Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The enteric nervous system (ENS) was first referred to as “the brain-in-the-gut” in a 1981 issue of the Annual Review of Physiology. Its status as an independent integrative nervous system, which determines motility and secretory behavior of the digestive tract, has since become a widely accepted concept. There is strong implication that, like the central nervous system, neurophysiology of the ENS is organized hierarchically from lower to higher levels like rungs leading from bottom to top of a ladder. Output projecting from the hierarchical order to the gut's effector systems determines minute-to-minute behavior of the nonstriated gastrointestinal musculature, secretory glands, and blood vasculature in the functioning gut. Integrated output to the musculature and secretory glands is reflected by specific patterns of motility and secretion that characterize different states of behavior at the level of each organ. Behavioral states that will be considered in this chapter are: (1) physiological ileus ; (2) postprandial ; (3) interdigestive ; (4) defensive ; and (5) emetic ; (6) haustration .
Absence of muscular contractile activity in the longitudinal and circular muscle coats is the identifying characteristic of physiological ileus. The postprandial state is the digestive behavior after a meal that consists of segmenting (mixing) motility integrated with set-point feedback control of luminal pH and osmolarity. The interdigestive state is generally called the “migrating motility complex” and is associated with set-point feedback control of luminal pH and osmolarity. The defensive state is characterized by copious neurogenic hypersecretion and ortho- or retrograde “power propulsion” functioning in concert to expel quickly, any luminal threat that threatens bodily integrity. In the large intestine of humans, four coordinated neurogenic patterns of behavior can be recognized as: (1) haustral formation, (2) physiological ileus, (3) defecatory power propulsion, and (4) defense.
Neurons and synaptic transfer of information from neuron to neuron take place at the bottom rung of the ENS hierarchical ladder. Synaptic connectivity of neurons in a single hardwired polysynaptic reflex, which becomes the basis for all patterns of propulsive motility and presumably any associated secretory behavior, occupy intermediate rungs on the ladder, as do neuronal pattern generators that underlie the timing of repetitive activity of the propulsive motor reflex circuit. Near the top rungs of the ladder-like hierarchy are programs for behaviors that emerge from the organization of the different kinds of neurons, their synaptic connections and their connectivity into circuits in the lower rungs. A library containing a neural program for each of the digestive behavioral states, listed above, is found in top rungs of the ladder. The function of each program in the library is reminiscent of 21st-century mobile “apps,” which are software applications that run on smart phones, tablet computers, and comparable devices. Therefore, we shall refer the various programs in the library as “apps” in this chapter, in recognition of a bright medical student who suggested this analogy to me in class, 1 day. “Apps,” of course, is a tongue-in-cheek expression that will be used throughout the chapter to emphasize the analogy with computational neural programs in the ENS.
Professor C. Ladd Prosser, at the University of Illinois, Champaign-Urbana campus, taught students in comparative neurophysiology that acquisition of complete knowledge at the lower rungs of a hierarchical ladder in a nervous system does not predict kinds of effector system behavior that will emerge from the highest rungs of the ladder. On the other hand, this viewpoint does not permit ignorance of details of properties of single neurons and the synaptic connections of individual neurons inside configurations of neural circuits and networks at lower rungs of the hierarchy of an independent integrative nervous system like the ENS. To understand “what goes wrong in the gut,” understanding of how the ENS works at all the levels of organization is essential.
Like central nervous systems elsewhere, the brain-in-the-gut functions with chemical synaptic connections between interneurons and motor neuronsin lower hierarchical rungs. Networks of interneurons store a library of apps, the output of which synaptically connects with and synaptically controls the activity of three kinds of motor neurons in the ENS. These are identified as musculomotor, secretomotor, and vasculomotor neurons. They are controlled by output from microcircuits formed by assemblies of synaptically interconnected interneurons ( Fig. 15.1 ).
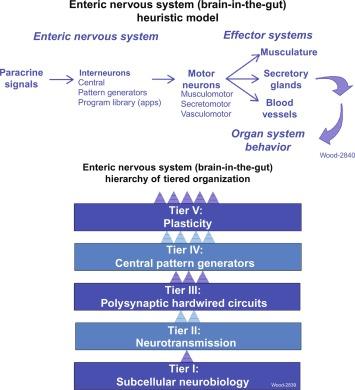
The gastrointestinal smooth musculature is innervated by excitatory and inhibitory musculomotor neurons in lower rungs of hierarchical organization. Excitatory musculomotor neurons release neurotransmitters that evoke contraction and increased tension in the musculature, when they fire. Inhibitory musculomotor neurons release neurotransmitters that suppress contractile activity of the musculature.
Secretomotor neurons stimulate secretion of chloride, bicarbonate, H 2 O, and mucus from mucosal secretory glands, when they fire. Secretomotor control of chloride secretion determines the osmolarity and liquidity in the small and large intestine. Neurogenic control of bicarbonate secretion sustains an optimal physiological pH set point for digestive enzymatic activity in the lumen of the upper small intestine, as well as mucosal protection against surges of acid entering from the stomach. A subset of secretomotor neurons project collaterals to periglandular arterioles and thereby enhance blood flow in support of stimulation of secretion when they fire.
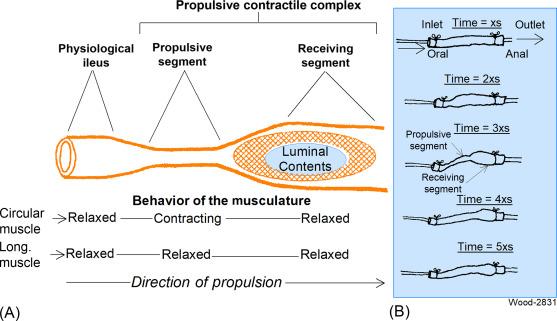
Propulsion of luminal contents is a fundamental motility function of the nonstriated musculature in the esophagus, stomach, small intestine, and large intestine. Propulsive motility in the esophagus, segments of the small intestine or a length of large intestine occurs as a stereotypically configured contractile complex with two linked components based on neurogenically controlled contractile behavior of the longitudinal and circular muscle coats ( Fig. 15.2 ). For descriptive clarity, the components are identified as a propulsive segment connected in the direction of propulsion with a receiving segment . The circular muscle coat of the propulsive segment is in a contractile state that reduces the circumference of the segment and exerts compressive forces on the contents. The circular muscle coat is in a relaxed state in the receiving segment, while contraction of the longitudinal muscle shortens the segment and expands its circumference. Compression of contents in the propulsive segment forces luminal contents into the dilated receiving segment. Because the intestine behaves geometrically like a cylinder with constant surface area, contraction of the circular muscle coat reduces the intestinal circumference and passively extends the longitudinal axis of the involved segment. Neurogenic relaxation of contractile tension in the circular muscle coat and contraction of the longitudinal muscle coat shortens the receiving segment, expands its circumference, and enlarges it into a luminal chamber that receives the forwardly moving luminal contents. Propulsion of luminal contents progresses in the oral or aboral direction along a length of tubular gut when multiple propulsive motor complexes are connected in series in one or the other direction. Orad propulsion occurs (e.g., during emesis in the small intestine) when the motor complexes are activated serially in the direction of the stomach. Aborad propulsion occurs (e.g., during defecation) when the motor complexes are activated in serial manner in the anal direction.
Activation of a single “hardwired” polysynaptic circuit, in the lower rungs of the ENS organizational hierarchy, accounts for formation of the stereotypic complex of muscle behavior that underlies propulsive motility. Behavior of longitudinal and circumferential muscle coats in the muscularis externa, evoked by the hardwired circuit, configures propulsive, and receiving segments. When the circuit is active, firing of excitatory musculomotor neurons to the longitudinal muscle coat and simultaneous firing of inhibitory musculomotor neurons to the circular muscle coat configure the receiving segment. At the same time, inhibition of inhibitory musculomotor neurons and firing of excitatory musculomotor neurons to the circular muscle coat takes place in the propulsive segment, as illustrated in Fig. 15.3 .
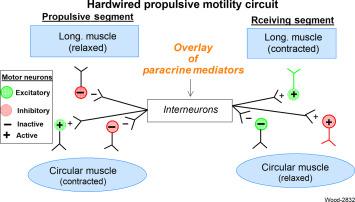
The polysynaptic propulsive motor circuit, in the lower echelons of ENS organization, is plastic in that it may be organized and reorganized for propulsion in the orad or aborad direction in the same length of bowel. Plasticity, for example, is observed during obstruction of a segment of large intestine when the direction of propulsion reverses and moves the luminal contents in the orad direction away from the obstruction. Retro-propulsion then stops and forward propulsion again propels the contents toward the obstruction. Directional plasticity of the propulsive motor circuit is seen, as movement of radiographic barium, during emesis in the upper small intestine when powerful reverse propulsion starts in mid-jejunum and rapidly propels the luminal contents over great distances in the direction of the opened gastric pylorus.
Central pattern generators (CPGs) are circuits in self-contained integrative nervous systems, like the ENS, that generate repetitive patterns of motor behavior independent of any sensory input or feedback. CPGs underlie many commonly recognized rhythmic motor behaviors such as walking, chewing, breathing, and feeding in both vertebrate and invertebrate animals. CPGs are linked with the hardwired propulsive motor circuit found in the networks at the lower rungs of the ENS hierarchical ladder ( Figs. 15.2 and 15.3 ). Rhythmic motor and secretory behaviors programmed by ENS apps, to be discussed later, reflect integration of CPG timing into programmed output at higher rungs of ENS hierarchical organization.
Firing of rhythmically timed bursts of action potentials by motor neurons identifies a CPG. The action potentials may be generated by an ensemble of neurons, in which case the activity is not traceable to any single identified neuron in the system. This is the situation for the Büdinger complex in mammals, which is a group of respiratory neurons located in the rostral ventrolateral medulla oblongata. In some other nervous systems, mainly in invertebrates, patterned motor discharge can be traced to endogenous firing of a single identifiable neuron. Stereotypic sequences of CPG driven repetitive motor behavior, such as walking and swimming, may be started by firing of a single “command neuron,” a classic example of which is defensive the “tail flick” escape behavior in crayfish. In the mammalian ENS, paracrine signaling to a CPG network, by enteric mast cells, behaves like a command that initiates continuous repetitive activation of propulsive motility linked with glandular secretion as defense against invading organisms, food allergens, and other threats.
Sensory feedback can start a CPG and fine tune ongoing motor behaviors, while they are being timed by a CPG; nevertheless, continuous cycling of a stereotypic sequence of motor behavior continues in the absence of sensory connectivity. This is the case for repetitious cycling of the linked propulsive and receiving segments of the ENS propulsive motility circuit, which continues spontaneously for extended periods several hours in the absence of sensory input in intestinal preparations in vitro ( Fig. 15.4 ).
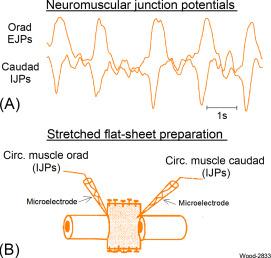
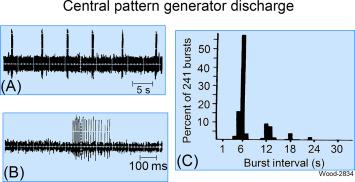
Electrophysiologically recorded results, obtained from single ENS neurons, point to ENS CPGs as endogenous neuronal oscillators that determine the timing of recurrent firing of ENS musculomotor and secretomotor neurons. This is the case for a subset of neurons in stretched intestinal whole-mount preparations from guinea pig, dog, and cat small intestine in vitro. The putative CPG neurons fire bursts of action potentials continuously over long-time periods with the spike bursts fired precisely at 6-s intervals or at fixed multiples of 6, 12, 18, or 24 for cat small intestine ( Fig. 15.5 ). Blockade of synaptic transmission does not change the timing of the burst-type discharge, which would be expected if the CPGs were endogenous “clocks.”
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here