Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A key paradigm in environmental carcinogenesis—that of gene-environment interactions, a concept used to describe the complex interplay between individual or population genetics and responses to chemical agents—has undergone an expansive transformation into a more contemporary understanding of the human “exposome.” The exposome is the cumulative lifelong burden of disease-contributing stressors, including exogenous and endogenous agents of all types, as well as an individual's genetic susceptibility and “omics” response profile (genomics, epigenomics, transcriptomics, proteomics, metabolomics) and their microbiome.
A classic sequential model of initiation, promotion, conversion, and progression has increasingly evolved into less linear models that may no longer classify carcinogens as initiators or promoters per se. It may be more important to understand the contribution of environmental agents to the various hallmarks of cancer: genomic instability, resisting cell death, deregulating cellular energetics, sustaining proliferative signals, evading growth suppressors, avoiding immune destruction, enabling replicative immortality, promoting tumor inflammation, activating invasion and metastasis, and inducing angiogenesis.
A traditional paradigm classified carcinogenic agents as environmental, lifestyle related, or occupational in origin, but these classifications are blurred by examples of complex interplay among all three types of exposure.
Carcinogen identification has evolved from historical observational studies, to occupational cohort epidemiology, to broad-scale screening and testing in experimental animals.
Next-generation approaches to carcinogen identification use exposome approaches of integrating sophisticated individual exposure monitoring and biosensing with highly sensitive “omics” approaches to detect early perturbation of disease-relevant signaling pathways.
Most human cancers probably result from the interaction of several exogenous carcinogenic influences (often unidentified, but many are dietary or lifestyle related in origin) along with intrinsic factors (e.g., inherited genes, hormones, inflammation and oxidative stress, immune status, energy balance).
Although the causes of most individual human cancers are not identifiable, there is incontrovertible evidence that certain chemical agents, radiation, and certain biologic agents are contributors to the overall incidence of human cancer.
Consumption of tobacco products, especially cigarette smoking, is responsible for nearly 30% of all cancers.
Chemical carcinogens include polycyclic aromatic hydrocarbons (PAHs), aromatic amines, benzene, aflatoxins, tobacco chemicals, and chemotherapeutic agents. Metal carcinogens are largely associated with occupational exposures and include specific forms of arsenic, nickel, and hexavalent chromium.
Radiation carcinogens include ultraviolet radiation, ionizing radiation, and radon.
Fibers such as asbestos and certain dusts are etiologic agents in lung cancers and mesothelioma.
Many components in the diet can influence the development of cancer through carcinogenic or anticarcinogenic mechanisms.
The identification of molecular biologic markers of exposure, effect, and susceptibility (reflecting early events that link exposure to adverse outcome, but before detection of clinical disease) will help further our understanding of human carcinogenesis.
In addition to genetic polymorphisms indicative of susceptibility, biomarkers of disease-relevant exposure will increasingly rely on highly sensitive, multiplatform, and multidimensional analysis of the impact of potential carcinogenic agents on epigenomics, transcriptomics, proteomics, metabolomics, and the microbiome.
Primary, secondary, and tertiary cancer prevention approaches will be greatly facilitated by the development of noninvasive biomarkers that identify high-risk individuals and by identification of the disease-consequential perturbations in molecular signaling that may also be targets for chemoprevention or cancer interception.
Direct causes of most individual human cancers will not be identifiable, but there is incontrovertible evidence that certain chemical agents, radiation, and certain biologic agents are contributors to the overall incidence of cancer. A traditional paradigm has classified carcinogenic agents as environmental or lifestyle related or occupational in origin, but these classifications are blurred by examples of complex interplay among all three “sources” of exposure. For example, cigarette smoking is likely responsible for 25% to 30% of human cancer: to smoke or not to smoke can be thought of as a lifestyle choice, but the act of smoking also gives rise to secondhand (environmental) tobacco smoke exposure, and smoking is pervasive in some occupational settings. Obesity is now also recognized as a major risk factor for certain cancers. Obesity is etiologically related to energy balance (lifestyle) and genetics, but certain environmental agents may act as “obesogens” and may indirectly contribute to cancer risk. Certain occupational chemical agents may also be released into the general environment, or unknowingly carried home to affect lifestyle, and occupation itself can be considered a lifestyle choice in some parts of the globe. A more recent paradigm in environmental carcinogenesis—gene-environment interactions, a concept used to describe the complex interplay between individual or population genetics and responses to chemical agents—has also undergone an expansive transformation into a more contemporary understanding of the human “exposome.” The exposome is the cumulative lifelong burden of disease-contributing stressors including exogenous and endogenous agents of all types, as well as an individual's “omics” profile (genomics, epigenomics, transcriptomics, proteomics, metabolomics), and his or her microbiome. Yet another traditional model of environmental carcinogenesis, that of the classic sequential model of initiation, promotion, conversion, and progression (history reviewed succinctly in Weiss ), has also increasingly been supplanted by less linear models that no longer classify carcinogens as initiators or promoters per se. Instead, the concepts of epigenomic and transcriptional reprogramming, including alternative RNA splicing, in response to microenvironmental shifts in allostatic load (cellular physiologic stress) are increasingly understood as drivers of early-stage carcinogenesis through the expansion of death-resistant cells. Thus a more apt approach may be to understand the contribution of chemical agents to the various hallmarks of cancer : genomic instability, resisting cell death, deregulating cellular energetics, sustaining proliferative signals, evading growth suppressors, avoiding immune destruction, enabling replicative immortality, promoting tumor inflammation, activating invasion and metastasis, and inducing angiogenesis. These evolving new thought paradigms hold promise for improved exposure monitoring, more accurate identification and earlier detection of disease-consequential exposures, and increasingly effective primary, secondary, and tertiary public health and clinical cancer prevention and interception measures.
Fig. 10.1 gives a linear representation of the traditional paradigm for progressive carcinogenesis. This model is useful in that it depicts what is thought to be the monoclonal nature of cancer (originating from a single cell) and the concept of clonal evolution and expansion. It evokes a chemical- or radiation-provoked initiating event, followed by promotion of clonal expansion to form a preneoplastic lesion. In this model, it is thought that a single cell of a preneoplastic lesion undergoes “conversion,” which theoretically equates to progeny cells acquiring enough cumulative genotypic and phenotypic alterations to express a malignant phenotype. Malignant cells, defined pathologically as “invasive,” can then undergo progression (volume expansion due to increased net cell proliferation) to a clinically relevant cancer with metastatic potential. This pictorial sequence is often punctuated with reference to mutagenic activation of oncogenes, inactivation of tumor suppressor genes, and, more recently, epigenetic alterations. Such a linear model is particularly illustrative of protective points of intervention beginning with avoidance of disease-consequential exposure and chemoprevention (primary prevention), early diagnosis and cancer interception (secondary prevention), and prevention of relapse and second cancers (tertiary prevention). It also helps give temporal perspective to the carcinogenic “lag” period, the presumptive period of time between an initiation event and clinical detection of a tumor.
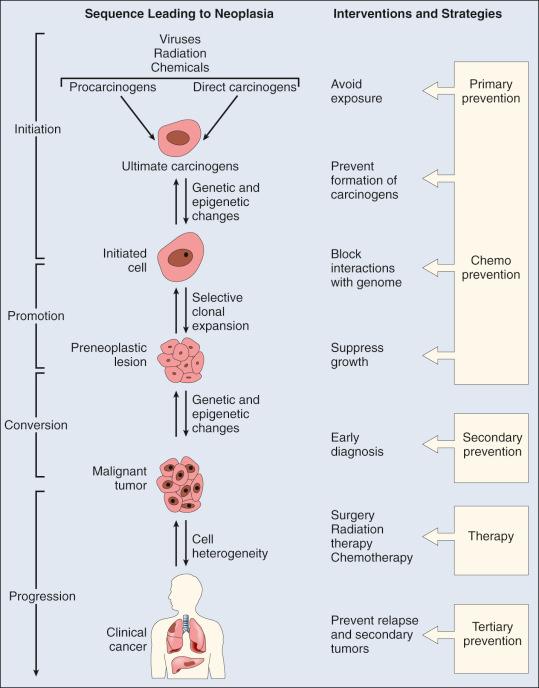
A consequence of this sequential model is that it tends to narrowly relegate environmental carcinogens as mutagenic “initiating” agents, and certain other chemical agents as nonmutagenic “promoting” agents, and does not account for the increasing weight of evidence that the process of early carcinogenesis at the cellular level is highly nonlinear and better understood as a perturbation of molecular homeostasis resulting in accelerated evolution toward acquisition of any of the 10 hallmarks of cancer. As shown in Fig. 10.2 , these hallmarks are best graphically portrayed in circular fashion because they do not necessarily follow a temporal sequence. This model elaborates our understanding of oncogenesis as a disruption of highly interconnected and complex signaling networks that intersect cytostasis and differentiation circuitry, cell viability circuitry, proliferation circuitry, and motility circuitry. It necessitates broadening our understanding of mechanisms of action in environmental carcinogenesis to include the potential ability of chemical carcinogens to provoke genomic instability, sustained proliferative signaling, acquisition of death resistance, evasion of growth suppressors, enabling of replicative immortality, and/or activated cellular motility and invasiveness.
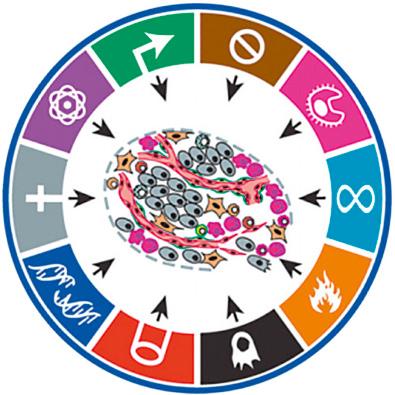
The “hallmarks of cancer” model also underscores the possible impact of environmental agents on deregulation of cellular energetics, avoidance of immune destruction by emerging tumor cells, promotion of tumor inflammation, and provocation of nonneoplastic additional cells in the tumor microenvironment, such as stromal cells, to contribute to the survival, development, and emergence of an early precancerous cell. One illustrative example of this complexity emerges from increased understanding of the role of cell death in cancer and carcinogenesis. Studies of genotoxin-treated cells in culture have drawn attention to the importance of an overlooked outcome in earlier attempts to measure mutagenesis as a surrogate marker for carcinogenic potential: the frequent emergence of death-resistant subclones of cells out of which an occasional cell carrying a mutated selectable gene could be detected as a low-frequency event. This carcinogen-provoked early acquisition of resistance to apoptosis has challenged the perspective that active cell death processes necessarily protect against cancer and carcinogenesis. To the contrary, the facile ability of cells to circumvent cell death when under stress actually renders the existence of such cell death signaling pathways as a risk factor for carcinogenesis. Indeed, it is now appreciated that additional modes of cell death, such as autophagy, can mediate either tumor cell survival or death; and even necrosis, often a consequence of toxic exposure, has been shown to be potentially proinflammatory and tumor promoting. In 2015 a series of review articles were published by an international consortium of scientists, collectively called the Halifax Project, which conducted detailed assessments of the potential of environmental chemicals to contribute causatively to the process of carcinogenesis by affecting each “hallmark” of cancer.
The methods and concepts for identifying chemical agents potentially carcinogenic to humans have evolved over time and continue to evolve as both the general public and regulatory agencies grapple with the fact that humans live in a chemical-laden society, which generally contributes to improved global health and increased longevity. In addition to consumption of and exposure to tobacco smoke, itself containing more than 30 potentially carcinogenic chemicals ( Box 10.1 ) delivered directly by inhalation of a chronically injurious toxic fume, humans are awash in a sea of low-level exposure to both natural and anthropomorphic chemicals. For example, polycyclic aromatic hydrocarbons (PAHs), a broad class of chemicals including some with carcinogenic potential, are released into the environment by both anthropomorphic fuel combustion and natural volcanic eruptions and forest fires, as well as consumed through smoking and eating flame-cooked meat. Such exposure complexity necessitates improved differentiation of “any” exposure from “disease-consequential” exposures, which itself represents a next-generation evolution of carcinogen identification from historical observational studies, to occupational cohort epidemiology, to broad-scale screening and testing in experimental animals, to exposome approaches of integrating sophisticated individual exposure monitoring with highly sensitive “omics” approaches to detect perturbation of disease-relevant signaling pathways.
A series of reports from the US Surgeon General since 1964 have argued that cigarette smoking is the most significant source of preventable morbidity and premature mortality in high-income countries. An estimated annual excess mortality of 400,000 is attributed to cigarette smoking in the United States. These deaths are the result of coronary heart disease, cancer, and various respiratory diseases. Cancers associated with smoking or smokeless tobacco use include those of the lung, oral cavity, esophagus, pharynx, larynx, and bladder. Additional associations have been reported for cancers of the pancreas, kidney, stomach, nasopharynx, and cervix. The overall increase in risk of disease among smokers compared with nonsmokers is about tenfold for lung cancer, sixfold for chronic obstructive pulmonary disease, and twofold for myocardial infarction. The combined effect of smoking-related diseases on the average life expectancy of smokers is a reduction of 5 to 8 years. On a worldwide basis, an estimated 6 million deaths per year are attributed to tobacco use, with upward of 80% of these deaths in low- and middle-income countries. If current use trends continue unabated, estimates as high as 8 million deaths due to tobacco use annually could be expected by the year 2030, and a total of 1 billion this century.
The addictive properties of tobacco smoking, due primarily to its nicotine content, cause both physiologic and psychologic dependence. Withdrawal symptoms can be severe and include irritability, aggressiveness, hostility, depression, difficulty concentrating, and a craving for tobacco. These pharmacologic factors are reinforced by social factors such as peer pressure, emulation of family role models, and cultural influences. Because most smokers begin smoking, and often form lifelong smoking habits, in their teenage years, preventive educational measures should be focused on this age group.
Smoking control measures use various strategies: cessation programs, clinical or community interventions, governmental or private-sector regulations, taxation of tobacco products, warning labels, and smoking prevention programs. Although the majority of ex-smokers have achieved abstinence without extensive personal assistance from organized cessation programs, other control measures (e.g., national health education programs, physician counseling, and indoor smoking regulations) and family pressure are important influences contributing to smoking cessation. Smoking prevention programs, particularly in schools, and government taxation have been somewhat effective in reducing the initiation of smoking among children and adolescents in developing countries. These approaches will need to be applied more vigorously, especially in low- to middle-income countries, to stem the expansion of smoking worldwide. New challenges also are arising with the creation of new and emerging tobacco products such as dissolvable tobacco products, e-cigarettes, and hookah tobacco. The Family Smoking Prevention and Tobacco Control Act (2009) in the United States has given the US Food and Drug Administration broad authority to regulate the manufacturing, distribution, and marketing of these and more traditional tobacco products to protect public health.
The carcinogenic effects of a sizable number of environmental or industrial chemicals were first described observationally in humans. The influences of occupation and lifestyle in cancer occurrence trace back at least to the 16th century when Ramazzini, in 1700, noted that nuns showed a higher frequency of breast cancer than was observed among other women. Also in that century, Paracelsus and Agricola described Bergkrankheiten (lung cancer) in German miners, probably caused by uranium and its decay product radon. In 1761, Hill observationally associated the use of tobacco snuff with cancer in the nasal passage, and in 1775 Pott noted the occurrence of soot-related scrotal cancer among chimney sweeps. In 1895, Rehn published evidence that occupational exposure to aromatic amines was associated with bladder cancer, and Unna in 1894 associated sunlight exposure with skin cancer.
These observational associations gradually gave way to more proactive approaches to attempt to identify potential carcinogens both computationally (retrospective epidemiology, usually of occupationally exposed or catastrophe-exposed cohorts) and by animal experimentation. However, it was not until the early 20th century that animal models for chemical carcinogenesis were developed, beginning in 1915 when Yamagiwa and Ichikawa demonstrated the production of skin tumors after topical application of crude coal tar to the ears of rabbits. These early studies gradually led to the standardization of animal carcinogenesis bioassays, with the goal of testing chemical class prototypes for carcinogenic potential, ostensibly before human exposure.
Contrary to experiences in earlier centuries, with the advent of animal bioassay programs, evidence of carcinogenicity in experimental animals has sometimes preceded evidence obtained from epidemiologic studies or case reports. Although the term carcinogen means “giving rise to carcinomas,” loosely referring to malignancies in general, more operational definitions are used for carcinogens in animal bioassays. In this context, a carcinogen may be defined as an agent whose administration to previously untreated cells or animals leads to a statistically significant increased incidence of malignant neoplasms, compared with the incidence in appropriate untreated control cells or animals. Data from such studies, combined with epidemiologic and mechanistic studies, are employed by regulatory agencies to try to protect humans from known chemical, radiation, and infectious hazards. From a mechanism of action (MOA) perspective, the regulatory thrust has been to determine whether a carcinogenic agent was mutagenic or nonmutagenic; the latter involved recognition that chronic tissue toxicity itself, in the absence of DNA damage, was potentially carcinogenic. Synthetic and naturally occurring chemicals comprise the largest group of known human carcinogens. More than 100 chemicals, chemical mixtures, biologic agents, physical agents, or industrial processes have been classified as Class 1 human carcinogens ( Table 10.1 ) by the International Agency for Research on Cancer (IARC), and more than 300 chemicals have been designated as probable (Class 2A) or possible (Class 2B) human carcinogens. For perspective, these numbers of potential carcinogenic hazards derive from an environmental milieu of nearly 10 million chemicals.
| Agent or Process | Common Organ or Tissue Sites of Cancer |
|---|---|
| AMBIENT AND DIETARY EXPOSURE | |
| Acetaldehyde (from alcoholic beverages) | Upper digestive tract |
| Aflatoxins | Liver |
| Areca nut | Oral cavity |
| Aristolochic acid (from plants) | Renal pelvis and ureter |
| Arsenic and arsenic compounds | Lung, skin |
| Erionite | Pleura, peritoneum |
| CULTURAL HABITS | |
| Alcoholic beverages | Oral cavity, pharynx, larynx, esophagus, liver |
| Betel quid with tobacco | Oral cavity |
| Tobacco products, smokeless | Oral cavity |
| Tobacco smoke | Respiratory tract, urinary bladder, renal pelvis, pancreas |
| Salted fish, Chinese style | Nasopharynx |
| Solar radiation | Skin |
| OCCUPATIONAL EXPOSURES | |
| Aluminum production | Lung, urinary bladder |
| 4-Aminobiphenyl | Urinary bladder |
| Asbestos | Lung, pleura, peritoneum, larynx, gastrointestinal tract |
| Auramine production | Urinary bladder |
| Benzene | Leukemia |
| Benzidine | Urinary bladder |
| Benzo( a )pyrene | Lung |
| Beryllium | Lung |
| Boot and shoe manufacture and repair | Nasal sinus |
| Cadmium | Lung |
| Chromium (VI) compounds | Lung |
| Coal combustion (indoors) | Lung |
| Coal gasification | Lung, urinary bladder, scrotum |
| Coal-tar pitches | Skin, scrotum, lung |
| Coal tars | Skin, lung |
| Coke production | Skin, scrotum, lung, urinary bladder |
| Dioxin | Multiple sites |
| Ethylene oxide | Lymphatic, hematopoietic |
| Fission products | Multiple sites |
| Formaldehyde | Liver |
| Furniture and cabinet making | Nasal sinus |
| Hematite mining | Lung |
| Ionizing radiation (all types) | Multiple sites |
| Iron and steel founding | Lung |
| Isopropyl alcohol manufacture (strong acid process) | Nasal sinus |
| Leather dust | Nasal sinus |
| Magenta, manufacture of | Urinary bladder |
| Methyl ether | Lung |
| Mineral oils (untreated and mildly treated) | Skin, scrotum |
| Mustard gas | Lung, larynx and pharynx |
| 2-Naphthylamine | Urinary bladder |
| Nickel and nickel compounds | Lung, nasal sinus |
| Painting | Lung |
| 2,3,4,7,8-Pentachlorodibenzofuran | Soft tissue sarcoma, non-Hodgkin lymphoma, cancer of the lung |
| Polychlorinated biphenyl (PCB-126) | Liver, bile duct, lymphoid tissue |
| Rubber industry | Urinary bladder, leukemia |
| Shale oils | Skin, scrotum |
| Silica, crystalline | Lung |
| Soots | Skin, scrotum, lung |
| Strong inorganic acid mists | Larynx |
| Talc containing asbestiform fibers | Lung |
| Toluidine | Urinary bladder |
| Underground mining with exposure to radon | Lung |
| Vinyl chloride | Liver, lung, gastrointestinal tract, brain |
| Wood dust | Nasal cavities, paranasal sinuses |
| THERAPEUTIC AGENTS | |
| Analgesics mixtures containing phenacetin | Renal, urinary bladder |
| Azathioprine | Leukemia |
| N,N -bis(2-chloroethyl)-2-naphthylamine | Urinary bladder |
| Busulphan | Lymphohematopoietic tissue |
| 1,4-Butanediol dimethanesulfonate | Leukemia |
| Chlorambucil | Leukemia |
| Chlornaphazine | Urinary bladder |
| Cyclophosphamide | Urinary bladder, leukemia |
| Cyclosporin | Lymphoma |
| Diethylstilbestrol | Breast, cervix |
| Estrogen replacement therapy | Endometrium, breast |
| Estrogens, nonsteroidal | Cervix and vagina, breast, endometrium, testes |
| Estrogens, steroidal | Endometrium, breast |
| Etoposide | Lymphohematopoietic tissue |
| Melphalan | Leukemia |
| 8-Methoxypsoralen plus UV radiation | Skin |
| MOCA (Methylene bis[2-chloroaniline]) | Urinary bladder |
| MOPP combination therapy | Leukemia |
| Oral contraceptives (combined) | Liver |
| Oral contraceptives (sequential) | Endometrium |
| Phenacetin | Renal pelvis, ureter |
| Semustine (methyl-CCNU) | Leukemia |
| Sulfur mustard | Lung |
| Tamoxifen | Endometrium |
| Thiotepa | Leukemia |
| Treosulfan | Leukemia |
| INFECTIOUS AGENTS | |
| Clonorchis sinensis | Liver, bile duct |
| Epstein-Barr virus | Lymphoma |
| Helicobacter pylori | Stomach |
| Hepatitis B virus | Liver |
| Hepatitis C virus | Liver |
| Human immunodeficiency virus type 1 | Kaposi sarcoma |
| Human papillomavirus types 16, 18, others | Cervix |
| Human T-cell lymphotropic virus type I | Adult T-cell leukemia/lymphoma |
| Kaposi sarcoma herpesvirus | Kaposi sarcoma |
| Opisthorchis viverrini | Liver (cholangiocarcinoma) |
| Schistosoma haematobium | Urinary bladder |
Substantial growth has occurred in our understanding of how these extrinsic factors (chemicals, radiation, infectious agents) interact with intrinsic factors (e.g., genetics of inherited genes, “omics” profile, diet, body mass index (BMI), hormones, immune status, and microbiome) to determine overall susceptibility and risk. Indeed, a key concept underpinning the role of the environment in cancer risk relates to the impact of geography on lifestyle adoption and adaptation and cancer susceptibility, and derives from the following series of epidemiologic observations:
Although the overall incidence of cancer development is reasonably constant among countries, incidences of specific cancer types can vary up to several hundredfold.
Large differences in tumor incidence exist within populations of a single country.
Migrant populations assume the cancer incidence of their new environment within one to two generations.
Cancer rates within a population can change rapidly.
It should be noted, however, that a major aspect of geography-influenced carcinogenesis is related to exposure to unique dietary and lifestyle behaviors and infectious agents, and the possible additive or synergistic interactions between infectious agents and diet. For example, the incidence of cancer of the esophagus, the sixth most common cause of death from cancer worldwide but the third most common in developing countries after stomach and lung cancer, varies from less than 5 per 100,000 persons per year in Central America and Western Africa to more than 50 per 100,000 persons per year in parts of China and central Asia. Putative causes for such “hot spots” have been identified, such as consumption of hot yerba maté in Uruguay, Brazil, and northeast Argentina; however, the causative factors in other high-risk areas remain unknown. Other cancers demonstrating unique geographic distribution include biliary cancer (cholangiocarcinoma) associated with liver fluke infection in some Asian (especially Thailand) and African countries; gastric adenocarcinoma associated with virulent strains of Helicobacter pylori infection in South Korea, Japan, and parts of China; and hepatocellular carcinoma (HCC) associated with hepatitis B virus (HBV) and aflatoxin exposure in parts of western Africa and eastern China. Other connections between geography and lifestyle/environment are documented by studies of shifting cancer organ-site rates over generations following immigration, such as the decrease in stomach cancer rates and increase in prostate cancer rates in offspring of Japanese immigrants to North America.
Chemical carcinogens comprise a diverse array of chemical structures, including both organic and inorganic compounds, the one common characteristic being that either the chemical itself, or its derivatives, are reactive species capable of interacting with biomolecules. Because the innate reactivity of such compounds also tends to make them unstable, relatively few carcinogens are direct acting. Instead, most carcinogens require metabolic activation to reactive species, often inside of the target cells. Metabolic pathways can be strongly influenced by a variety of extrinsic and intrinsic factors and are important determinants of both interindividual and target organ susceptibilities to carcinogens. Because the mammalian species, including humans, developed in a complex milieu of naturally occurring toxic agents, a panoply of innate mechanisms of extracellular and intracellular resistance to such toxic insults developed, ranging from circulating and intracellular sulfhydryl-rich peptides and antioxidants to extensive DNA repair pathways (see Chapter 11 ). These must be overcome before toxic and carcinogenic properties are manifest, underscoring the importance of dose to toxicology.
Once formed intracellularly, the reactive intermediates can interact with both protective and vulnerable molecular targets related to carcinogenesis. As described earlier, in classic linear models of carcinogenesis, mutagenicity was considered a necessary property of carcinogenic “initiators.” DNA was considered the principal molecular target of carcinogens, and it was presumed that nearly all carcinogens interacted with DNA to produce genetic lesions (DNA damage) that can result in mutation of critical cellular genes, including oncogenes and tumor suppressor genes. More recently, the potential interactions of reactive chemicals with signaling pathways governing the various hallmarks of cancer, and the cell's molecular responses to those interactions, have come to be recognized as part of the early carcinogenic process. One such example is the potential connection between compounds known as environmental estrogens, chronic receptor-mediated growth signaling, and hormonally related cancers ( Box 10.2 ). Other critical examples, discussed later, include the nonmutagenic impact of chemically reactive species on gene regulation through epigenomic, metabolomic, and/or transcriptional reprogramming.
Women with a high lifetime exposure to estrogen, such as that occurring with early onset of menarche and late menopause, may be at higher risk for breast cancer. In obese menopausal women, adipose tissue becomes the major source of estrogens, and higher blood estrogen levels among postmenopausal women have shown that higher levels are associated with increased risk of subsequent breast cancer. These observations provoke an important question: do xenoestrogens (synthetic chemicals that can act like estrogens) or other environmental factors such as phytoestrogens (plant estrogens) play a role in the risk for breast cancer? There is clear evidence that these molecules may interfere with the interactions of human estrogens with their receptors, and perturb signaling pathways that not only promote proliferative signaling, but possibly even early resistance to antiestrogens. However, thus far no epidemiologic “smoking gun” has been identified. The Long Island Breast Cancer Study was unable to identify any environmental factors that could be responsible for the elevated incidence of breast cancer on Long Island or other locations and no evidence emerged for organochlorine pesticides, which have long been known to have weak estrogenic activities. Bisphenol A, an industrial chemical present in some polycarbonate plastics used in bottles and infant food packaging, has come under recent scrutiny as a xenoestrogen. Consumer advocacy and industry action rather than regulatory mandates seem to be reducing exposures to bisphenol A at this time. Some studies have shown that women who have diets high in phytoestrogens have lower rates of breast cancer. These phytoestrogens may actually oppose the actions of natural estrogen and sometimes lead to lower serum estrogen levels. The role of early life exposures as risk factors for late-in-life events, potentially via epigenomic effects, is emerging as a key area of investigation in environmental carcinogenesis.
Increased understanding of the mechanistic basis of carcinogenesis provides opportunities for the identification of molecular biologic markers that reflect both disease-relevant exposure and/or events occurring between exposure and clinical disease. It is hoped that the use of biologic markers will help define the roles of environmental agents (particularly at low levels and in complex mixtures) in the etiology of human cancer. These molecular biologic markers can be classified into three major categories:
Markers of exposure reflecting a biologically effective dose of a carcinogen
Markers of effect indicating a disease-relevant biologic response to an exposure
Markers of susceptibility that characterize the inherent susceptibility of an individual to a carcinogenic agent
The detectable persistent interaction of a carcinogen with macromolecules was first demonstrated by Miller and Miller in 1947, who showed azo dye residues bound to liver proteins of treated rats. Later studies indicated the importance of carcinogen modification of DNA (either directly or after metabolic activation) in the cancer process. The measurement of carcinogen metabolites, carcinogen-DNA adducts, or carcinogen-protein adducts in human tissues or fluids provides the basis for development and application of individual exposure monitoring, molecular dosimetry research, and the rapidly expanding field of “molecular” cancer epidemiology. The potential advantage of this approach is that more accurate assessments of individual or group dose may be achieved than through estimates of carcinogen exposure in the environment or workplace.
Carcinogen-DNA and carcinogen-protein adducts have been detected in tissues from a variety of human populations with known or suspected carcinogen exposure. PAH-DNA adducts are elevated in white blood cells from persons occupationally exposed to airborne PAHs, including coke oven workers, foundry workers, and aluminum plant workers, and in lung tissue from heavy smokers. DNA isolated from exfoliated bladder epithelial cells of cigarette smokers has been shown to contain 4-aminobiphenyl adducts. Alkylation damage is detected in DNA from esophageal tissue of persons with documented dietary exposure to nitrosamines. Aflatoxin adducts are found in hepatic DNA after dietary exposure to this mycotoxin. Cisplatin-DNA intrastrand adducts have been measured in white blood cells of patients undergoing cancer chemotherapy.
Carcinogen-DNA adducts excreted in urine provide a potentially noninvasive means for quantifying DNA damage. Urinary concentration of aflatoxin-guanine adducts is strongly correlated with dietary aflatoxin intake. In addition to their use as indicators of previous carcinogen exposure, DNA adducts have the potential for use as direct indicators of future cancer risk. The first prospective test of this application appeared in 1992, when detectable levels of aflatoxin-guanine adducts and several other aflatoxin metabolites in urine were shown to be predictive of the development of liver cancer.
Carcinogen-protein adducts have also been studied as biomarkers of human exposure. Alkylation damage in hemoglobin has been used as an exposure index in risk assessment analyses of persons occupationally exposed to ethylene oxide. It has been found that 4-aminobiphenyl adducts in hemoglobin are highly specific (and sensitive) markers of exposure to cigarette smoke. The level of 4-aminobiphenyl hemoglobin adducts is related to the number of cigarettes smoked, the type of tobacco smoked, and the metabolic phenotype of the smoker. The levels of these adducts drop markedly after smoking cessation.
Unreacted carcinogen metabolites excreted in urine also are used to monitor human exposure to and uptake of carcinogens and related compounds. Concentrations of hydroxylated PAHs (e.g., 1-hydroxypyrene) in urine are elevated in smokers, in patients after topical treatment with coal tar, in road pavers, in coke oven workers, in aluminum plant workers, and in persons ingesting PAHs from food. In occupational settings, the concentration of urinary 1-hydroxypyrene is highly correlated with estimated or measured concentrations of airborne PAHs.
These noninvasive approaches to exposure assessment have potential applications as biomonitoring tools, but at present they have major limitations, and by themselves do not address the key question of parsing out disease-relevant exposure. In all of these studies, significant differences in adduct or metabolite levels are often observed between persons with similar carcinogen exposure, likely due to individual biologic variability, exposure misclassification, and confounding variables such as diet, physical activity, smoking, or personal environment. It is hoped that the exposome approach, at the intersection between high-sensitivity biomarker detection and perturbation of disease-relevant signaling pathways, may improve capabilities for detection of meaningful exposures as a function of individual susceptibility.
The concept of the cumulative lifelong human exposome is the most recent integrative approach to understanding the complex interplay among natural and anthropogenic chemicals, the environment-lifestyle-occupational continuum of exposures, and the response of host cells to exposure. This includes the intersection with diet and energy balance, the interplay between extrinsic and intrinsic exposures in the context of cellular physiologic stress (illustrated by the conundrum of the necessity of oxygen for life in juxtaposition with the toxic consequences of oxidative stress), and the concept of disease-consequential exposure, especially in the context of dose. The importance of dose is critical, a steady holdover from the 16th century alchemist and father of toxicology, Paracelsus: “All things are poison and there is nothing without poison; the right dose differentiates a poison from a remedy” or “the dose makes the poison.” Herein lies the central conceptual challenge of exposome research: to drive innovation in increasingly sensitive methods to monitor and quantify individual environmental and internal human exposures, and to combine those data with leading-edge methodologies to detect subclinical perturbations in disease-relevant signaling pathways that directly contribute to disease evolution.
In traditional risk assessment paradigms, exposure science focused on providing an exposure estimate that would then be benchmarked against algorithm-driven regulatory guidance values in order to maximally protect public health. More recently, exposure science has shifted toward development and application of advanced analytic methods including remote and on-person sensors, mechanistic and computational exposure biology, and advanced “systems-omics” analysis as next-generation biomarkers for meaningful exposure. These approaches may revolutionize conventional testing strategies and risk assessment, particularly as larger numbers of chemicals are being detected in biologic samples at lower and lower levels. Systems level “omics” approaches to biomarkers are shedding light on early biologic effects of external and intrinsic stressors on adverse health outcomes, including tobacco, diet, occupational exposures, and environmental pollutants. As described by Escher and colleagues, “a mechanistic understanding of the causal links between exposure and adverse effects on human health and the environment can be improved by integrating the exposome approach with the adverse outcome pathway (AOP) concept that structures and organizes the sequence of biologic events from an initial molecular interaction of a chemical with a biologic target to an adverse outcome. Complementing exposome research with the AOP concept may facilitate a mechanistic understanding of stress-induced adverse effects, examine the relative contributions from various components of the exposome, determine the primary risk drivers in complex mixtures, and promote an integrative assessment of chemical risks for both human and environmental health.” Similarly, additional model constructs for understanding the potential impact of environmental carcinogens point out that different chemicals in chemical mixtures might affect different target cells (somatic or stem cell) with different but complementary procarcinogenic effects on signaling pathways governing the nexus between cell senescence and immortality.
Some of these concepts were also recently highlighted by a Working Group convened by the National Institute of Environmental Health Sciences (NIEHS) to give guidance on addressing the challenges of “low-dose” carcinogenesis. The Working Group concluded, “The scientific community will need to acknowledge limitations of animal-based models in predicting human responses; evaluate biologic events leading to carcinogenesis both spatially and temporally; examine the overlap between measurable cancer hallmarks and characteristics of carcinogens; incorporate epigenetic biomarkers, in silico modelling, high-performance computing and high-resolution imaging, microbiome, metabolomics, and transcriptomics into future research efforts; and build molecular annotations of network perturbations. The restructuring of many existing regulatory frameworks will require adequate testing of relevant environmental mixtures to build a critical mass of evidence on which to base policy decisions.”
The epigenome is a principal determinant of cellular transcriptional programming, and perturbations in the epigenomic are increasingly understood as instrumental in cancer development. In general, “epigenetics” refers to heritable changes in gene expression that are not caused by alterations in the primary DNA sequence but are associated with altered DNA methylation, multiple histone modifications, and tight regulation of multiple species of noncoding RNAs. These factors, functionally characterized as epigenetic chromatin writers, readers, or erasers, govern the dynamics of chromatin conformation and transcription, and their complex net function can be perturbed by both genotoxic and nongenotoxin carcinogens. A number of studies have identified carcinogen-induced, rapid alterations in epigenomic “marks,” some of which are also present in tumors from that tissue, and smoking-associated DNA methylation changes in buccal cells have been associated with DNA methylation changes in smoking-related epithelial cancers.
Studies have shown that environmental estrogens can disrupt estrogen receptor (ER) signaling, alter DNA and histone methylation, and reprogram the epigenome. Some of this epigenomic reprogramming may occur as a result of adverse exposure to environmental estrogens during development, and possible disruption of normal mammary gland development, resulting in changes in disease susceptibility across the life course. The mechanism of disruption can involve perturbation of genomic signaling through altered binding of ligand-associated ER with its DNA binding site, and/or nongenomic signaling of membrane-bound ER resulting in activation of growth-regulating or growth-deregulating kinase cascades. A study suggested that environmental estrogens such as bisphenol A activates growth-promoting proliferation and resistance to therapeutic drugs that target the epidermal growth factor receptor (EGFR) pathway. Likewise, exposure of the developing prostate to endocrine-disrupting chemicals can increase risk of adult prostate cancer, and this has been associated with epigenomic disruption via phosphatidylinositol 3-kinase (PI3-K) effects on histone methyltransferase.
A complex interplay between genetic (mutational) and epigenetic contributions to carcinogenesis is highlighted by multidimensional analysis of the genome, epigenome, and transcriptome of glioblastoma. Mutated EGFR appears to promote epigenomic remodeling and transcriptome reprogramming in EGFR-dependent glioblastoma multiforme (GBM) pathogenesis. Interesting to note, the MOA of carcinogenic forms of arsenic and nickel seems to involve epigenetic modification of chromatin structure and function, in addition to genotoxic and actions. Epigenetic dysregulation through changes in the methylome and histone modification has also been observed in virus-associated neoplasms.
Important to note, the epigenome is also a potential target for chemoprevention; for example, inhibitors of bromodomain proteins that serve as epigenetic “readers” inhibit in vitro neoplastic transformation by 12- O -tetradecanoylphorbol-13-acetate (TPA), ostensibly by inhibiting TPA-induced expression of prosurvival genes. Likewise, a number of anticarcinogenic phytochemicals have a profound effect on DNA methylation, histone modification, and regulation of noncoding RNAs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here