Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Carbohydrates, fats, and proteins can all be used by cells to synthesize large quantities of adenosine triphosphate (ATP), which is used as an energy source for almost all other cellular functions. For this reason, ATP has been called an energy “currency” in cell metabolism. Indeed, the transfer of energy from foodstuffs to most functional systems of the cells can be performed only through this medium of ATP (or the similar nucleotide guanosine triphosphate [GTP]). Many of the attributes of ATP are presented in Chapter 2 .
An attribute of ATP that makes it highly valuable as an energy currency is the large quantity of free energy (≈7300 calories—or 7.3 Calories [kilocalories]—per mole under standard conditions, but as much as 12,000 calories under physiological conditions) vested in each of its two high-energy phosphate bonds. The amount of energy in each bond, when liberated by decomposition of ATP, is enough to cause almost any step of any chemical reaction in the body to take place if appropriate energy transfer is achieved. Some chemical reactions that require ATP energy use only a few hundred of the available 12,000 calories, and the remainder of this energy is lost in the form of heat.
In previous chapters, we discussed the transfer of energy from various foods to ATP. To summarize, ATP is produced through the following processes:
Combustion of carbohydrates —mainly glucose, but also smaller amounts of other sugars such as fructose; this combustion occurs in the cell cytoplasm through the anaerobic process of glycolysis and in the cell mitochondria through the aerobic citric acid (Krebs) cycle.
Combustion of fatty acids in the cell mitochondria by beta-oxidation.
Combustion of proteins , which requires hydrolysis to their component amino acids and degradation of the amino acids to intermediate compounds of the citric acid cycle and then to acetyl coenzyme A and carbon dioxide.
Among the most important intracellular processes that require ATP energy is the formation of peptide linkages between amino acids during synthesis of proteins. The different peptide linkages, depending on which types of amino acids are linked, require from 500 to 5000 calories of energy per mole. Recall from Chapter 3 that four high-energy phosphate bonds are expended during the cascade of reactions required to form each peptide linkage. This expenditure provides a total of 48,000 calories of energy, far more than the 500 to 5000 calories eventually stored in each of the peptide linkages.
ATP energy is also used for synthesizing glucose from lactic acid and for synthesizing fatty acids from acetyl coenzyme A. In addition, ATP energy is used for the synthesis of cholesterol, phospholipids, the hormones, and almost all other substances of the body. Even the urea excreted by the kidneys requires ATP for its formation from ammonia. One might wonder why energy is expended to form urea, which is simply discarded by the body. However, remembering the extreme toxicity of ammonia in the body fluids, one can see the value of this reaction, which keeps the ammonia concentration of the body fluids at a low level.
Muscle contraction will not occur without energy from ATP. Myosin, one of the important contractile proteins of the muscle fiber, acts as an enzyme to cause breakdown of ATP into adenosine diphosphate (ADP), thus releasing the energy required to cause contraction. Only a small amount of ATP is normally degraded in muscles when muscle contraction is not occurring, but this rate of ATP usage can rise to at least 150 times the resting level during short bursts of maximal contraction. The mechanism by which ATP energy is used to cause muscle contraction is discussed in Chapter 6 .
In Chapter 28, Chapter 4, Chapter 66 , active transport of electrolytes and various nutrients across cell membranes and from the renal tubules and gastrointestinal tract into the blood is discussed. We noted that active transport of most electrolytes and substances such as glucose, amino acids, and acetoacetate can occur against an electrochemical gradient, even though the natural diffusion of the substances would be in the opposite direction. Energy provided by ATP is required to oppose the electrochemical gradient.
The same principles apply to glandular secretion as to the absorption of substances against concentration gradients because energy is required to concentrate substances as they are secreted by the glandular cells. In addition, energy is required to synthesize the organic compounds to be secreted.
The energy used during propagation of nerve impulses is derived from the potential energy stored in the form of concentration differences of ions across the neuronal cell membranes. That is, a high concentration of potassium inside the neuron and a low concentration outside the neuron constitute a type of energy storage. Likewise, a high concentration of sodium on the outside of the membrane and a low concentration on the inside represent another store of energy. The energy needed to pass each action potential along the fiber membrane is derived from this energy storage, with small amounts of potassium transferring out of the cell and sodium into the cell during each of the action potentials. However, active transport systems energized by ATP then retransport the ions back through the membrane to their former positions.
Despite the paramount importance of ATP as a coupling agent for energy transfer, this substance is not the most abundant store of high-energy phosphate bonds in the cells. Phosphocreatine , which also contains high-energy phosphate bonds, is three to eight times more abundant than ATP. Also, the high-energy bond (∼) of phosphocreatine contains about 8500 calories per mole under standard conditions and as many as 13,000 calories per mole under conditions in the body (37°C and low concentrations of the reactants). This amount is slightly greater than the 12,000 calories per mole in each of the two high-energy phosphate bonds of ATP. The formula for creatinine phosphate is the following:
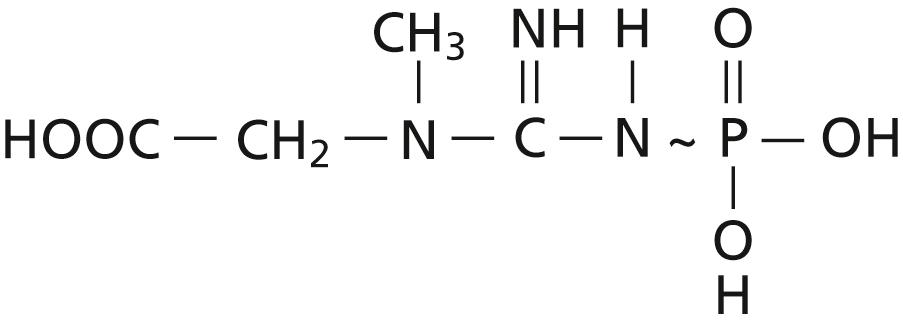
Unlike ATP, phosphocreatine cannot act as a direct coupling agent for energy transfer between the foods and the functional cellular systems, but it can transfer energy interchangeably with ATP. When extra amounts of ATP are available in the cell, much of its energy is used to synthesize phosphocreatine, thus building up this storehouse of energy. Then, when the ATP begins to be used up, the energy in the phosphocreatine is transferred rapidly back to ATP and then to the functional systems of the cells. This reversible interrelation between ATP and phosphocreatine is demonstrated by the following equation:
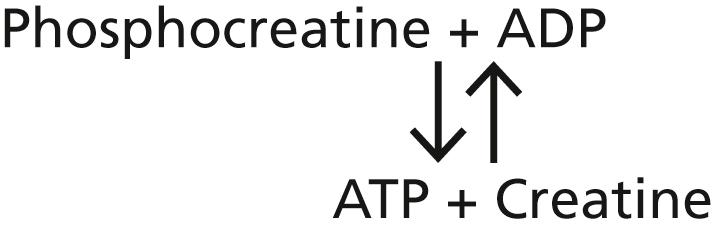
The higher energy level of the high-energy phosphate bond in phosphocreatine (1000–1500 calories per mole greater than that in ATP) causes the reaction between phosphocreatine and ADP to proceed rapidly toward the formation of new ATP whenever the slightest amount of ATP expends its energy elsewhere. Therefore, the slightest usage of ATP by the cells calls forth the energy from the phosphocreatine to synthesize new ATP. This effect keeps the concentration of ATP at an almost constant high level as long as any phosphocreatine remains. For this reason, we can call the ATP-phosphocreatine system an ATP “buffer” system. One can readily understand the importance of keeping the concentration of ATP nearly constant because the rates of almost all the metabolic reactions in the body depend on this constancy.
Anaerobic energy means energy that can be derived from foods without the simultaneous utilization of oxygen; aerobic energy means energy that can be derived from foods only by oxidative metabolism. In Chapter 68, Chapter 69, Chapter 70 , we noted that carbohydrates, fats, and proteins can all be oxidized to cause synthesis of ATP. However, carbohydrates are the only significant foods that can be used to provide energy without utilization of oxygen ; this energy release occurs during glycolytic breakdown of glucose or glycogen to pyruvic acid. For each mole of glucose that is split into pyruvic acid, 2 moles of ATP are formed. However, when stored glycogen in a cell is split to pyruvic acid, each mole of glucose in the glycogen gives rise to 3 moles of ATP. The reason for this difference is that free glucose entering the cell must be phosphorylated by using 1 mole of ATP before it can begin to be split; this is not true of glucose derived from glycogen because it comes from the glycogen already in the phosphorylated state, without the additional expenditure of ATP. Thus, the best source of energy under anaerobic conditions is the stored glycogen of the cells .
One of the prime examples of anaerobic energy utilization occurs in acute hypoxia. When a person stops breathing, a small amount of oxygen is already stored in the lungs and an additional amount is stored in the hemoglobin of the blood. This oxygen is sufficient to keep the metabolic processes functioning for only about 2 minutes. Continued life beyond this time requires an additional source of energy. This energy can be derived for another minute or so from glycolysis—that is, the glycogen of the cells splitting into pyruvic acid, and the pyruvic acid becoming lactic acid, which diffuses out of the cells, as described in Chapter 68 .
Skeletal muscles can perform extreme feats of strength for a few seconds but are much less capable during prolonged activity. Most of the extra energy required during these bursts of activity cannot come from the oxidative processes because they are too slow to respond. Instead, the extra energy comes from anaerobic sources: (1) ATP already present in the muscle cells, (2) phosphocreatine in the cells, and (3) anaerobic energy released by glycolytic breakdown of glycogen to lactic acid.
The maximum amount of ATP in muscle is only about 5 mmol/L of intracellular fluid, and this amount can maintain maximum muscle contraction for no more than a second or so. The amount of phosphocreatine in the cells is three to eight times this amount, but even by using all the phosphocreatine, maximum contraction can be maintained for only 5 to 10 seconds.
Release of energy by glycolysis can occur much more rapidly than can oxidative release of energy. Consequently, most of the extra energy required during strenuous activity that lasts for more than 5 to 10 seconds but less than 1 to 2 minutes is derived from anaerobic glycolysis. As a result, the glycogen content of muscles during strenuous bouts of exercise is reduced, whereas the lactic acid concentration of the blood rises. After the exercise is over, oxidative metabolism is used to reconvert about four fifths of the lactic acid into glucose; the remainder becomes pyruvic acid and is de graded and oxidized in the citric acid cycle. The reconversion to glucose occurs principally in the liver cells, and the glucose is then transported in the blood back to the muscles, where it is stored once more in the form of glycogen.
After a period of strenuous exercise, a person continues to breathe hard and to consume large amounts of oxygen for at least a few minutes and sometimes for as long as 1 hour thereafter. This additional oxygen is used to (1) reconvert the lactic acid that has accumulated during exercise back into glucose, (2) reconvert adenosine monophosphate and ADP to ATP, (3) reconvert creatine and phosphate to phosphocreatine, (4) re-establish normal concentrations of oxygen bound with hemoglobin and myoglobin, and (5) raise the concentration of oxygen in the lungs to its normal level. This extra consumption of oxygen after exercise is called repaying the oxygen debt .
The principle of oxygen debt is discussed further in Chapter 85 in relation to sports physiology. The ability of a person to build up an oxygen debt is especially important in many types of athletics.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here