is reflected in the complex changes that are seen regarding hepatic enzyme efficiency and metabolic performance that occur throughout gestation. In the early gestation period, the liver is the primary site of hematopoiesis. Hepatic hematopoiesis develops in utero at 5 to 6 weeks gestation, followed closely by protein synthesis. The ability to metabolize carbohydrates and lipids begins by 10 weeks gestation, followed by the development of drug-metabolizing systems. Several patterns of hepatic enzyme development have been described that correlate with the needs of the developing fetus.
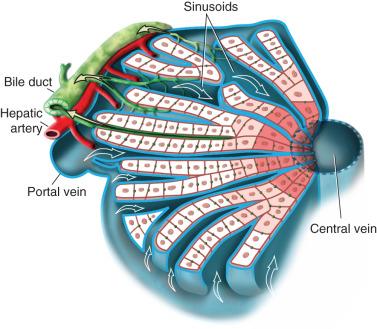
At the time of delivery, the liver weighs between 120 and 160 g but remains structurally and physiologically immature. Peripheral branches of the intrahepatic biliary system require an additional 4 to 8 weeks before they can be identified histologically. The liver is composed of eight structurally independent segments, each with a feeding hepatic artery, portal vein, draining hepatic vein, and bile duct. Segment 1 is the caudate lobe. Segments 2 and 3 form the left lateral segment, and with segment 4, the left lobe of the liver is defined. Segments 5, 6, 7, and 8 constitute the right lobe of the liver.
The liver receives blood from two sources: the portal vein that drains the spleen and intestine, and the hepatic artery that provides systemic oxygenated blood directly to biliary epithelium and to the hepatic sinusoids. The portal vein accounts for approximately 70% of the blood flow to the liver. In the hepatic sinusoids, the hepatic arterial and portal venous blood mix and intercalate among hepatocytes, fenestrated sinusoidal cells, and a host of resident immune cells (e.g., Kupffer cells). Sinusoids drain into terminal hepatic venules, which eventually coalesce to form the left and right hepatic veins. The veins merge into the inferior vena cava immediately before entering the right atrium. At any given time, the liver contains approximately 13% of the circulating blood volume.
During the neonatal period, liver function is immature and its ability to metabolize and clear most xenobiotics is poor. Factors believed to affect the clearance of medications include hepatic blood flow and the developmental status of hepatic transport and enzyme systems. Size alone does not account for this observed degree of immaturity, because the fetal and neonatal liver account for a greater percentage of body weight than the adult counterpart (3.6% of body weight vs. 2.4% in adults). The neonatal liver contains approximately 20% fewer hepatocytes than the adult liver, and the cells are almost one-half the size of adult hepatocytes. These structural features may play some role in the functional deficiencies exhibited by infant livers. Cellular growth and hypertrophy of the liver continue at a rapid pace into young adulthood.
The structural unit of the liver parenchyma is the lobule, a hub-and-spoke structure with the central vein serving as the hub that is bordered by portal tracts, which contain a bile duct and tributaries of the portal vein and hepatic artery. While the mixed venous and arterial blood flows from the portal triad to the central vein, bile flows in the opposite direction through a canalicular matrix that then enters the bile ductule in the portal tract. The functional unit of the liver is the hepatic acinus, which is centered on the portal track and extends in three concentric zones (i.e., zones of Rappaport) outward to the central vein ( Fig. 30.1 ). The more central zones (zones 1 and 2) are most active in oxidative processes, whereas the distal zone 3, which is closer to the central vein, depends on glycolysis and is more susceptible to ischemic and toxic injury.
Lipid solubility, an important and desired feature of many anesthesia drugs, allows passive diffusion across cellular membranes. Lipophilic drugs are also difficult to excrete. They have a propensity to accumulate in the body's fat stores and then slowly recirculate from deep compartments back into plasma. Renal and biliary excretion of lipid-soluble compounds can result in their resorption across their respective membranes. A major role of the liver is to transform lipid-soluble drugs into water-soluble compounds that become easily excreted metabolites. An interesting example of the need for this biotransformation is the anesthetic compound thiopental, which if not transformed into its less lipophilic counterpart, would have a plasma half-life of approximately 25 years.
The primary family of liver enzymes assigned the task of metabolizing these exogenous substances is the cytochrome P-450 (CYP) family. P designates a red pigment , which is related to a heme molecule and absorbs light at a wavelength of 450 nm. The primary reactions involved in the drug biotransformation and metabolism are hydroxylation and conjugation. Hydroxylation prepares the metabolite for conjugation (i.e., phase II reaction). The CYP family of enzymes is responsible for most phase I reactions, and the members were first thought to be chemically similar to mitochondrial cytochromes (see Chapter 7 ).
The CYP enzymes likely evolved as a mechanism by which the host was able to protect itself from toxins ingested from the environment. Most enzymes involved in hepatic drug metabolism are categorized in three distinct families: CYP1, CYP2, and CYP3. Each family is further divided into subfamilies that are designated with capital letters and numbered in the order in which they were discovered. The CYP enzymes are generally conserved across species, but their regulation and catalytic activity vary among species, which highlights the challenges associated with laboratory analysis of drug metabolism.
Genetic and nongenetic factors contribute to the variability in the enzymatic activity seen across all CYP enzymes. Genetic factors that have been shown to impact CYP variability include specific polymorphisms, gene expression regulation, and sex. For example, approximately 5% of Caucasian populations lack CYP2D6 activity, which is associated with altered metabolism of some drugs. A lack of CYP2D6 activity enhances the effect of drugs such as haloperidol and metoprolol that require the enzyme for efficient metabolism, whereas codeine, which is metabolized to morphine by CYP2D6, provides little analgesia in a child with CYP2D6 deficiency. Furthermore, ethnicity is emerging as an important factor contributing to CYP pharmacokinetics and significant differences are now being appreciated between cohorts with differing racial makeup (see Chapter 6 ).
Nongenetic factors that influence CYP activity include concomitant disease states, malnutrition, and exposure to a host of pharmacologic and naturally occurring compounds. Many drugs can inhibit or stimulate the enzyme system ( Table 30.1 ). Inhibition of the CYPs occurs when drugs compete for the same enzyme. The degree to which this competition becomes clinically important depends on five factors: the relative amount of the specific CYP, the concentrations of each drug, the degree of pharmacologically active metabolite generated through this system, the importance of the enzyme in elimination of the drugs, and the therapeutic index of the drug.
| P-450 | Substrate | Inhibitors | Inducers |
|---|---|---|---|
| CYP1A2 | Caffeine | Fluvoxamine | Omeprazole |
| Clozapine | Furafylline | Tobacco smoke | |
| Estradiol | |||
| Theophylline | |||
| CYP2A6 | Halothane | Methoxsalen | |
| Nicotine | |||
| CYP2C8 | Rosiglitazone | Phenytoin | |
| Taxol | Rifampin | ||
| CYP2C9 | Diclofenac | Sulfaphenazole | Rifampin |
| Ibuprofen | Secobarbital | ||
| Tolbutamide | |||
| Warfarin | |||
| CYP2C19 | Omeprazole | Fluvoxamine | |
| Ketoconazole | |||
| CYP2D6 | Codeine | Fluoxetine | |
| Chlorpromazine | Quinidine | ||
| Desipramine | |||
| Dextromethorphan | |||
| Encainide | |||
| Haloperidol | |||
| Metoprolol | |||
| CYP2E1 | Acetaminophen | Disulfiram | Ethanol |
| Halothane | Isoniazid | ||
| CYP3A4 | Cyclosporin A | Delavirdine | Carbamazepine |
| Estradiol | Erythromycin | Phenobarbital | |
| Indinavir | Grapefruit juice | Phenytoin | |
| Lovastatin | Ketoconazole | Rifampin | |
| Midazolam | Ritonavir | St. John's wort | |
| Nifedipine | Troleandomycin | Troglitazone | |
| Quinidine | |||
| Docetaxel |
Enhanced CYP expression occurs after amplified transcription of the specific gene that is induced by a variety of compounds. For example, rifampin and phenytoin induce CYP3A4 by binding the cytosolic human pregnenolone-X-receptor (hPXR) or the steroid and xenobiotic receptor (SXR). The activated receptor translocates into the nucleus, where it binds the regulator elements of the CYP3A4 gene and promotes increased transcription of CYP3A4, which can lead to toxic concentrations of intermediate compounds, as is the case with erythromycin, or to subtherapeutic concentrations of cyclosporine in transplant recipients.
The superfamily of CYP enzymes is divided into subfamilies based on sequence homology and on demonstration of broad substrate specificities. An important example is the CYP3A subfamily, which is the most abundant group of cytochromes involved in the metabolism of xenobiotics. The three identified isoforms are CYP3A4, CYP3A5, and CYP3A7. CYP3A4 is the most abundant single enzyme in the human liver, accounting for the metabolism of approximately 50% of clinically used pharmaceuticals. CYP3A5 is more commonly found in the kidneys and lungs and to a lesser degree in the liver. CYP3A7 is the predominant isoform in the neonatal liver but is replaced after birth by CYP3A4. Given its critical involvement in hepatic biotransformation of xenobiotics, the CYP3A4 family of enzymes is used to study estimated hepatic drug clearance in various age and gender groups.
Changes in the distribution and activity of the CYP enzyme systems occur with hepatic growth and maturation ( Fig. 30.2 ). The CYP3A family is homogeneously distributed across the liver parenchyma in the fetal liver and shortly after birth. However, during postnatal growth, expression of the CYP3A protein shifts toward the periportal region of the acinus (i.e., Rappaport zone 1). By adulthood, expression of CYP2A becomes increasingly limited to the zone 1 and zone 2 hepatocytes, with sparse expression occurring in zone 3. Other examples of developmental changes in the CYP system include the CYP2C and CYP 3A3/4 subfamilies, which have negligible expression in the first few weeks of life. CYP2D6 reaches adult activity levels within 1 month chronological age; variability thereafter is determined primarily by genetic polymorphisms.
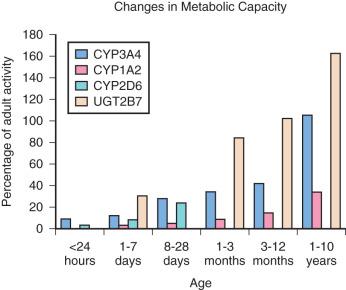
Changes in activity of CYP enzyme families and subfamilies have correlated with drug clearance. For example, midazolam clearance correlates with changes in CYP3A4 activity, with decreased clearance in fetal neonatal livers and with adult clearance rates achieved by 3 months of age. In contrast, CYP3A7 activity peaks at about 1 week postpartum and steadily diminishes during the first year of life, reaching approximately 10% of fetal liver activity by adulthood. In all, the advancements in the understanding of CYP ontogeny have led to improved integration of physiologic, developmental, and physiochemical knowledge to design more accurate pharmacokinetic modeling systems and guide optimal dosing regimens for children.
Conjugation of lipophilic compounds increases their water solubility to facilitate renal excretion. Conjugation reactions (i.e., glucuronidation, sulfation, glutathione conjugation, acetylation, and methylation) are generally decreased in infants compared with adults.
Glucuronidation is catalyzed by uridine 5′-diphosphate (UDP)–glucuronosyltransferase (UGT) family of enzymes, which are derived from assimilation of proteins from separate genes or alternate splicing from single-gene transcripts. UGT enzymes are responsible for the metabolism of several drugs, including phenols, estrogens, and opioids (see Chapter 7 ). As with the CYP enzymes, individual UGT enzymes demonstrate substrate specificity and can act in concert to metabolize single compounds. Glucuronidation is not fully active in neonates because of decreased mRNA transcript production. As such, this population is at risk for toxic drug accumulation (e.g., chloramphenicol causing the grey baby syndrome). Hepatic UGT enzyme concentrations are reduced during fetal and early postnatal development. At 3 months of age, the levels of many UGTs are 25% of those in adults.
UGT1A enzyme activity, which is involved in the conjugation of bilirubin and ethinylestradiol, is decreased in the fetus, but it increases to adult rates within 3 to 6 months after a term delivery. UGT1A6, which conjugates acetaminophen and naproxen, has 10% of adult activity in the fetus and neonate, and it achieves only 50% of adult activity by 6 months of age. UGT2B7 is active in the metabolism of the nonsteroidal antiinflammatory drugs (NSAIDs) naloxone, morphine, and lorazepam. Fetal activity of this enzyme approaches 10% to 20% of adult levels, with a rapid increase to adult levels by 2 months of age.
Sulfation is accomplished by sulfotransferases, a family of cytosolic enzymes that are divided into two categories: catechol and phenol sulfotransferase. These enzymes conjugate inorganic sulfate from 3′-phosphoadenosine-5′-sulfophosphate (PAPS) with compounds containing functional hydroxyl groups. The catechol transferases develop earlier in fetal life than the phenol counterparts and appear to exhibit decreased activity in the developing neonate. Although specific sulfotransferase substrates require identification, the activity of these enzymes is increased in fetuses and neonates compared with adults and theorized to be an efficient conjugation pathway in this age group. Interestingly, with the obesity epidemic, UDP-UGT and sulfotransferase expression and function are altered in patients with nonalcoholic fatty liver disease.
Glutathione S -transferases (GSTs) conjugate glutathione with a broad spectrum of lipophilic and electrophilic compounds. The family of GSTs is composed of up to five different groups in various classes designated µ, α, θ, σ, and π, which are derived from at least three genetic loci. Tissue-specific expression of these enzymes has been demonstrated, with the liver expressing the greatest amount of protein. Variable time-dependent expression has also been shown, with α- and π-class GSTs having enhanced expression between 16 and 24 weeks gestation, whereas only the α-class enzymes predominate in the neonate and adult liver. The hepatic π-class enzymes disappear from their hepatocellular location by 6 months of age and can be found only in the epithelial cells of the biliary canaliculi. Variations in the developmental expression of this class of enzymes have made it challenging to fully appreciate what is likely a multitude of clinical interactions.
Acetylation reactions are catalyzed by N -acetyltransferases, which transfer an acetyl group from acetyl coenzyme A to a variety of substrates (e.g., p -aminobenzoic acid, p -aminosalicylic acid, procainamide). Two genes, NAT1 and NAT2 , are responsible for yielding two specific enzymes with different allelic forms. Despite having 87% sequence homology, these enzymes exhibit different substrate specificities. Both are cytosolic enzymes involved in the biotransformation of several drugs and the bioactivation of several human carcinogens. NAT1 is present in multiple fetal and postnatal tissues and accounts for the most N -acetyltransferase substrate metabolism in children younger than 1 year of age. NAT2 is located primarily in the liver and becomes the dominant acetylator after 1 year of age. NAT2 has polymorphisms with enzyme kinetics that differentiate patients with slow or rapid acetylation capabilities. Infants younger than 1 year of age usually are slow acetylators; subsequent age-dependent alterations lead an individual's targeted acetylator status. Individuals who are genetically destined for rapid acetylation manifest this feature by 2 to 4 years of age.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here