Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thoracic aortic pathology is one of the leading causes of death in the world in those over the age of 65 and in the United States accounts for more than 11,000 deaths annually. Thoracic aortic aneurysms (TAAs) occur less frequently than abdominal aortic aneurysms. The estimated incidence ranges from 5.6 to 10.4 cases per 100,000. The incidence is difficult to discern because TAAs are relatively silent, and sudden fatalities are more often associated with alternative diagnoses. The prevalence of asymptomatic TAA, based on population studies, ranges between 0.16% and 0.34%. With the aging population combined with advances in imaging technology, there has been a steady increase in the number of patients diagnosed with TAA. Epidemiologic data suggest an increasing incidence of TAAs, which may be related to the aging population, improved educational awareness of the general public and primary care pracitioners. The natural history of TAAs is not well characterized, although it has been shown that approximately 70% of patients who forgo treatment will progress to rupture, with a fatality rate approaching 90%.
Traditionally open surgical repair of TAAs was performed with or without distal aortic perfusion. These procedures carry significant morbidity and mortality, associated with the need for thoracotomy, single-lung ventilation, aortic cross clamping, and prolonged visceral or renal ischemia. Reported mortality rates of open TAA repair at centers of excellence range from 3% to 8% and are associated with paraplegia rates of 3% to 5%. The first endovascular exclusion of a thoracic aorta was reported by Dake in 1994. The US Food and Drug Administration (FDA) approved the first thoracic endograft in March 2005. after a nonrandomized trial showed that thoracic endovascular repair (TEVAR) compared favorably with the standard open surgical repair. Since then, there has been a paradigm shift in the treatment of descending thoracic aneurysms (DTAs), with most patients preferentially offered TEVAR, thus expanding the pool of patients that could undergo treatment. Despite the lack of randomized trial data, TEVAR has become the preferred method of treatment for all patients with DTAs who meet the anatomic criteria. A recent Cochrane Review of thoracic endograft use for the treatment of TAAs determined that there was a paucity of randomized control studies, but limited midterm data suggested clinical equipoise between TEVAR and open repair. However, numerous nonrandomized studies have shown that TEVAR is effective and durable in the short term, which may justify the paradigm shift.
The decision to repair a DTA depends on multiple factors; aortic diameter, anatomic location, expansion rate, underlying pathology, associated symptoms, and the patient's overall medical condition. The Society for Thoracic Surgeons Endovascular Task Force, in the absence of high-quality data, has recognized that thoracic aortic pathology is increasingly being treated with TEVAR. The decision to treat a TAA with TEVAR or open repair must be made with the same rigor. Societal guidelines emphasize an individualized approach. Generally open repair of an asymptomatic TAA should be considered at a diameter greater than 5.5 to 6.0 cm and if the risk of rupture exceeds the risks associated with endovascular repair. Saccular, symptomatic aneurysms, those associated with connective tissue disorders, and select acute aortic emergencies may require repair at a smaller diameter. For genetically mediated conditions, a diameter greater than 5.0 cm is suggested. In addition, aneurysms that grow rapidly (>1.0 cm per year, or >0.5 cm in 6 months) should be considered for early repair. Guidelines propose that TEVAR is beneficial for the treatment of a variety of thoracic pathology; acute type B dissection, blunt thoracic aortic injury, penetrating aortic ulcerations, embolizing aortic thrombus, and intramural hematomas ( Box 37.1 ). This chapter is limited to the treatment of TAAs.
Thoracic aortic aneurysm diameter >5.5 to 6.0 cm
Aneurysm growth >1.0 cm per year
Aneurysm growth >0.5 cm in 6 months
Blunt traumatic aortic injury
Complicated or uncomplicated type B dissection
Embolizing aortic wall thrombus
Intramural hematoma
Large penetrating aortic ulcers
Symptomatic descending thoracic aneurysm
Ruptured descending thoracic aneurysm
Anatomic considerations including aneurysm extent, availability of suitable landing zones and nature of the underlying pathology dictate repair options. The decision to proceed with TEVAR requires detailed imaging for procedural planning. Important anatomic characteristics, which require imaging assessment, include aortic arch anatomy, landing zones, aortic angulation, branch vessel location, and access vessel diameter and quality. Computed tomographic angiography (CTA) of the chest, abdomen, pelvis and femoral vessels has become the preferred imaging modality for the evaluation of aortic pathology and preoperative TEVAR planning. Multiplanar reconstructions in addition to three-dimensional modeling provide invaluable information regarding vessel sizes, angulation, length, and anatomic relationships as well as severity of calcification and presence of thrombus. The availability of advanced software such as Vitrea (Vital Images, Minnetonka, MN) and Aquarius (TeraRecon, San Mateo, CA) has empowered physicians, allowing them to determine accurate endograft sizing measurements and to perform predeployment planning. For patients who cannot undergo CTA due to contraindications for administration of intravenous iodinated contrast media, magnetic resonance angiography has been used successfully, although patients with renal failure are at risk for nephrogenic systemic fibrosis.
Accurate measurements of the proximal and distal seal zones and access vessel diameter are of critical importance in planning TEVAR. Thoracic aortic diameters vary according to gender, anatomic location, pathology, age, and body habitus. According to industry-developed instructions for use (IFU), most thoracic aortic endografts require 10% to 20% oversizing in relation to the native aortic diameter. Overzealous endograft sizing has been associated with device infolding, gutter formation, endoleaks, and accelerated aneurysmal degeneration. Undersized endografts can lead to device migration and/or endoleak formation. Aortic diameters are most accurately measured in an orthogonal plane to the centerline of flow. Distal landing zone diameters are often smaller than the proximal landing zone, requiring the use of tapered endografts to compensate for the size discrepancy. Lengths of aortic coverage can be difficult to assess and are underestimated by centerline measurements because endografts are often positioned along the outer curvature of the aortic wall. The natural tortuosity of the aorta coupled with the large cavity of an aneurysm and the tendency of the endograft to locate against the greater aortic curvature usually results in a longer length for graft coverage. These factors must be taken into consideration during endograft sizing.
The thoracic aortic diameter is typically larger than the abdominal aorta and requires larger devices and delivery systems. The largest devices are constrained in 24 to 26 French sheaths that require an iliac access greater than 8 mm for safe insertion and deployment. More recently, 46-mm low-profile Alpha thoracic stent-grafts (Cook Medical, Bloomington, IN) in 20 French sheaths have become available. Particular attention should be given to the proximal external iliac artery, as this area is prone to atherosclerotic narrowing and calcification. This is more important in women, who expectedly have smaller vessels. In situations where access is restricted, the common iliac artery can be directly accessed via a small retroperitoneal incision or an iliac conduit can be sewn in place. Conduit diameters measuring 10 mm are preferred and can be accessed via the retroperitoneal incision or tunneled to the femoral region to avoid angulation in large individuals. Occasionally this conduit serves as a bypass at the end of the procedure in cases of advanced external iliac artery occlusive disease. On rare occasions, the infrarenal aorta can be accessed at the time of exploratory laparotomy. The use of an internal endoconduit to address unfavorable iliac anatomy has been described. This technique requires the placement of covered stents or stent-grafts across the diseased iliac segment followed by aggressive balloon angioplasty for a controlled rupture that will facilitate insertion of the endograft. The Terumo Solopath Balloon Expandable Transfemoral Access Sheaths (Terumo Medical Corporation, Somerset, NJ) provides an alternative approach to managing difficult iliac artery access. The Solopath Sheath is a 14 to 21 French hydrophilic sheath with an external noncompliant radially expanding balloon that gently dilates diseased vessels. The balloon decompresses before sheath removal, thus reducing the risk of iliac artery trauma.
The tortuosity and high shear force in the thoracic aorta require a slightly longer seal zone than that employed in the infrarenal aneurysmal repair. A seal zone of at least 20 mm is recommended at each end of the endograft to prevent migration and minimize endoleak. The seal zones should be extended when endografts are placed in areas of severe angulation, conical aorta, calcified segments, or areas where mural thrombus is present. However, it is important to recognize the potential consequences of extending aortic endograft coverage, as the greater the number of excluded intercostal arteries and branch vessels impact the rate of paraplegia.
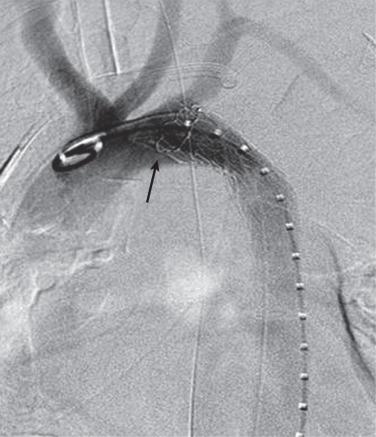
The proximal attachment zones are classified as zones 0 to 5 and the distal attachment zones as zones 5 to 11 ( Fig. 37.1 ). Each zone is divided by a tangential line along the distal aspect of each great vessel; zone 0 is proximal the innominate artery origin, zone 1 is distal to the innominate and proximal to left common carotid artery (LCCA), zone 2 is distal to the LCCA and proximal to the origin of the left subclavian artery (LSCA), zone 3 is less than 20 mm from the LSCA without covering it, and zone 4 is greater than 20 mm from the LSCA and above the T6 vertebral body. The location of the proximal landing zone depends on aneurysmal morphology; however, the ideal location in terms of anatomic accommodation of the endograft is in the straight aortic segments (zones 3 and 4). An important consideration for the proximal seal zone is the angle of the aortic arch. Endografts placed within the aortic arch (zones 1, 2, and 3), especially those with acute angles, may fail to oppose to the inner curvature of the aortic arch ( Fig. 37.2 ). These situations have occasionally resulted in untoward effects, such as device migration and proximal endograft collapse, mostly in nonaneurysmal applications. Recent endograft modifications such as the Zenith Pro-Form and Alpha (Cook, Bloomington, IN) and the C-TAG (W. L. Gore, Flagstaff, AZ) have been designed to address this problem.
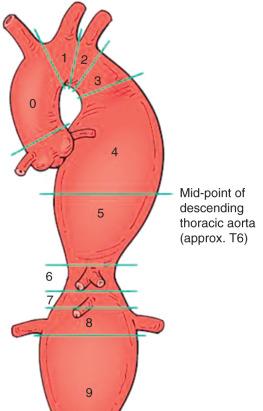
The distal attachment zones are classified as zones 5 to 11. Zone 5 starts in the distal half of the descending thoracic aorta but proximal to the celiac access, zone 6 extends from the celiac origin to the proximal aspect of the SMA, zone 7 is the SMA extending to the suprarenal aorta, zone 8 covers at least one renal artery, zone 9 covers the infrarenal aorta, zone 10 covers the common iliac artery, and zone 11 continues into the external iliac artery.
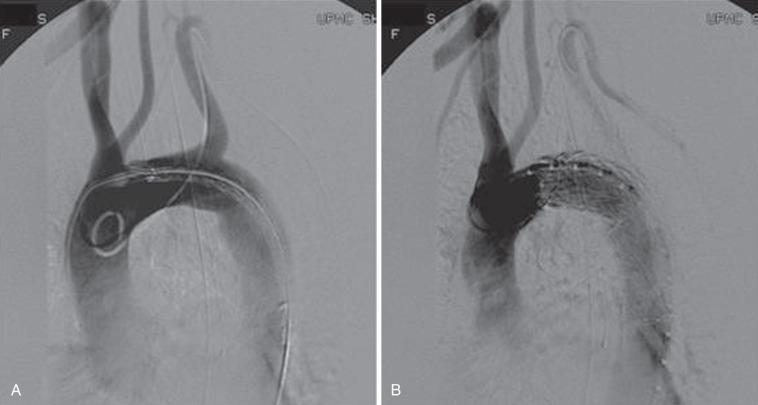
Current practice guidelines from the Society for Vascular Surgery (SVS) recommend preoperative left SCA revascularization in elective TEVAR cases. In approximately 20% of regulatory cases, adequate coverage required extending the endograft into zone 2, excluding the LSCA ( Fig. 37.3 ). The management strategy of the LSCA has evolved since the inception of TEVAR. Prophylactic revascularization of the LSCA with a carotid subclavian bypass or transposition is prescribed. The practice of LSCA coverage has come under severe scrutiny, with EUROSTAR data linking it to an increased risk of spinal cord ischemia (SCI) and brain stem strokes. Peterson and colleagues observed a 63% (5/8) stroke and upper extremity ischemic symptom rate in patients who had LSCA coverage without revascularization. A review of the literature disclosed a 23% complication rate with LSA coverage, compared with a 3% rate when flow to the LSCA was maintained. Additional reports of posterior circulation infarctions, arm ischemia, and SCI have reinforced the recommendations of preserving the LSCA. Intentional coverage without revascularization appears to be tolerated in some cases and can be performed in emergent settings in the presence of good collateral circulation. A review of 22 patients who underwent intentional coverage of the LSCA determined that almost 70% of patients remained asymptomatic, with 30% suffering mild symptoms of left arm claudication. Absolute contraindications to LSCA coverage include a dominant left vertebral artery, left internal mammary–to–coronary artery bypass, left arm hemodialysis access, aberrant right SCA, anomalous origin of the left SCA from the aortic arch, hypoplastic right vertebral artery, termination of the left vertebral artery in the posteroinferior cerebellar artery, and/or occluded internal iliac arteries. In addition, extended thoracic aortic coverage combined with prior abdominal aortic repair should be considered higher risk for SCI, and LSCA revascularization should be performed. Current SVS guidelines recommend routine preoperative revascularization in elective TEVAR and expectant management in acute settings, excluding those with absolute contraindications.
In order to precisely deploy an endograft at the level of the LSCA, it is often helpful to gain access to the aortic arch via the left brachial artery. This improves visualization of the junction between the subclavian and the aorta and allows for bailout stenting in the event of inadvertent coverage. Alternative techniques to maintain LSCA perfusion include endovascular debranching and in situ fenestration. Endovascular debranching with a chimney technique has been described for the left SCA and left common carotid artery. The goal for each is to extend the proximal landing zone and decrease operative morbidity. In situ fenestration was first described by McWilliams in 2004. The transluminal technique was performed using the back end of a stiff 0.018-inch guidewire and cutting balloons. Since that time, reports of in situ laser fenestration (Turbo Elite Laser Catheter, Spectranetics, Colorado Springs, CO) have surfaced. The laser is similarly inserted via brachial access, juxtaposed to the endograft, a short laser energy burst is applied, and the fenestration is dilated with standard noncompliant balloons. Early technical reports and midterm follow-up were both favorable.
The distal aortic landing zone places the endograft at least 2 cm cephalad to the celiac axis. When aneurysmal disease is more extensive, coverage of the celiac artery is an option, thus extending the distal seal zone. Visceral ischemia can occur, but reports suggest that adequate collateralization through the gastroduodenal and pancreaticoduodenal arcades minimize the physiologic insult. A recent review reported selective coverage of the celiac axis in 31 patients during TEVAR. Of these patients, two (6%) had bowel ischemia and five (16%) had an endoleak that required reintervention. A large review of worldwide data suggested a relatively low rate (6% to 8%) of foregut ischemia with celiac artery coverage and was associated with an overall mortality rate of 9%. These findings illustrate that celiac coverage is feasible in most cases, but the variable and unpredictable arcade of collaterals between the celiac and SMA require an individualized approach. Tailoring patient care based on an assessment of preexisting comorbidities, operative morbidity of celiac revascularization, and physician skill set are all critically important. Patients with a prominent gastroduodenal artery arcade, replaced right hepatic artery, preexisting celiac stenosis, and/or well-developed SMA appear to have the most favorable results after covering the celiac origin. Open surgical or endovascular revascularization can be performed, if needed, with a relatively low morbidity. In the elective situations, open celiac debranching should be performed prior to TEVAR. Inflow may originate from a nondiseased segment of the infrarenal aorta or iliac artery. An end-to-end celiac artery anastomosis or end-to-side hepatic artery bypass should be considered. In the latter situation, ligation of the celiac origin should be performed to prevent a type II endoleak. Endovascular techniques are dictated by aneurysmal extent and anatomy, including parallel grafts (chimney, snorkel, sandwich, or periscope) and fenestrated or branched stent-grafts. Custom-made branch endografts are not FDA-approved for use in the United States.
Since FDA approval of the first TEVAR device in 2005, there has been a rapid increase in the number of stent-grafts available, with new modifications and designs along the way. In the past 2 decades, original TEVAR devices have undergone several rounds of modification: improved device materials, reduction of delivery size, advancement of device conformability, creation of longer lengths and tapered configurations, and development of larger diameter grafts. These developments have established TEVAR as the standard of care for most thoracic aortic pathology and increased the indications for use.
The Conformable Gore TAG thoracic endoprosthesis (W.L. Gore, Flagstaff, AZ), the most recent advancement of the first FDA-approved endograft for the treatment of DTA ( Fig. 37.4 ), has been the most widely implanted device in both on- and off-label use in the United States. The C-TAG endograft is an expanded polytetrafluoroethylene (ePTFE) tubular graft reinforced with a layer of fluorinated ethylene propylene (FEP) and externally supported by Nitinol self-expanding stents. This endograft has lower porosity, which helps to avoid the sac enlargement associated with the earlier version of the device. The FEP wrap has replaced the longitudinal deployment wire of the original device. Effective proximal and distal seals are achieved with flared edges of the graft as well as an additional ePTFE cuff. The graft has an inlaid gold band that marks the proximal and distal edges. The C-TAG device has been modified to increase conformability, especially on the lesser curvature of the aortic arch, by modifying the wire form's strength and pattern. The proximal covered scallops have also been replaced by short open bare metal stents ranging in length from 3 to 6.5 mm.

The C-TAG device is constrained in a sleeve of ePTFE/FEP film that is bound by a longitudinal seam running the entire length of the device. The device is deployed by pulling the string, which unzips the seam deploying the endograft from the middle to both ends. The device is available in diameters of 21 to 45 mm and lengths of 10, 15, or 20 cm. Two tapered devices (26 to 21 mm and 31 to 26 mm) are available and provide added flexibility in sizing. The device is delivered through an appropriately sized sheath with a pressurized hemostatic valve to minimize blood loss, these range in size from 18, 20, 22, or 24 French. The C-TAG device is designed to be oversized by 7% to 22%, incorporated in the IFU device sizing guide.
Advantages of the C-TAG device include a simple deployment mechanism and flexibility, which allows easy accommodation of tortuous anatomy. The endograft deploys from the middle of the graft out toward both ends. This design theoretically avoids the “wind sock” effect, which can occur if the proximal end is released first. Once the graft is deployed, the seal zones are ballooned with a trilobed balloon.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here