Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
* Adapted and updated from Rhodes ET, Ferrari LR, Wolfsdorf JI. Perioperative management of pediatric surgical patients with diabetes mellitus. Anesth Analg. 2005;101:986–999.
The incidence of type 1 diabetes mellitus in children is increasing worldwide, and while type 2 diabetes in children remains a less common disorder, its prevalence is also increasing. The use of insulin pumps and various multicomponent insulin regimens has increased the complexity of perioperative management of children with diabetes. Anesthesiologists must carefully consider the pathophysiology of the disease, as well as each child's specific diabetes treatment regimen, glycemic control, intended surgery, and anticipated postoperative course, when devising an appropriate perioperative management plan. Standardized algorithms for perioperative diabetes management improve care without significantly increasing costs ; several guidelines and studies of perioperative management of children with diabetes are available in the literature and examples are included.
Type 1 and type 2 are the most common, but not the only, forms of diabetes ( Table 27.1 ). Type 1 diabetes is characterized by absolute deficiency of insulin, which usually results from immune-mediated destruction of pancreatic beta cells. In contrast, type 2 diabetes is characterized by a combination of insulin resistance and a relative deficiency of insulin. Children with type 2 diabetes typically are overweight and frequently have a first- or second-degree relative with type 2 diabetes. However, the high prevalence of obesity in children has blurred the distinction between type 1 and type 2 diabetes. Children with phenotypic characteristics of type 2 diabetes may have pancreatic autoimmunity, and approximately 35% of children with diabetes who require exogenous insulin at diagnosis are overweight or obese ; these data are consistent with the prevalence of childhood overweight and obesity in the general U.S. pediatric population.
| Genetic Defects of Beta Cell Function |
| Monogenic diabetes (formerly referred to as maturity-onset diabetes of the young [MODY]) |
| Permanent neonatal diabetes |
| Mitochondrial disorders |
| Disease of the Exocrine Pancreas |
| Cystic fibrosis–related diabetes |
| Drug-Induced Diabetes |
| Steroids |
| Chemotherapeutic Agents |
| Genetic Syndromes |
| Prader-Willi syndrome |
| Down syndrome |
| Turner syndrome |
| Wolfram syndrome |
| Endocrinopathies |
| Autoimmune polyglandular syndrome |
| Cushing syndrome |
Other forms of diabetes are less commonly encountered (see Table 27.1 ). Monogenic diabetes, formerly referred to as maturity-onset diabetes of the young (MODY), occurs in approximately 2% to 3% of the pediatric diabetes population, and with improvements in care, patients with cystic fibrosis–related diabetes account for a small, but significant, fraction of the diabetes population at major pediatric medical centers. Additional modifications to the perioperative treatment regimen may be necessary when diabetes is associated with genetic syndromes and/or other endocrinopathies, such as adrenal insufficiency (see later discussion).
Although the worldwide incidence of type 1 diabetes is quite variable, the incidence is increasing in almost all populations. The SEARCH for Diabetes in Youth Study, which began in 2000, provides the most comprehensive estimates of the prevalence and incidence of type 1 and type 2 diabetes among U.S. youth younger than 20 years of age. Type 1 diabetes remains the most common form of diabetes observed among U.S. youth, with the greatest prevalence (2.55 per 1000) among non-Hispanic white youth. The epidemic of obesity contributed to a progressive increase in the incidence and prevalence of type 2 diabetes in U.S. children. In the SEARCH study, the prevalence of type 2 diabetes in 10- to 19-year-old youth ranged from 0.17 per 1000 among non-Hispanic white youth to 1.20 per 1000 among Native American youth, and the incidence ranged from 3.7 per 100,000 per year among non-Hispanic white youth to 27.7 per 100,000 per year among Native American youth. Increases in the prevalence and incidence of type 2 diabetes have also been noted in other parts of the world.
Understanding both the pharmacokinetic and pharmacodynamic properties of insulin preparations and antihyperglycemic medications is critical to developing an appropriate perioperative plan.
Type 1 diabetes always requires treatment with insulin. However, an increasing number of insulin preparations ( Table 27.2 ) and delivery systems are available. The least complex regimens consist of two or three insulin injections per day. They incorporate a combination of an intermediate-acting insulin (e.g., neutral protamine Hagedorn [NPH]) and/or a long-acting insulin (e.g., insulin detemir [Levemir] or insulin glargine [Lantus]) for basal coverage with a short- or rapid-acting insulin (e.g., regular, insulin aspart [NovoLog], insulin lispro [Humalog], or insulin glulisine [Apidra]) to provide prandial glycemic coverage. More intensive multicomponent insulin regimens are being used with greater frequency and typically consist of insulin glargine, a long-acting insulin, which provides a relatively constant 24-hour basal concentration of circulating insulin without a pronounced peak to simulate basal insulin secretion, in conjunction with rapid-acting insulin administered with food. Some studies have demonstrated superior glycemic control with such regimens compared with regimens using NPH insulin and regular insulin, although the ideal insulin regimen for children with type 1 diabetes remains controversial and outcomes may differ geographically. Another long-acting insulin, insulin detemir, may be used in intensive insulin regimens and has demonstrated more predictable glucose-reducing effects than both NPH and insulin glargine. The newest long-acting insulin, degludec (Tresiba), is approved for use in adults in many parts of the world and recently received FDA approval for use in children and adolescents.
| Insulin Type | Onset (h) | Peak (h) | Duration (h) |
|---|---|---|---|
| Rapid-Acting b | |||
| Insulin lispro (Humalog) c | <0.25 | 0.5–2.5 | ≤5 |
| Insulin aspart (Novolog) c | <0.25 | 1–3 | 3–5 |
| Insulin glulisine (Apidra) c | <0.25 | 0.5–1.5 | 3–5 |
| Short-Acting b | |||
| Regular (soluble) | 0.5–1 | 2–4 | 5–8 |
| Intermediate- and Long-Acting b | |||
| NPH (isophane) | 1–2 | 2–8 | 14–24 |
| Insulin glargine (Lantus) c | 2–4 | No peak | 20–24 |
| Insulin detemir (Levemir) c | 1–2 | 3–9 | Up to 24 |
| Insulin degludec (Tresiba) c | 1–2 | No peak | >42 |
a Times of onset, peak, and duration of action vary within and between patients and are affected by numerous factors, including dosage, site, depth of injection, dilution, temperature, and other factors.
b Premixed combinations of intermediate-acting and either rapid- or short-acting insulins are available whose pharmacodynamic profiles have a bimodal pattern reflecting the two insulin components.
c Insulin analog developed by modifying the amino acid sequence of the human insulin molecule.
Many children with type 1 diabetes are managed with an insulin pump. This device administers a continuous subcutaneous infusion of insulin (typically a rapid-acting insulin, see Table 27.2 ) at a basal rate that is supplemented by bolus doses of rapid-acting insulin given with meals and snacks. In appropriately selected children, pump therapy has shown superiority over injection regimens. Standard insulin preparations are U100, meaning that there are 100 units of insulin per milliliter. However, very young patients with type 1 diabetes may require diluted insulin (e.g., U10) to achieve accurate dosing. Parents of preschool-aged children, especially toddlers, with type 1 diabetes should be specifically questioned and educated about their use of diluted insulin.
Most children with type 2 diabetes are managed with insulin and/or oral metformin, the only oral agent approved for use in children with diabetes in the United States. Metformin's primary action is to decrease hepatic glucose production and, secondarily, to increase insulin sensitivity in peripheral tissues. Occasionally, other oral agents including sulfonylureas, which promote insulin secretion, and thiazolidinediones, which increase insulin sensitivity in muscle and adipose tissue, are used in adolescents. Nutritional therapy is always included in the management of children with type 2 diabetes. The T reatment O ptions for Type 2 D iabetes in A dolescents and Y outh (TODAY) trial, evaluated the optimal treatment for type 2 diabetes in children and adolescents, age 10 to 17 years. In the TODAY trial, monotherapy with metformin was associated with durable glycemic control in approximately 50% of children and adolescents with type 2 diabetes. The addition of rosiglitazone, but not an intensive lifestyle intervention, was superior to metformin alone.
Increasingly, other medications used to manage adults with type 2 diabetes are being evaluated for use in children, although none are yet approved in the United States. Incretins, including glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1), are gastrointestinal hormones released after eating that stimulate insulin secretion and are necessary for normal glucose tolerance. GLP-1 acts through a G protein–coupled receptor to promote glucose-dependent insulin secretion, suppression of glucagon secretion, slowing of gastric emptying, and reduction in food intake. Exenatide (Byetta) is a GLP-1 receptor agonist widely used in adults with type 2 diabetes as an adjunctive therapy for those taking metformin and/or a sulfonylurea. Inhibitors of dipeptidyl peptidase-IV, the enzyme that degrades GLP-1, are also increasingly being used for the treatment of type 2 diabetes in adults, but have not been approved for use in children. Pramlintide acetate (Symlin) is a synthetic amylin receptor agonist that can be used as an adjunct to insulin therapy in adults with type 1 or type 2 diabetes. Amylin, a 37-amino acid polypeptide islet hormone cosecreted with insulin from islet beta cells, has three effects: delay of gastric emptying, inhibition of glucagon secretion, and modulation of satiety.
Trauma of any kind, and surgery in particular, triggers a complex neuroendocrine stress response that includes suppression of insulin secretion and increased production of counterregulatory hormones (frequently referred to as “stress hormones”), particularly cortisol and catecholamines. Insulin is the primary anabolic hormone that promotes glucose uptake in muscle and adipose tissue while suppressing glucose production (glycogenolysis and gluconeogenesis) by the liver. The counterregulatory hormones, which include epinephrine, glucagon, cortisol, and growth hormone, exert the opposite effects, resulting in resistance to insulin action and an increase in blood glucose concentration by (1) stimulating glycogenolysis and gluconeogenesis in the liver, (2) increasing lipolysis and ketogenesis, and (3) inhibiting glucose uptake and utilization in muscle and fat. Glucagon, secreted by alpha cells in the pancreatic islets, suppresses insulin secretion while stimulating hepatic glycogenolysis, gluconeogenesis, and ketogenesis. Epinephrine, which acts via β 2 - and α 2 -adrenergic receptors, stimulates glucagon production, increases glycogenolysis and gluconeogenesis, stimulates lipolysis, decreases insulin secretion, and decreases glucose utilization in insulin-sensitive tissues. Cortisol stimulates gluconeogenesis, proteolysis, and lipolysis and decreases glucose utilization. Growth hormone augments glucose production, decreases glucose utilization, and accelerates lipolysis. Proinflammatory cytokines may further stimulate secretion of counterregulatory hormones and alter insulin receptor signaling. These changes increase catabolism, as evidenced by increased hepatic glucose production and breakdown of protein and fat. In the patient with diabetes with absolute or relative insulin deficiency, the enhanced catabolism resulting from surgical trauma can lead to marked hyperglycemia and even diabetic ketoacidosis. These metabolic effects may be exacerbated by a prolonged fast before surgery.
Although adequate analgesia is essential to minimize the neuroendocrine stress response to surgery, some anesthetics may also independently contribute to perioperative hyperglycemia. Inhalation anesthetics, such as isoflurane and sevoflurane, may cause hyperglycemia by inhibiting insulin secretion ; the hyperglycemia results from both impaired glucose uptake and increased glucose production. In contrast, epidural analgesia with local anesthetics prevents this hyperglycemic effect through an inhibitory effect on endogenous glucose production. Similarly, intravenous (IV) anesthesia with opioids mitigates the hyperglycemic response to surgery through its apparent neutral effect on endogenous glucose production but decrease in glucose clearance. Although these differences are important to consider, the metabolic effects of anesthesia per se are relatively minor compared with the direct effects of surgery.
Hyperglycemia can impair wound healing by hindering collagen production, which may decrease the tensile strength of the surgical wound. Hyperglycemia may also have adverse effects on neutrophil function, including decreased chemotaxis, phagocytosis, and bactericidal killing. Evidence from controlled experimental studies in rabbits demonstrated that these effects may be reversed, in part, by glycemia-independent effects of insulin. However, the overall benefits of intensive insulin therapy, including a reduction in mortality, were derived mainly from maintenance of normoglycemia, whereas glycemia-independent actions of insulin exert only minor, organ-specific effects.
Several pediatric clinical trials have investigated whether a strategy of tight glycemic control with IV insulin should be used to normalize blood glucose concentrations in nondiabetic children after major surgery. Unless combined with continuous glucose monitoring, this approach increases the risk of severe hypoglycemia. The preponderance of published data indicates that tight glycemic control is not recommended as standard treatment for children who have undergone cardiac surgery because it does not significantly change the infection rate, mortality, length of stay, or measures of organ failure compared with standard care. Similarly, in a 35-center trial in critically ill children, excluding postcardiac surgery patients, there was no evidence of observed benefit of tight glycemic control and the possibility of harm.
Clinical studies in adults with diabetes mellitus have not consistently supported the relationship between perioperative glycemic control and short-term risk of infection or morbidity. However, several studies in adults with diabetes undergoing surgery have shown an association between postoperative hyperglycemia and infectious complications. A meta-analysis showed that patients in surgical intensive care units (ICUs) benefit from intensive insulin therapy, whereas patients in other ICU settings do not. These outcomes have obfuscated the specific glycemic targets and the means for achieving them in both critically and noncritically ill patients. A recent consensus statement recommended that an insulin infusion should be used to control hyperglycemia in the majority of critically ill patients in the ICU setting, with a starting threshold of no greater than 180 mg/dL. Once IV insulin therapy has been initiated, the serum glucose concentration should be maintained between 140 and 180 mg/dL. There have been no prospective, randomized controlled trials to establish guidelines in noncritically ill patients treated with insulin. The current clinical strategy for these patients includes premeal glucose targets of less than 140 mg/dL and random blood glucose values less than 180 mg/dL, as long as these targets can be safely achieved. To avoid hypoglycemia, one should reassess the insulin regimen if the blood glucose concentration decreases below 100 mg/dL. The insulin regimen should be modified if the blood glucose concentration is less than 70 mg/dL, unless the event is easily explained by other factors (such as a missed meal). These perioperative blood glucose goals are also recommended in an Endocrine Society Clinical Practice Guideline for the management of hyperglycemia in hospitalized patients in the noncritical care setting and are supported by the International Society for Pediatric and Adolescent Diabetes (ISPAD) Clinical Practice Consensus Guidelines 2014 for management of children and adolescents with diabetes requiring surgery.
In the 1980s and 1990s the dogma of tight glucose control replaced an era in which the mantra of caring for diabetic surgical patients was “keep them sweet,” but in more recent years perioperative glucose control for patients with diabetes has become controversial and is unresolved, with conflicting evidence. A systematic review of the literature concluded that there are insufficient data regarding the best strategy or regimen to attain target blood glucose concentrations in ambulatory surgical patients with diabetes mellitus. The recommended practice for subspecialties, such as cardiac, neurosurgical, and solid organ transplant surgical patients, requires still more study, and it must be highlighted that few of the published investigations and meta-analyses have focused specifically on children.
When feasible, children with diabetes should not undergo elective surgery until they are metabolically stable ( Fig. 27.1 )—that is, there is no ketosis, serum electrolytes are normal, and the glycated hemoglobin (HbA1c) value approximates the recommended goal of less than 7.5% across all age groups. A more stringent target of less than 7% is recommended for young adults provided this can be achieved without excessive hypoglycemia ; a similar target applies for those with type 2 diabetes. The preoperative consultation to assess the adequacy of metabolic control should be scheduled at least 10 days before the procedure (see Fig. 27.1 ). If metabolic control is poor, surgery should be delayed if possible. Both the endocrinology and anesthesiology services should participate in this assessment. Whenever possible, surgery for children with diabetes should be scheduled as the first case in the morning so that prolonged fasting is avoided and diabetes treatment regimens may be most easily adjusted.
![FIGURE 27.1, Clinical practice guideline for perioperative management of diabetes mellitus. Split-mixed insulin regimen refers to a regimen combining multiple daily injections of intermediate-acting insulins (e.g., neutral protamine Hagedorn) and multiple injections of rapid- or short-acting insulins (regular, insulin lispro [Humalog], insulin aspart [NovoLog], or insulin glulisine [Apidra]). ECG, electrocardiogram. FIGURE 27.1, Clinical practice guideline for perioperative management of diabetes mellitus. Split-mixed insulin regimen refers to a regimen combining multiple daily injections of intermediate-acting insulins (e.g., neutral protamine Hagedorn) and multiple injections of rapid- or short-acting insulins (regular, insulin lispro [Humalog], insulin aspart [NovoLog], or insulin glulisine [Apidra]). ECG, electrocardiogram.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/EssentialsofEndocrinology/0_3s20B9780323429740000276.jpg)
Children who present for emergent surgery (e.g., trauma or acute surgical conditions) require a multidisciplinary preoperative assessment with collaborative involvement of both the endocrinology and anesthesiology services. Surgery often cannot be delayed even if metabolic control is poor—for example, a child requiring emergent surgery who presents in diabetic ketoacidosis. This has implications for the intraoperative management of such children; described later under “Special Surgical Situations.”
The regimen for managing diabetes before, during, and after a surgical or diagnostic procedure that requires the child to fast should aim to maintain near-normoglycemia—that is, a blood glucose concentration of 100 to 200 mg/dL. Blood glucose in this range reduces the risks of osmotic diuresis, dehydration, electrolyte imbalance, metabolic acidosis, infection, and hypoglycemia in the sedated child who may be unable to communicate with staff. The child need not be admitted before the day of surgery, but rather early on the same morning as the surgery or procedure. Parents should receive explicit written instructions regarding appropriate modifications of their child's diabetes regimen before and for the day of surgery. On admission to the hospital, metabolic control should be assessed, including a preoperative determination of the blood glucose concentration. On the morning of surgery, no rapid- or short-acting acting insulin should be administered unless the blood glucose concentration exceeds 250 mg/dL. However, admission to the hospital before major surgical procedures in children and adults has been recommended if the metabolic status needs to be optimized preoperatively. If the surgery must be delayed for any reason, frequent blood glucose monitoring is mandatory to prevent perioperative hypoglycemia or hyperglycemia.
If the blood glucose exceeds 250 mg/dL and no rapid-acting insulin has been given in the prior 3 hours, a conservative dose of rapid-acting insulin (e.g., insulin lispro, insulin aspart, or insulin glulisine) is administered to restore near-normoglycemia. This is achieved using the child's usual insulin “correction factor,” which refers to the decrease in blood glucose concentration expected after administering 1 unit of rapid-acting insulin. This can be calculated using the “1500 rule”: divide 1500 by the child's usual total daily dose (TDD) of insulin. For example, if a child typically receives 30 units of insulin daily, this child's “correction factor” would be 1500 ÷ 30 = 50. In this example, 1 unit of rapid-acting insulin would be expected to decrease the child's blood glucose concentration by approximately 50 mg/dL. Various correction factors have been described, including a “1500 rule” for short-acting insulin (regular) and an “1800 rule” for rapid-acting insulin, such as insulin lispro. For simplicity and because of insulin resistance caused by surgical stress, the “1500 rule” is appropriate in this setting, even with the use of a rapid-acting insulin. To then calculate an appropriate corrective dose of insulin to restore near-normoglycemia, the anesthesiologist should aim for a target blood glucose concentration of 150 mg/dL.
A “correction factor” rather than a sliding scale is also used to manage a child with hyperglycemia and restore the blood glucose concentration to 150 mg/dL. For example, if the child has a correction factor of 1 unit of rapid-acting insulin to reduce the blood glucose concentration by 50 mg/dL, and the current blood glucose value is 300 mg/dL, to reduce the blood glucose concentration from 300 mg/dL to 150 mg/dL, a total dose of (300 − 150)/50 or 3 units of insulin would be required. At the start of the procedure, the “correction” dose may be administered subcutaneously (using rapid-acting insulin) or by IV infusion using short-acting (regular) insulin in those children who will be managed with an IV insulin infusion during the procedure. For children with type 2 diabetes who do not require insulin (but who are, by definition, insulin resistant), an insulin dose of 0.1 unit/kg of rapid-acting insulin may be administered subcutaneously to correct a blood glucose concentration greater than 250 mg/dL.
More detailed preoperative recommendations must be based on the individual child's usual treatment regimen. For most children with diabetes undergoing minor outpatient surgical procedures, insulin can be provided perioperatively with subcutaneous injections. In adults, especially those with type 2 diabetes, this practice is commonly, although not universally, preferred. Others routinely recommend insulin infusions even for minor outpatient surgical procedures in children. Several reports suggest that better glycemic control can be achieved in the perioperative period with a continuous IV infusion rather than subcutaneous insulin administration. However, these studies were conducted before the availability of rapid-acting insulins whose rapid and reproducible effects after subcutaneous administration may more closely match the ability to titrate IV-administered short-acting insulin. Subcutaneous insulin lispro is as effective as regular IV insulin to treat uncomplicated diabetic ketoacidosis in children. Whatever the management strategy for the diabetes, it is most important that it is coordinated between the anesthesiology and endocrinology services, and that the modifications to the child's diabetes regimen at home need to be communicated clearly and in a timely manner to the family.
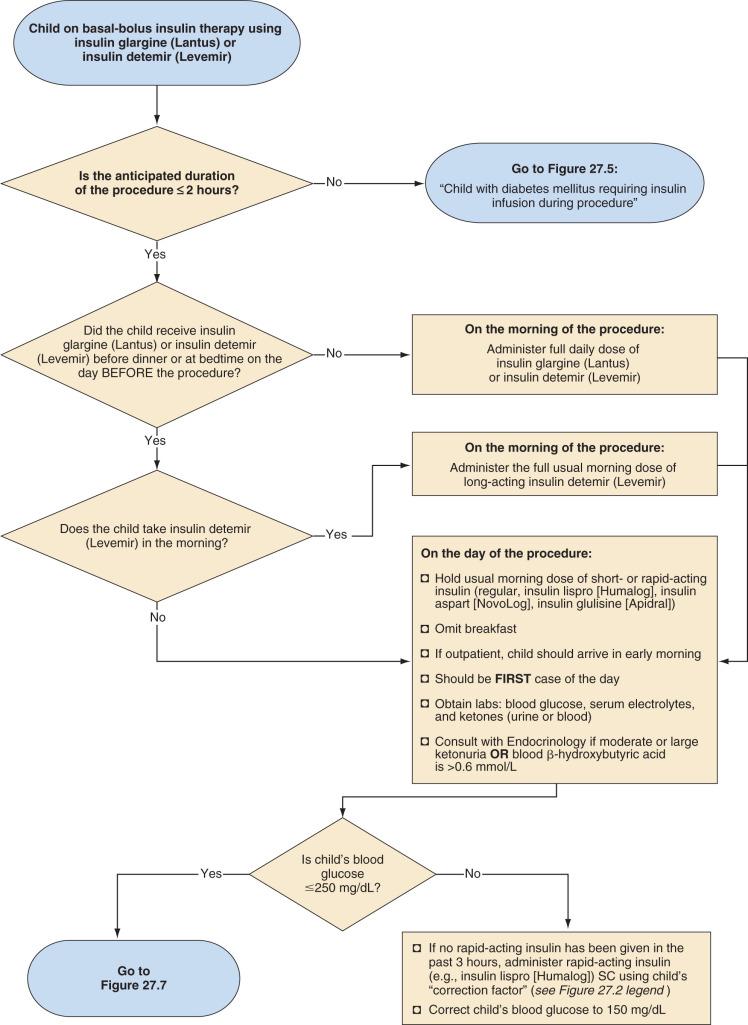
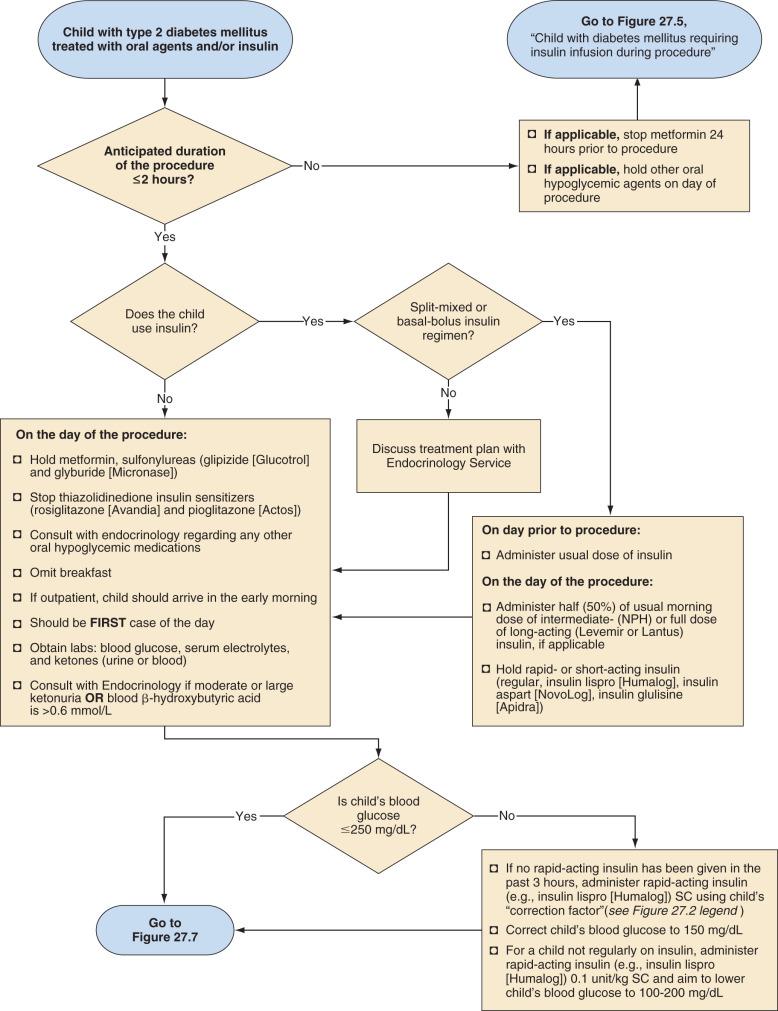
Split-mixed insulin regimens involve two to three injections per day with a combination of intermediate-acting insulin (NPH) plus a rapid- or short-acting insulin. For children who use such a regimen, 50% of the usual morning dose of NPH should be administered on the morning of the procedure ( Fig. 27.2 ). For the child using a basal-bolus regimen that includes rapid-acting insulin with meals and basal insulin once-daily with insulin glargine or once- or twice-daily with insulin detemir, a dose of basal insulin may be required on the morning of the surgery based on the child's regimen and what insulin he or she received on the evening of the previous day ( Fig. 27.3 ). For example, if insulin glargine or detemir is typically administered only once-daily in the morning, the full dose must be administered on the morning of the procedure to prevent hyperglycemia and ketosis.
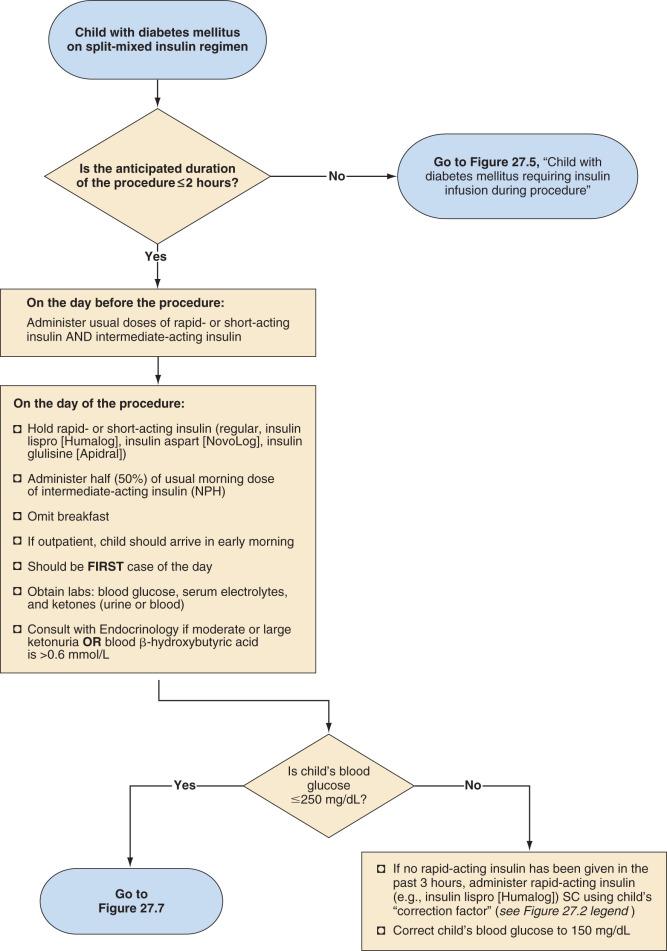
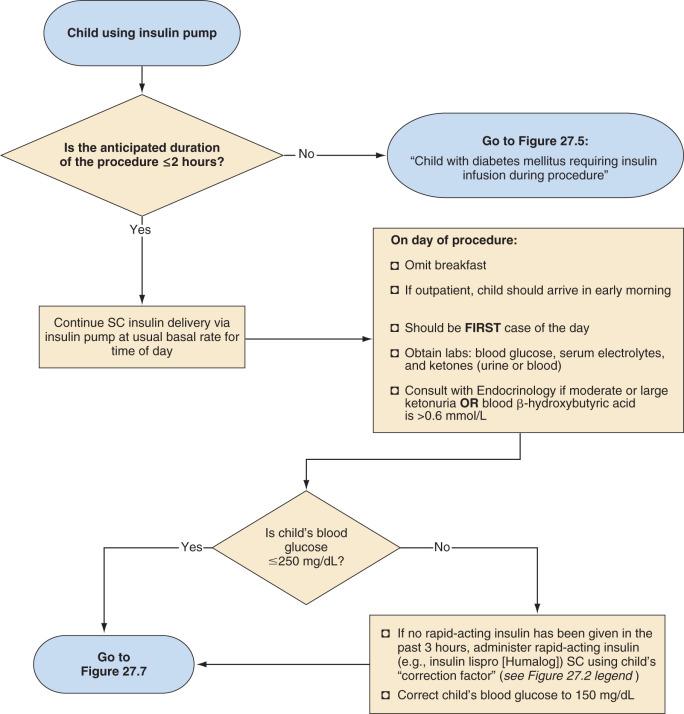
Management of the child with an insulin pump depends on the duration of the surgical procedure ( Fig. 27.4 ). Those undergoing minor procedures expected to last 2 hours or less can continue to receive their usual basal rate via their insulin pump. However, this approach requires the anesthesiologist to become familiar and comfortable with the use of an insulin pump in the operating room. Other protocols include transitioning patients who use an insulin pump to IV insulin infusion or subcutaneous insulin glargine. For procedures expected to last longer than 2 hours, children should be transitioned to an IV insulin infusion, as described in the next section.
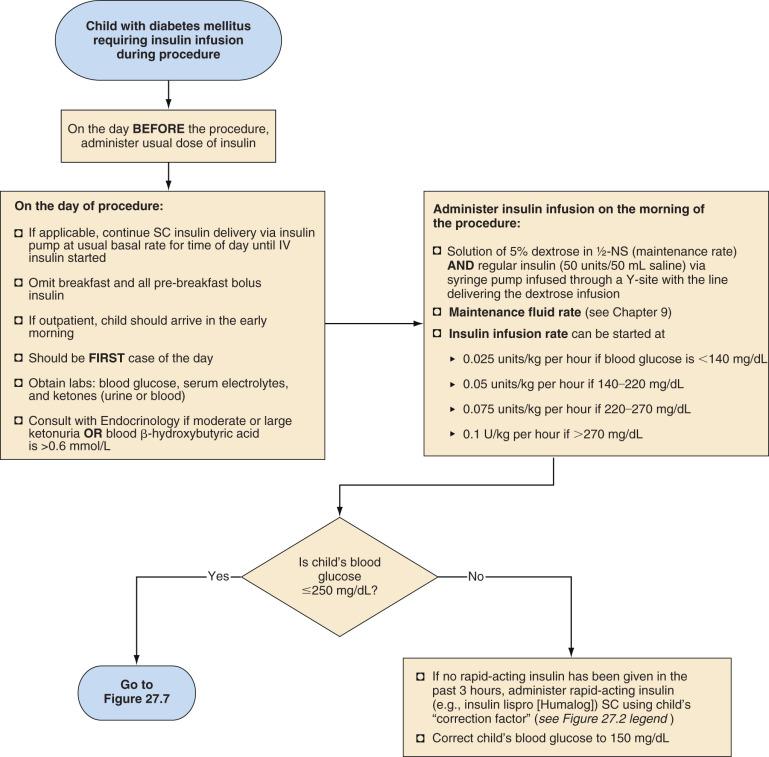
Children who require major surgery, especially procedures anticipated to last more than 2 hours, should have an IV insulin infusion as the preferred perioperative diabetes management plan ( Fig. 27.5 ). Studies in children and adults have demonstrated that glycemic control is superior with infusions of IV insulin compared with subcutaneous injections. These children should receive their usual doses of insulin on the day before the procedure. On the morning of the procedure, an IV infusion of 5% dextrose in half-normal saline should be started at a maintenance rate, and an IV insulin infusion should also be provided to accommodate the dextrose infusion to maintain blood glucose in the target range of 100 to 200 mg/dL (see Fig. 27.5 ). The maintenance rate for IV fluids depends on body size: 20–40 mL/kg lactated Ringer's solution over 2–4 hours of surgery and then 2, 1, and 0.5 mL/kg for each 10 kg body weight if the patient remains NPO postoperatively (see Chapter 9 ). The insulin dose varies with the child's pubertal status; prepubertal children are relatively more sensitive to insulin than pubertal adolescents. In prepubertal children with type 1 diabetes, after the remission (honeymoon) period, the insulin requirement is typically 0.6 to 0.8 unit/kg per day, whereas in adolescents, the requirement is 1 to 1.5 units/kg per day. Children with type 2 diabetes may require even greater doses of insulin because of their insulin resistance. The IV insulin infusion can be started at 0.025 units/kg per hour if blood glucose is less than 140 mg/dL, 0.05 units/kg per hour if blood glucose is between 140 and 220 mg/dL, 0.075 units/kg per hour if blood glucose is between 220 and 270 mg/dL, and 0.1 units/kg per hour if blood glucose is more than 270 mg/dL. Only regular insulin should be used for IV infusions.
Children with type 2 diabetes may require insulin or one of several oral antihyperglycemic agents ( Fig. 27.6 ). Metformin should be discontinued 24 hours before a major surgical procedure (e.g., expected to last more than 2 hours) because of its long duration of effect and the risk of lactic acidosis in the presence of dehydration, hypoxemia, or poor tissue perfusion. For minor procedures, expected to last 2 hours or less, metformin may be discontinued on the day of the procedure. Similarly, other oral agents, such as sulfonylureas and thiazolidinediones, may be discontinued on the morning of the procedure. Fig. 27.6 outlines additional recommendations for children with type 2 diabetes who use a split-mixed or basal-bolus insulin regimen. Adjustments for other insulin regimens or oral hypoglycemic agents in type 2 diabetes should be determined in consultation with an endocrinologist or diabetologist.
The insulin and fluid regimen during and after surgery depends on the duration of the procedure. If the procedure is likely to be brief (e.g., ≤1 hour) and one can reasonably anticipate that the child will be able to drink soon after the procedure, it may not be necessary to start a glucose-containing IV infusion. If the duration of fasting is likely to be more prolonged, an IV infusion should be started at a maintenance rate as described earlier ( Fig. 27.7 ). Intraoperative maintenance fluid should then be replaced with a comparable glucose-containing solution. Replacement of insensible losses and intravascular volume owing to blood or other body fluid losses should be with an appropriate isotonic solution (e.g., lactated Ringer's solution or normal saline solution).
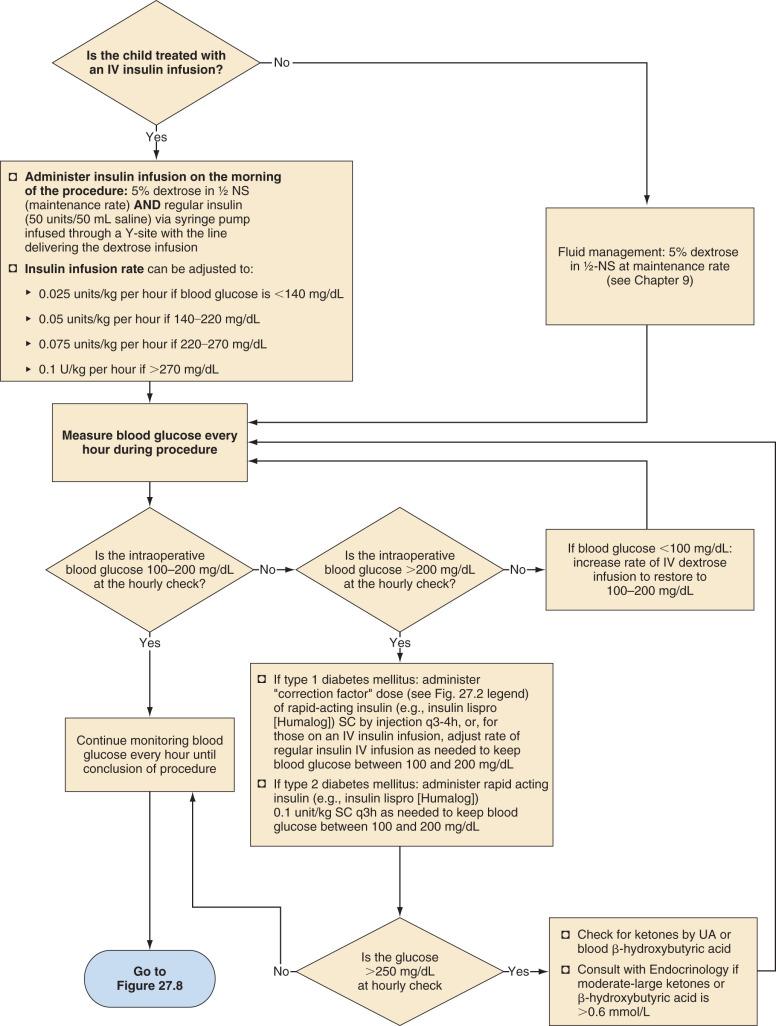
Although some protocols include potassium chloride in the maintenance IV fluid solution, this practice should generally be avoided because of the danger of inadvertent intraoperative administration of large quantities of potassium during fluid resuscitation. Children undergoing a brief procedure with a baseline normal serum potassium concentration and well-controlled diabetes have a small risk of hypokalemia. Those undergoing more prolonged surgeries or emergent surgeries during which metabolic decompensation is more likely, require intraoperative assessment of electrolytes and appropriate adjustment of the electrolyte composition of their IV solution. In all cases, blood glucose concentrations should be measured hourly and either insulin or dextrose adjusted, as necessary, to maintain blood glucose in the target range of 100 to 200 mg/dL. If the blood glucose exceeds 250 mg/dL, urine or blood ketones should also be measured (see Fig. 27.7 ). Either an increase in the rate of continuous insulin infusion or subcutaneous administration of a rapid-acting insulin analog is used to correct intraoperative hyperglycemia. Intraoperative IV bolus of regular insulin is not recommended because this causes a rapid supraphysiologic increase in serum insulin concentration, which, owing to insulin's short half-life (approximately 5 minutes), will have a short-lived effect on blood glucose concentration. In contrast, subcutaneously administered rapid-acting insulin analogs have a typical and reproducible pharmacologic profile.
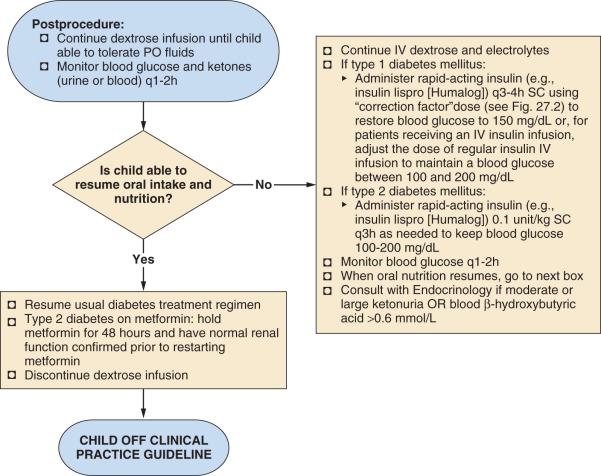
As soon as the child is able to resume drinking and eating normally, the usual diabetes regimen, including insulin and/or oral agents, may be reinstituted and the dextrose infusion discontinued ( Fig. 27.8 ). One exception to this approach is for children with type 2 diabetes who take metformin; metformin should be withheld for 48 hours and renal function must be within normal limits before its resumption. IV dextrose and electrolyte solution should be continued until oral intake is restored. An infusion of IV short-acting insulin (regular) or intermittent subcutaneous rapid-acting insulin should be administered, not more frequently than every 3 hours, to maintain blood glucose in the target range of 100 to 200 mg/dL. Frequent blood glucose monitoring and monitoring blood or urine ketones is essential because of the variable effects of surgical trauma, inactivity, pain, anxiety, nausea and/or vomiting with poor oral intake, medications, and postoperative infection. At the time of discharge from the hospital, children and their parents or care providers should be given appropriate guidelines regarding these issues. Those who are admitted to the hospital overnight after surgery should be managed in consultation with the endocrinology service, if possible, to coordinate appropriate scheduling and subsequent dosing of insulin.
Children with diabetes who need urgent surgery must have a full clinical and biochemical assessment. Frequently, the problem necessitating surgery may have led to metabolic decompensation that must first be corrected and stabilized, unless the need for surgery is immediate. These children are often dehydrated; in addition to administering insulin, rehydration and electrolyte replacement is critical to address their metabolic derangements and restore normal glomerular filtration and renal function. In most cases, these children require emergent surgery that should be managed with an IV infusion of insulin as described earlier (see Fig. 27.5 ). Children with diabetic ketoacidosis require close collaboration between the anesthesiology and endocrinology services.
Children undergoing neurosurgical procedures for tumors in or near the pituitary gland, especially craniopharyngioma, often require management of diabetes insipidus (DI), as do children with known DI who require anesthesia for surgical or radiologic procedures. The perioperative management of these children requires meticulous attention to fluid and electrolyte balance to prevent either overhydration or underhydration and electrolyte disturbances.
Central DI (also referred to as neurohypophyseal, neurogenic, or vasopressin-sensitive DI) is caused by a deficiency of the antidiuretic hormone, arginine vasopressin, which produces antidiuresis by stimulating V2 receptors on the principal cells of the kidney to promote reabsorption of water. Central DI can be caused by disorders of vasopressin gene structure; accidental or surgical trauma to vasopressin neurons; congenital anatomic hypothalamic or pituitary defects; neoplasms; infiltrative, autoimmune, and infectious diseases affecting vasopressin neurons or fiber tracts; and increased metabolism of vasopressin. The etiology is unknown in approximately 50% of children with central DI.
It is important to distinguish polyuria (>2 L/m 2 per day) caused by the onset of acute postsurgical central DI from polyuria resulting from diuresis of salt and fluid given during surgery. In both cases, children may have a large volume (exceeding 200 mL/m 2 per hour) of urine. Serum osmolality is increased, associated with inappropriately dilute urine (low urine osmolality), in DI but will be normal in the child excreting excess salt and water. In a solute diuresis, the urine osmolality is usually greater than 300 mOsmol/kg, in contrast to the dilute urine characteristic of DI. A meticulous examination of the intraoperative and postoperative records and careful bedside assessment of volume status (jugular venous distention, capillary refill) helps to distinguish between these two entities.
Of special interest is the triphasic pattern of vasopressin secretion often observed after neurosurgical procedures that interfere with the supraoptic-hypophyseal tract. An initial phase of transient DI may be observed that lasts between 12 hours and 2 days after surgery. This may be explained by local edema that interferes with normal vasopressin secretion. If significant vasopressin-secreting cell damage has occurred, release of stored vasopressin from damaged neurons leads to a second phase that involves water retention. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) may last up to 10 days. Finally, a third phase, permanent neurogenic DI, may follow if more than 90% of vasopressin cells are destroyed. Pronounced SIADH in the second phase generally portends permanent DI in the final phase of the triple response. In children with vasopressin and cortisol deficiency (e.g., in combined anterior and posterior hypopituitarism after neurosurgical treatment of craniopharyngioma), symptoms of DI may be masked because cortisol deficiency impairs renal free water clearance. Institution of glucocorticoid therapy may precipitate polyuria, leading to the diagnosis of DI ( Fig. 27.9 ).
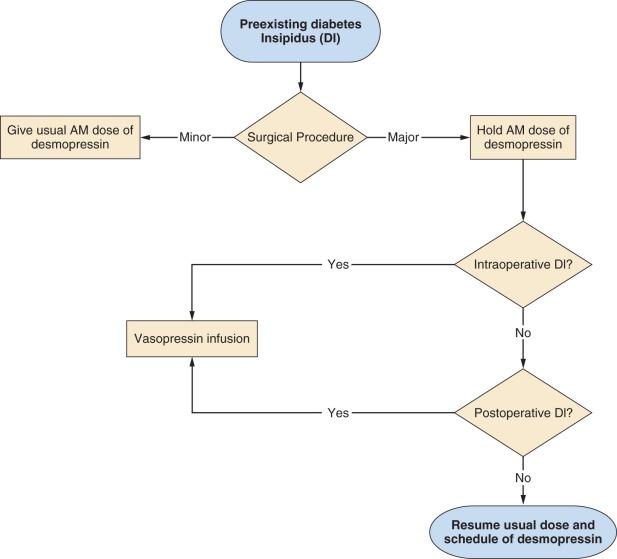
Children with preexisting DI who are scheduled for a minor procedure—that is, a procedure without significant blood loss and not followed by a period of further fasting or fluid shifts (e.g., myringotomy, radiologic imaging, peripheral orthopedic procedures)—are treated differently from those undergoing a more major procedure associated with blood loss or fluid shifts and delayed postoperative resumption of fluid intake (see Fig. 27.9 ).
Children undergoing minor procedures with anesthesia should receive their usual morning dose of desmopressin (DDAVP) ( Table 27.3 ). The anesthetic technique is tailored to the procedure (e.g., IV sedation for magnetic resonance imaging [MRI], general endotracheal anesthesia for tonsillectomy). Arterial and urinary catheters are not required, and recovery takes place in the postanesthesia care unit (PACU). Once the morning dose of desmopressin has been administered, intraoperative and postoperative fluids should be restricted to the rate of 1 L/m 2 per 24 hours (~two-thirds of usual maintenance fluid requirement) to match insensible free water losses and obligatory urine output. Oral fluids may be offered once the child is awake. Although modern PACU policies often no longer require successful fluid intake before discharging the ambulatory patient, discharging the child with DI should be delayed until the child is able to freely take oral fluids without vomiting. Subsequent doses of DDAVP should be administered according to the child's usual preoperative schedule.
| Desmopressin acetate 100-, 200-µg tablets; nasal Rhinal tube 10 µg/0.1 mL; nasal spray 10 µg/0.1 mL can only deliver fixed doses of 10 µg (0.1 mL) per spray | |
| Oral | Dose: 100–400 µg q8–12 hours |
| Oral doses are 10–20 times intranasal doses of desmopressin | |
| Onset of action: ~1 hour | |
| Duration: 6–8 hours; dose dependent (0.4 µg has antidiuretic effect up to 12 hours) | |
| Intranasal | Dose: ~10–20 µg/dose q12–24 hours |
| Onset of action: within 1 hour | |
| Duration: 5–21 hours | |
| Vasopressin (Pitressin) (20 units/mL; 0.5-mL, 1-mL, and 10-mL vials). Dilute in normal saline or 5% dextrose in water | |
| Intravenous | Dose: 1.5 mU/kg per hour; titrate upward in 0.5 mU/kg per-hour increments to target urine output <2 mL/kg per hour |
| Onset of action: rapid; maximum effect is achieved within 15 minutes of initiation of continuous infusion | |
| Duration: action ceases within 20 minutes of stopping intravenous infusion | |
A major surgical procedure is defined as an operation associated with the potential for significant blood loss; intraoperative or postoperative hemodynamic, neurologic, or respiratory instability; entry into a body cavity (e.g., craniotomy, abdominal surgery, thoracic surgery); craniofacial or airway surgery; major orthopedic surgery (e.g., spine surgery, tumor resection or amputation, major osteotomies); and/or surgery followed by delayed resumption of unrestricted fluid intake. The child with DI should be scheduled as the first case of the day. On the day before the procedure, the child treated with DDAVP should receive the usual morning dose, but only 50% of the usual evening or bedtime dose (see Fig. 27.9 ). On the morning of the procedure, DDAVP is withheld. The child undergoing a major procedure should receive general anesthesia with conventional monitoring, as well as insertion of arterial and urinary catheters when appropriate, and after surgery is admitted to a high-care facility (e.g., ICU). A central venous catheter for monitoring central venous pressure, although not indicated solely for the management of DI, is useful in the postoperative period.
At the start of the procedure, an infusion of aqueous vasopressin (available as Pitressin 20 U/mL in a 1-mL vial, diluted to 20 U/1000 mL to yield a final concentration of 20 mU/mL) should be started at 1.5 mU/kg per hour (0.0015 U/kg per hour). IV fluids, using normal saline with 5% dextrose, should total 1 L/m 2 per 24 hours to approximate insensible losses and obligate urine output. Additional IV saline, isotonic fluids, or blood products may be given to replace the blood and surgical fluid loss, to offset the third-space fluid losses, and to maintain hemodynamic stability. Fluid intake and output should be continuously monitored and serum sodium concentration measured frequently to ensure water balance is maintained. Urine output should not routinely be replaced in the child receiving a vasopressin infusion. Accidental overdoses resulting from inadequate dilution of the highly concentrated stock solution result in severe abdominal cramping, diarrhea, vomiting, and pallor owing to action at V1 receptors on smooth muscle in the gastrointestinal tract and blood vessels.
The new diagnosis of intraoperative or postoperative DI is based on clinical and laboratory findings, including a serum sodium concentration greater than 145 mmol/L, polyuria (>4 mL/kg per hour) for 30 minutes or more, increased plasma osmolality (>300 mOsm/kg) in association with hypotonic urine (<300 mOsm/kg), and after excluding the presence of glycosuria and diuretic or mannitol administration as possible causes of polyuria.
When DI occurs, an infusion of aqueous vasopressin (20 U/1000 mL) is initiated at 1.5 mU/kg per hour (0.0015 U/kg per hour); because aqueous vasopressin has a brief plasma half-life (approximately 10–20 minutes), the rate of infusion is increased every 30 minutes until urine output decreases to less than 2 mL/kg per hour, indicating that antidiuresis has been achieved. Once a urine output of less than 2 mL/kg per hour is achieved, the vasopressin infusion is maintained at a constant rate.
IV DDAVP should not be used in the acute management of postoperative central DI because it offers no advantage over aqueous vasopressin and because its long half-life (8–12 hours) compared with that of vasopressin, increases the risk of water intoxication and precludes dose titration.
The child with DI should be cared for in an ICU after major surgery. Fluid input and output, serum electrolytes, and osmolality are monitored closely (hourly if necessary) in the intraoperative and postoperative periods. Until stability is achieved, it is important to have a urinary catheter in place to distinguish postoperative urinary retention from oliguria. The vasopressin infusion initiated intraoperatively is continued in the ICU. Fluid administration is not adjusted according to urine output; however, fluid deficits are replaced and blood pressure supported until antidiuresis (urine output <2 mL/kg per hour) is clearly established. In the patient with maximum antidiuresis, total (oral and IV) maintenance fluids should not exceed insensible plus obligatory urinary losses (i.e., approximately 1 L/m 2 per 24 hours). In the postoperative period, appropriate maintenance fluid is generally 5% dextrose in half-normal saline solution with 0 to 40 mEq/L of potassium chloride (depending on the serum potassium concentration). Blood loss should be replaced with normal saline solution, 5% albumin, or blood products, as appropriate.
Aqueous vasopressin in a dose of 1.5 mU/kg per hour results in a supranormal blood vasopressin concentration of approximately 10 pg/mL, twice that needed for full antidiuretic activity. The effect of vasopressin is maximal within 2 hours after starting an infusion.
After hypothalamic, but not transsphenoidal surgery, greater initial concentrations of vasopressin are occasionally required to treat acute DI. This may be attributed to the release of a substance related to vasopressin from the damaged hypothalamo-neurohypophyseal system, which acts as an antagonist to normal vasopressin activity. Much greater rates of vasopressin infusions, resulting in plasma concentrations greater than 1000 pg/mL, should be avoided because they may cause cutaneous necrosis, rhabdomyolysis, and cardiac rhythm disturbances.
Children treated with vasopressin for postneurosurgical DI should be switched from IV to oral fluid intake at the earliest opportunity. With an intact thirst mechanism and access to free water, the patient will better regulate blood osmolality. Once oral intake has been resumed without nausea and vomiting (often by the morning of the day after surgery), the vasopressin infusion should be stopped; all IV infusions should be stopped to avoid iatrogenic fluid overload, and oral fluids are permitted freely. DDAVP is then reinstituted (nasally or orally) in the child with preexisting DI or begun in the child who has new-onset DI (see Table 27.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here