Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Technological developments in hysteroscopy and laparoscopy favor a smaller footprint to facilitate ambulatory procedures, with a trend toward smaller-caliber endoscopes and instruments and creative specimen removal with attention to safety.
Indications for hysteroscopy include removal of endometrial polyps or submucous myomas, retained intrauterine devices or Essure coils, the diagnosis and treatment of intrauterine adhesions, and resection of a uterine septum or cesarean niche. Hysteroscopy can serve as a diagnostic examination in the setting of recurrent pregnancy loss, infertility, myomectomy planning, and persistent abnormal uterine bleeding with inability to rule out endometrial pathologic conditions by ultrasound/blind biopsy.
Indications for gynecologic laparoscopy include hysterectomy, myomectomy, treatment of endometriosis, removal of ectopic pregnancy, removal of adnexal pathologic tissue, evaluation of pelvic pain with treatment of any pathologic condition amenable to conservative surgery in a person interested in future childbearing, risk-reducing adnexal surgery, sterilization, appendectomy, drainage of pelvic/intraabdominal abscess, release of pelvic/intraabdominal adhesions, repair of cesarean section niche and congenital anomalies, and oncologic surgical staging.
An appropriately placed paracervical block with local anesthetic to affect the hypogastric nerves in close proximity to the uterosacral ligaments is more effective than topical or intracervical anesthesia for hysteroscopy. A longer-acting paracervical block can be included for postoperative pain management with hysterectomy or myomectomy and in endometriosis surgery as part of an enhanced recovery after surgery protocol. Local instillation of anesthetic at the laparoscopic port sites at the beginning or conclusion of surgery is also beneficial to the patient.
Vaginoscopic technique, minimizing speculum usage, avoidance of a tenaculum, use of a small-caliber hysteroscope, and short operating time correlate with patient comfort during hysteroscopy. Mechanical morcellation is increasingly being used to remove intrauterine pathologic tissue, and operative times are shorter compared with resectoscopic procedures. Interestingly, the use of either energy modality for even a simple polyp removal has become more commonplace, potentially increasing cost in the office environment.
Saline is the standard as a hysteroscopic distention medium, with an allowable fluid deficit of up to 2.5 L. Electrolyte disturbance and complications of fluid overload can occur at less than that deficit level, so be diligent about hysteroscopic fluid management.
Complications of hysteroscopy include uterine perforation with risk of injury to the surrounding vascular and visceral structures, pelvic infection, bleeding, and overabsorption of the distending media.
Complications specific to laparoscopy include trocar entry injuries to blood vessels or internal organs, port site hernias, hypercapnia from absorption of carbon dioxide, and ventilatory compromise from the compressive effects of the pneumoperitoneum and/or steep Trendelenburg position.
The primary laparoscopic trocar placement can cause up to 50% of vascular and bowel injuries in gynecologic laparoscopy. It is important for the laparoscopic surgeon to strategically plan for the where and the how of initial trocar placement and be familiar with the three entry techniques.
Laparoscopic surgery is usually multiport, with a trend to small-diameter instruments. Single-site surgery has a learning curve and can be done through an umbilical or vaginal approach; the robotic platform has both single-site and multiport options. Any surgery, of course, can be a hybrid of different approaches. Examples of this include hand-assist surgery and laparoscopic-assisted vaginal surgery.
Thermal or mechanical bowel or urinary tract injuries can occur in laparoscopic surgery and might not be recognized intraoperatively. Sequelae from a delay in diagnosis can have serious consequences; diligent teamwork is required for optimal postoperative care. Cystoscopy can provide reassurance about bladder integrity and ureteral patency but not necessarily show a thermal injury.
As a risk-reducing measure to prevent future malignant transformation to ovarian carcinoma, bilateral salpingectomy should be offered to all women desiring sterilization and to all women undergoing hysterectomy. If the ovaries are preserved, care should be taken to remove the entire fimbria because this is the site of greatest risk for malignant transformation, and to avoid disruption of the ovarian blood supply from the infundibulopelvic ligament.
Safe specimen removal in laparoscopic surgery requires mindfulness and careful preoperative review with the patient regarding options. Specimen extraction using nonpower morcellation is often done from within a bag from a vaginal approach; alternatives include extension of an abdominal port, usually midline at a suprapubic incision or the umbilicus.
Simulation training in endoscopic surgery improves surgical skills and can results in improved “muscle memory” with regard to the handling of laparoscopic and hysteroscopic equipment, dissection techniques, suturing, and knot tying.
Accompanying video for this chapter is available on ExpertConsult.com .
Endoscopic procedures such as laparoscopy and hysteroscopy are performed with a telescope and a light source, which when linked to a camera and video monitor provide for visualization and documentation of internal structures. Laparoscopy refers to viewing the peritoneal cavity, including the pelvis, and hysteroscopy refers to the uterine cavity. Endoscopy is an integral component of minimally invasive gynecologic surgery, providing an important mode of access for the diagnosis and operative management of many gynecologic conditions.
The traditional hysteroscopic access to the uterine cavity and abdominal multiport laparoscopic approach to the peritoneal cavity are the backbone of a growing array of access modalities, instrumentation, and operative procedures in gynecologic surgery. These include the diagnosis and management of the following:
Benign and malignant neoplasms
Acquired and congenital anomalies
Endometriosis
Pelvic infection and adhesive disease
Infertility and pregnancy complications
Pelvic pain
Abnormal bleeding
Removal of adnexal structures
Pelvic prolapse
Myomectomy
Hysterectomy
Gynecologists also perform cystoscopy during laparoscopic and urogynecologic procedures to evaluate the bladder and visualize the ureteral orifices, to examine for intraoperative injury and inadvertent needle or mesh placement, and to assess for invasive pathologic conditions such as deep infiltrative endometriosis, mesh erosion, or metastatic gynecologic cancer affecting the urinary tract . Retroperitoneal placement of an endoscope allows for extraperitoneal lymph node dissection in oncologic surgery. Robotic assistance facilitates complex laparoscopic surgery such as oncologic staging and surgery in patients who are morbidly obese. With Vnotes (vaginal natural orifice transluminal endoscopic surgery), the surgeon uses the vagina as an access point for the endoscope, building on techniques used in single-site transumbilical laparoscopic surgery and using the vagina as a portal to the abdominal cavity instead of the umbilicus.
Endoscopy is a cornerstone of minimally invasive gynecologic surgery (MIGS) and has advanced dramatically since the pioneering work of Raoul Palmer and Kurt Semm in the 1960s and 1970s ( ; ). The emergence of educationally focused endoscopic organizations across the globe, enhanced collaboration between surgical specialties, practice focus on MIGS, and the establishment of MIGS fellowship programs and advanced training programs have positively affected the depth, breadth, and quality of research and technical advances in gynecologic endoscopy. The integration of MIGS into the fields of benign gynecology, gynecologic oncology, and urogynecology has served to move minimally invasive surgery into the mainstream as a standard of care for the majority of gynecologic surgical procedures. This chapter is an evidence-based overview of gynecologic endoscopy, intended to encourage skilled, cost-effective, appropriate endoscopic care, with an aim to optimize gynecologic outcomes.
Contemporary hysteroscopy and laparoscopic procedures are less often preoperatively placed in “diagnostic” or “operative” categories and more typically done as “see and treat” interventions, in part because of what can be accomplished during the preoperative workup and in part because of the expanded smorgasbord of what can be accomplished with the hysteroscope and laparoscope. The patient who presents with abnormal uterine bleeding, pelvic pain, a pelvic mass, or signs of a pelvic infection will have a thorough history and physical examination (H&P), appropriate laboratory studies, and almost universally some type of imaging study to inform the clinical decision-making process before surgery. An attentive, thorough H&P is paramount in the preoperative workup. This will guide decision making regarding which laboratory tests to order, be it a pregnancy test, blood count, thyroid study, chemistries, chlamydia and gonorrhea, or tumor markers. It will help determine which imaging tests are needed. The optimal preoperative evaluation incorporates a patient-centered approach to decision making. Careful attention to preoperative discovery helps guide the appropriate surgical intervention because operative findings can then be anticipated and the patient’s preferences are understood.
Surgical readiness is facilitated by preoperative imaging, which almost universally involves a sonographic study . Gynecologic ultrasound is a cost-effective, noninvasive, and accessible imaging technique that can help elucidate the nature and extent of pelvic disease ( ). Ultrasound is easily integrated into the process of gynecologic investigation and preoperative planning; for example, a transabdominal “visceral slide-test” can yield information about the likelihood of adhesive disease at the site of planned trocar placement, decreasing procedure-related complications and increasing patient safety ( ). The observer-dependent nuances of an ultrasound examination in real time can be informative for the gynecologic surgeon, assessing the presence and nature of uterine or adnexal pathologic changes, elucidating the likelihood of infiltrative endometriosis or pelvic adhesions, and helping to clarify what surgical options are reasonable or contraindicated. When ultrasound is performed as a point-of-care study, the patient becomes an integral part of the ongoing discussion of findings, and this enhances the process of shared decision making.
Minimally invasive surgical options for definitive diagnosis with the twin goal of symptom relief can be offered to the patient, guided by ultrasound findings and concomitant physical examination. Uterine fibroids might be visualized within the myometrium by ultrasound in a perimenopausal woman with heavy and painful menses, or the clinician might surmise that adenomyosis has infiltrated throughout the myometrium by the diffuse inhomogenous echotexture and thickened uterine wall apparent on transvaginal ultrasound. A classic ovarian endometrioma or an ovarian dermoid cyst might be visualized sonographically in the setting of a palpable pelvic mass. Should the ultrasound appearance of the adnexa be suspicious for an ovarian malignancy, prompt referral to a gynecologic oncologist can be arranged.
In the setting of abnormal bleeding, a thin endometrial lining of normal contour in a postmenopausal patient or in the early follicular phase of a premenopausal patient by ultrasound essentially rules out intracavitary pathologic conditions ( ). It is acceptable then to forego a potentially painful endometrial biopsy in such a patient and concentrate on causative factors for the abnormal bleeding other than endometrial pathologic conditions in the initial workup. An endometrial polyp or submucosal fibroid can be identified by a properly timed ultrasound as a mass within the uterine cavity and is often missed by an endometrial biopsy ( ). With ultrasound as first-line triage in the setting of abnormal uterine bleeding or infertility, when an endometrial mass is visualized, it is not unreasonable for hysteroscopy to be the next step, as the only invasive intervention for diagnosis and treatment of what is likely an endometrial polyp or myoma.
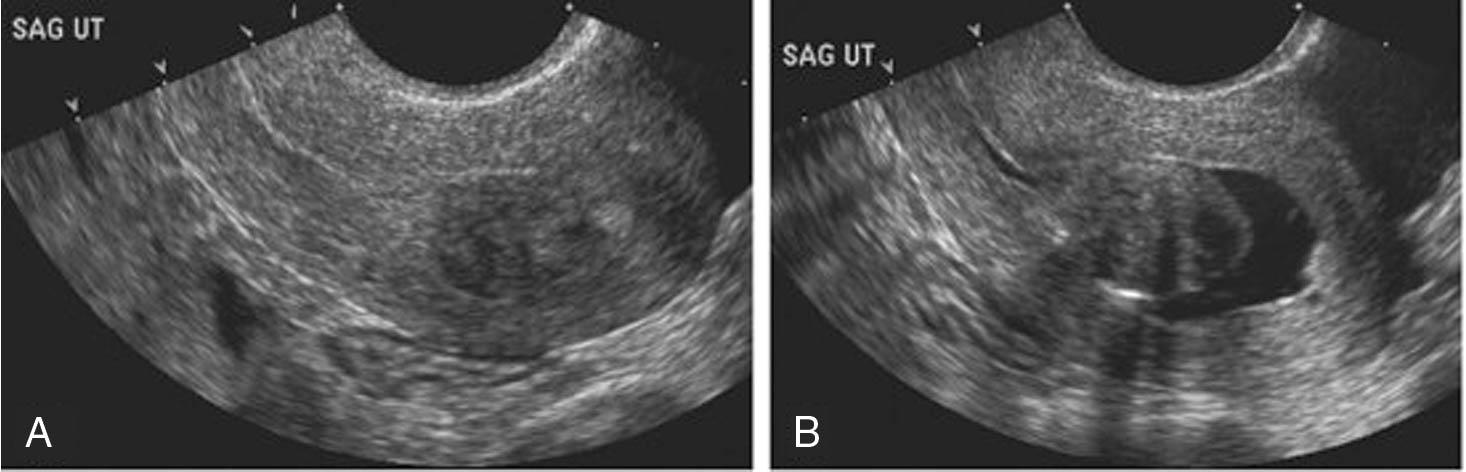
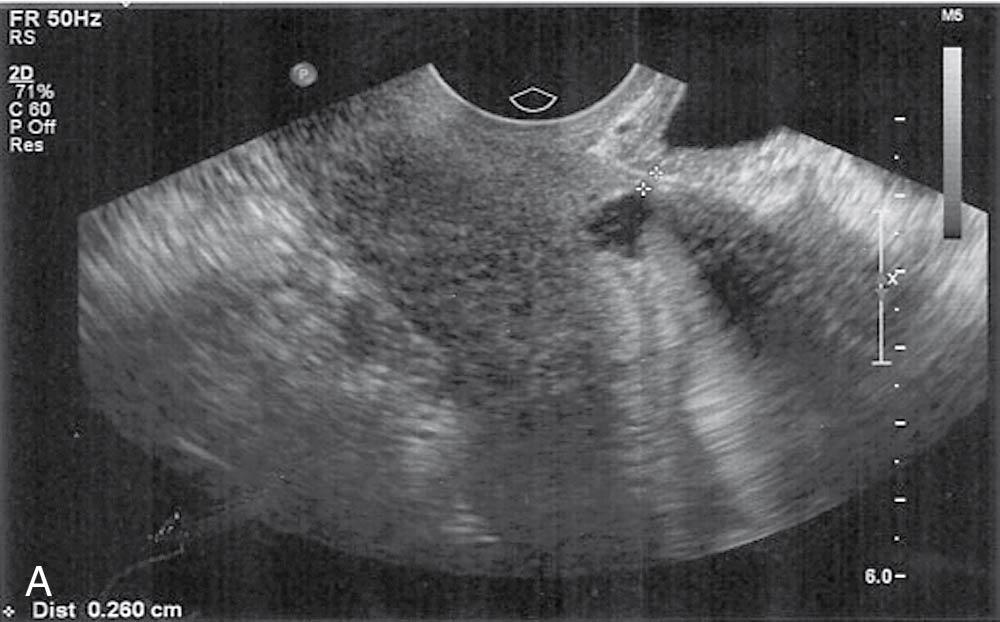
Adjuncts to the standard gray-scale ultrasound evaluation include Doppler flow to evaluate lesion vascularity in the uterus or adnexa, informing the gynecologist about the likelihood of an endometrial polyp versus intracavitary fibroid versus a mere blood clot and providing information indicating the likelihood of a benign versus malignant process in the adnexa. Sonohysterography involves placing a small catheter to instill either saline or gel into the uterine cavity under ultrasound guidance and can help rule out the presence of an endometrial mass in the setting of a relatively thickened endometrial appearance when no discrete mass is seen by standard ultrasound . If no mass is apparent, an endometrial biopsy could suffice for endometrial diagnosis and hysteroscopy deferred. Sonohysterography has been shown to be comparable to hysteroscopy in delineating the presence of intrauterine pathologic conditions such as polyp or submucosal fibroid formation ( Fig. 10.1 ), retained products of conception, a cesarean section scar defect, or uterine septum ( ). Three-dimensional (3-D) ultrasound is an excellent tool to ascertain the presence of uterine anomalies or an intrauterine device (IUD) location and can be used in lieu of magnetic resonance imaging (MRI) to delineate fibroids before a complex laparoscopic myomectomy; 3-D ultrasound with gel instillation has been referred to as “virtual hysteroscopy” and can be helpful as an adjunct to ultrasound for preoperative planning before a more complicated hysteroscopic myomectomy.
Preoperative MRI imaging is helpful in selective patients to assess a fibroid uterus for signs of adenomyosis or sarcoma or map fibroids before a laparoscopic or hysteroscopic myomectomy procedure . Computed tomography (CT) is less helpful in the pelvis for elucidating gynecologic pathologic conditions but can be informative with delineation of the urinary tract and gastrointestinal systems and in investigating pelvic abscess formation. Fluorescence studies are increasingly helpful intraoperatively in delineation of disease. In addition, intraoperative transabdominal or laparoscopic ultrasound can provide crucial information to guide surgical interventions.
Hysteroscopy is the direct visualization of the endometrial cavity and its contents via the cervix using an endoscope and a light source . Technological developments with a trend toward smaller-caliber hysteroscopes have enabled more office-based procedures and facilitated operating room procedures with intravenous (IV) sedation rather than general anesthesia.
Endometrial polyps are a common cause of abnormal uterine bleeding, found in up to 35% of patients with postmenopausal bleeding ( ) and not infrequently in women on tamoxifen. These usually benign endometrial growths can cause abnormal bleeding and subfertility in premenopausal woman. Submucosal fibroids within the endometrial cavity can cause profoundly heavy uterine bleeding and anemia in premenopausal women and can contribute to infertility and pregnancy loss, as well as IUD expulsion. Because both endometrial polyps ( Fig. 10.2 ) and submucosal fibroids are often missed by a blind endometrial biopsy, ultrasound is an evidence-based initial step in the investigation of abnormal uterine bleeding in both reproductive-age and postmenopausal patients, with hysteroscopic evaluation the gold standard for diagnosis and treatment of these intracavitary lesions. Indications for hysteroscopy include diagnosis and treatment in patients with abnormal bleeding and the finding of an endometrial mass on ultrasound or sonohysterography. In patients with nondiagnostic imaging, endometrial biopsy can be performed in lieu of or at the time of hysteroscopy to rule out endometrial carcinoma or precancerous endometrial pathologic conditions.
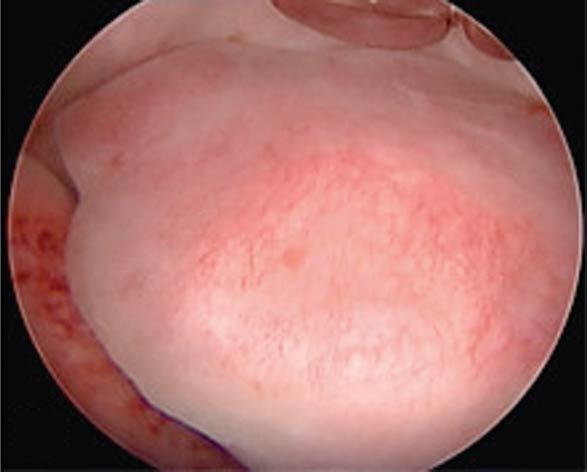
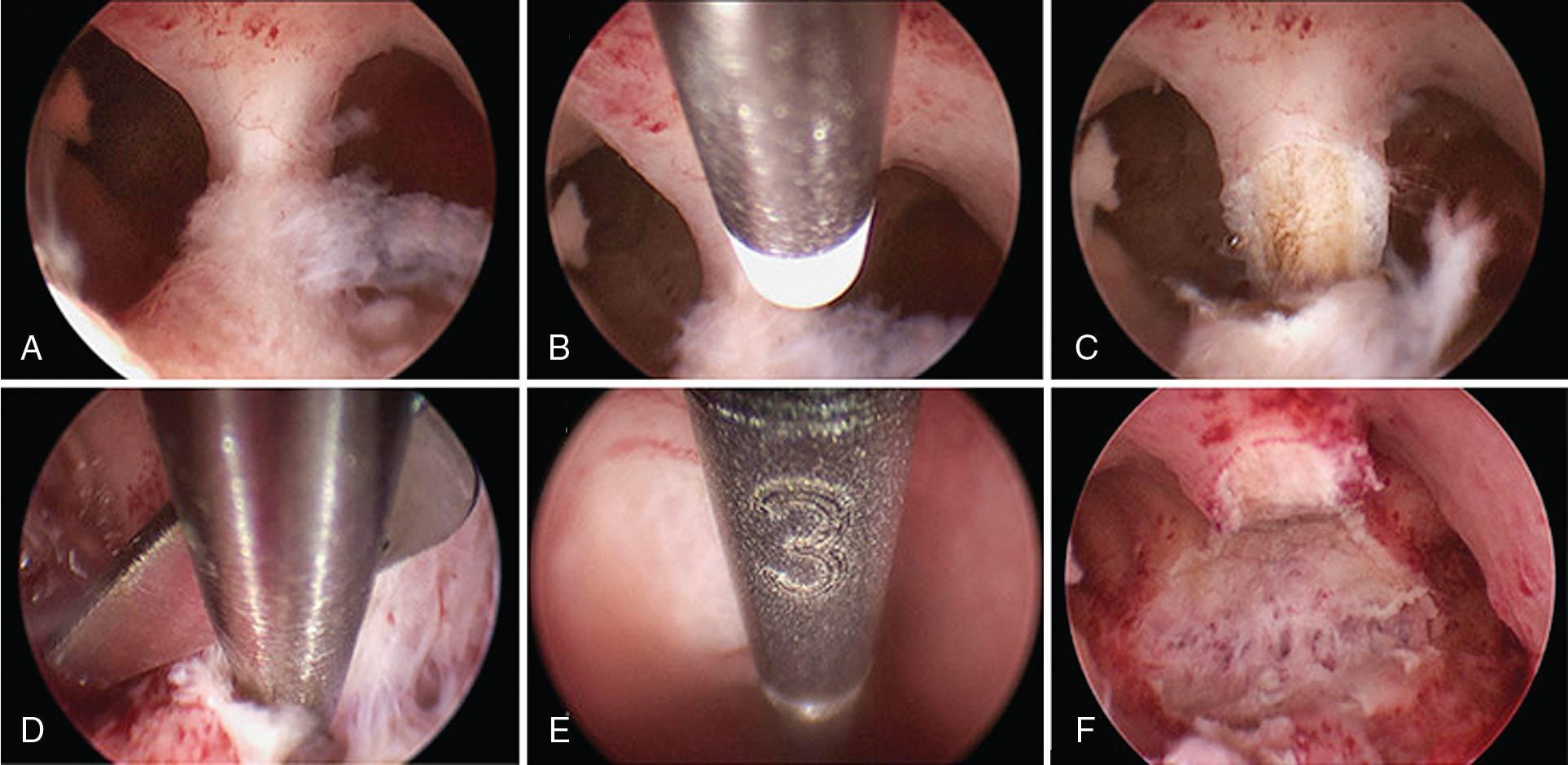
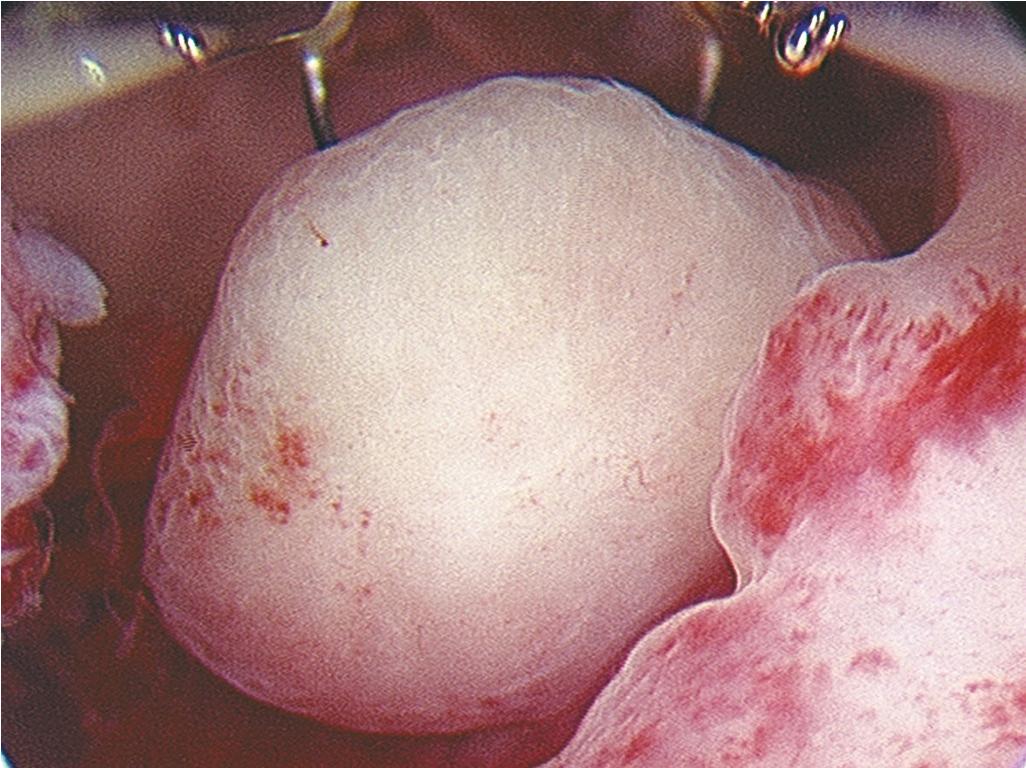
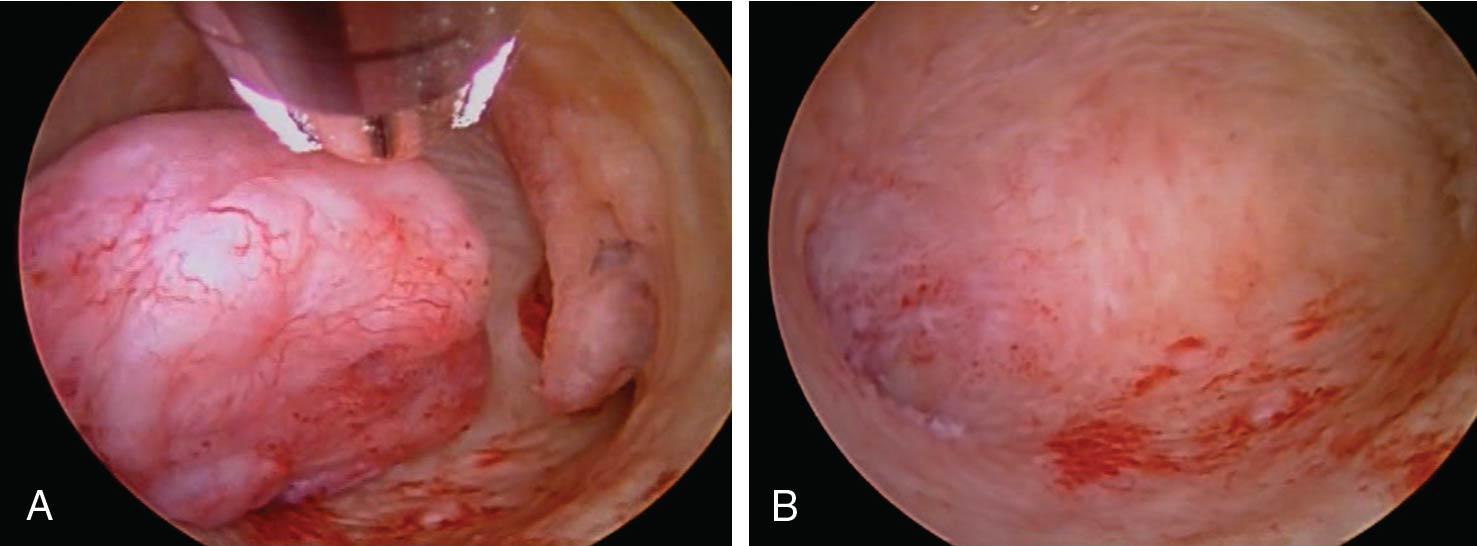
Intrauterine adhesions are usually caused by prior uterine instrumentation and can present with amenorrhea and infertility as a condition called Asherman syndrome ( ). This agglutination is best diagnosed by hysteroscopy and can be treated with scissors or instrumental dissection under hysteroscopic visualization. Another indication for hysteroscopy is the management of a retained or impacted intrauterine device, which can be removed under direct visualization with a hysteroscopic 5-French grasper. Uterine anomalies such as septal defects ( Fig. 10.3 ) can be confirmed hysteroscopically and resected with a small 5-French bipolar tip or hysteroscopic scissors; other energy sources can also be used, with care taken not to damage the endometrium. Retained products of conception remote from delivery or after a pregnancy loss might be amenable to cold-loop resection or mechanical morcellation under hysteroscopic guidance ( Fig. 10.4 ). A cesarean scar defect can be evaluated hysteroscopically concomitant with laparoscopic surgical correction or, if the patient is not interested in a future pregnancy and abnormal bleeding is a problem, treated with hysteroscopic resection under ultrasound guidance ( Fig. 10.5 ).
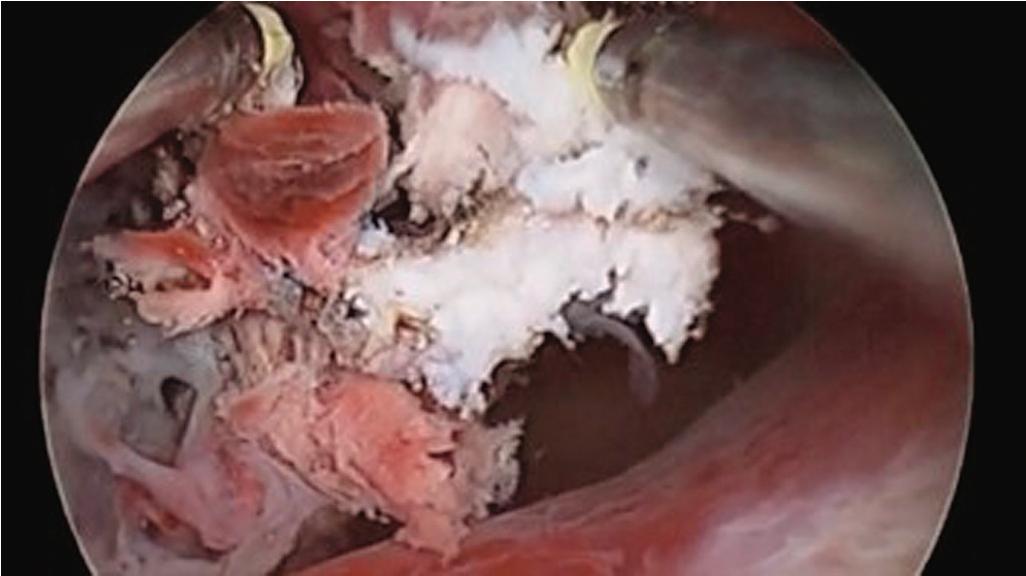
Hysteroscopic sterilization is anticipated to again be an option in the future; its global use from 2002 to 2018 is now historical because there is no longer a device on the market for blockage of the proximal aspect of the fallopian tubes by hysteroscopic means. Hysteroscopy can be used to remove Essure implants when a significant component of a retained sterilization device is present in the uterine cavity; familiarity with the implant is necessary to ensure the entire coil set is extracted.
Indications for hysteroscopy are listed in Box 10.1 . Relative contraindications for hysteroscopy include pregnancy and genital tract infections, including active herpes.
Endometrial diagnosis (abnormal uterine bleeding, recurrent pregnancy loss)
Endocervical and endometrial polypectomy
Hysteroscopic myomectomy
Lysis of intrauterine adhesions
Uterine septum repair
Assessment and possible resection of cesarean scar defect
Removal of foreign body, such as retained intrauterine device or intracavitary Essure implant
Removal of retained products of conception
Recurrent pregnancy loss
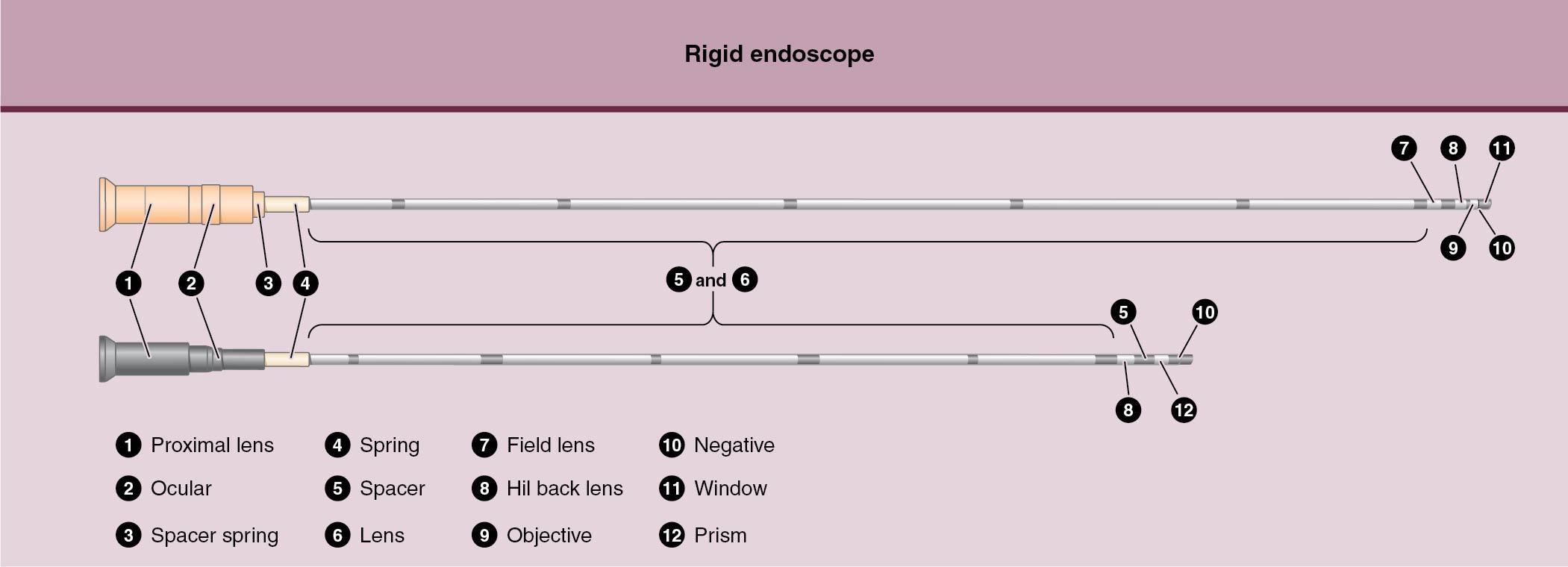
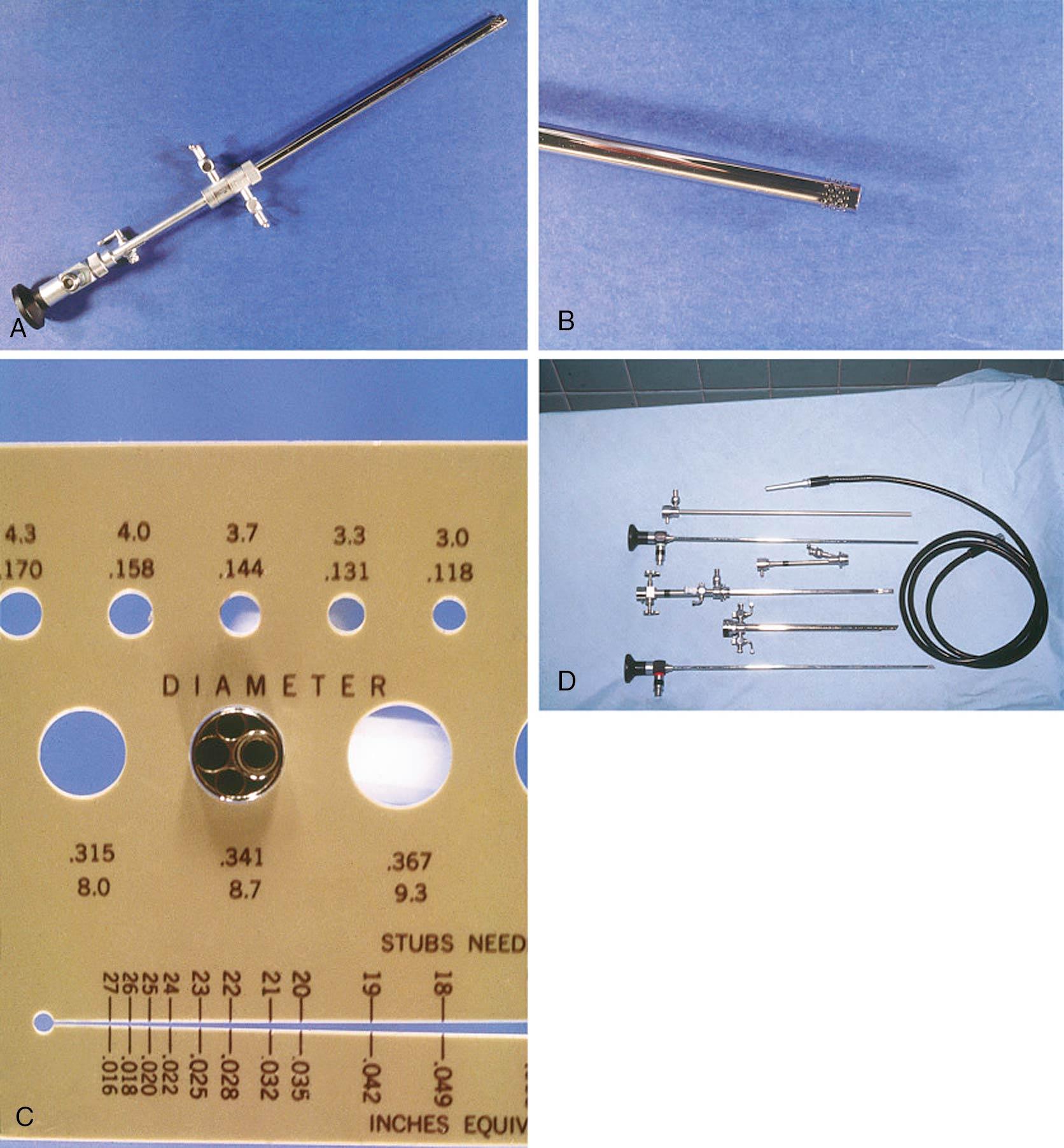
A hysteroscope can be rigid ( Fig. 10.6 ) or flexible, and the tip diameter can range in size from approximately 3 to 9 mm. The wider the tip of the hysteroscope, the greater the potential for patient discomfort and cervical trauma because progressive cervical dilation will likely be required for the larger hysteroscope to be advanced through the endocervical canal and into the endometrial cavity. The tip of a rigid hysteroscope can be 0 degrees or angled, depending on the operator’s preference; l2- to 30-degree lenses are most often used. A light source is required, and a camera with attached video monitor is usually included. The reimbursement profile for hysteroscopy has shifted to significantly favor office-based procedures, supporting the capital investment in equipment. One obstacle to office-based hysteroscopy can be the reprocessing of instruments, but this challenge is not insurmountable. A case-by-case expenditure is an alternative with disposable setups and hysteroscopes, although these are not as versatile as most reusable systems. Future trends in instrumentation will likely include reposable, hybrid systems with LED and Bluetooth mechanisms.
Hysteroscopy requires a mechanism for intrauterine distention to facilitate visualization . Most hysteroscopes have a mechanism for continuous flow of input and output fluid to optimize visualization within the uterine cavity. Isotonic solutions are considered the safest option, as opposed to electrolyte-free hypotonic solutions, which carry a higher risk of potentially life-threatening hyponatremia in association with excess fluid absorption. Saline has become the distention medium of choice for both office and operating room hysteroscopy.
Saline is an isotonic electrolyte solution that can be used with bipolar electrosurgical energy, laser energy, and mechanical interventions. Hypotonic electrolyte-free fluids are compatible with monopolar electrosurgical platforms and can also be used with mechanical instruments or laser . Carbon dioxide gas has had a role as a distention medium in diagnostic hysteroscopy but has the potential for gas embolism and is not conducive to operative interventions because visualization can be limited.
The continuous inflow of the distention solution to optimize visualization can have implications on patient hemodynamics. Fluid overload and the resultant altered physiology can cause life-threatening hyponatremia, cerebral edema, and congestive heart failure; therefore a team awareness of fluid balance with hysteroscopy is crucial. The lower safety profile of hypotonic fluids relative to isotonic solutions such as physiologic saline is due to the greater risk of hyponatremia and fluid overload per quantity absorbed. Because of well-documented complications that can arise from excessive hysteroscopic fluid absorption with any medium, an important safety measure with any hysteroscopic procedure includes judicious measurement of the fluid deficit, the difference between fluid in and fluid out.
Guidelines mandate discontinuation of a hysteroscopic procedure before a fluid deficit of 1 L with a hypotonic solution such as glycine or mannitol. Guidelines allow for a fluid deficit of 2.5 L with isotonic fluids such as saline ( ). Fluid management systems are available to provide a more precise measurement of fluid input and output and to heighten safety when high fluid volumes are anticipated. Certain fluid management systems allow for adjustment of flow pressures to optimize visualization. For more simple office-based procedures, fluid overload can be avoided by limiting from the onset the amount of fluid to be infused to an amount under the deficit guidelines.
Hysteroscopes have an operative port through which graspers, scissors, tenacula, or bipolar tips can be placed ( Fig. 10.7 ). These working instruments are usually 5 French in diameter, fit easily into a standard 5-mm hysteroscope, and can release, incise, grasp, or biopsy endometrial pathologic tissue under direct visualization. The polyp, small leiomyoma, or biopsy specimen can often then be extracted along with the hysteroscope. Endometrial polyp forceps and specialized myoma forceps can be used to gently grasp and remove any endometrial pathologic tissue inadvertently released within the uterine cavity, and ultrasound guidance can be used to assist with this process if desired. This simple technique works well with endometrial polyps and can also be used with fragments of myomas from resectoscopic surgery. These small 5-French instruments can also be used through an operative port to remove a retained IUD or Essure coil and to treat intrauterine adhesions or an intrauterine septum.
Specialized hysteroscopes are available that can incorporate an energy-based modality for operative intervention under direct visualization. These options for intracavitary treatment include the electrosurgical resectoscope ( Fig. 10.8 ) and the mechanical morcellator ( Fig. 10.9 ), and they are especially helpful with fibroid removal and also with larger polyps. Advanced technology has resulted in ever smaller prototypes of these hysteroscopes, essentially the equivalent of a 5-mm office hysteroscope, and so these procedures as well are being moved into the office setting. For challenging cases, however, such as those requiring significant dilation of a stenotic cervix or for which a large-bore hysteroscope is needed, IV sedation in the operating room setting might be imperative.
The office or clinic is a cost-effective venue for many hysteroscopic procedures, efficient for the gynecologist, and convenient for the patient ( Box 10.2 ). A meta-analysis comparing office hysteroscopy with operating room hysteroscopy showed no significant difference in adverse events or treatment success ( ). Office hysteroscopy was substantially less expensive in all randomized trials compared with the operating room counterpart. There was greater reported postoperative pain associated with the office venue, however, and the authors’ conclusion was that a shift to an office venue should be “thoughtfully considered.” Effective strategies can be employed to enhance patient comfort ( Box 10.3 ).
Hysteroscope of choice, with attendant light source and monitor
Saline solution, inflow/outflow tubing
Under-buttocks drape and blue pads
Accounting system for fluid management
Open-sided speculum, as thin as possible to reduce patient discomfort
Ring forceps with sterile gauze or cotton balls
Chlorhexidine 4% (off label) or povidone-iodine scrub
Paracervical block equipment
22-gauge spinal needle
Control syringe
Anesthetic solution
Tenaculum
Microdilators and standard cervical dilators on standby
Hysteroscopic grasping forceps preloaded into the scope
Scissors and tenaculum on standby
Endometrial polyp forceps
Energy devices, as deemed appropriate
Emergency kit for vagal response, Foley catheter for bleeding
Administer preprocedure analgesic, such as ibuprofen.
Ensure the patient is in a nonfasting state.
Use a small-diameter hysteroscope.
Know the uterine axis.
Use the vaginoscopic “no-touch” technique.
Paracervical block can be performed with a longer-acting agent such as chloroprocaine.
Minimize speculum use.
Use chlorhexidine as a cleansing solution.
Avoid tenaculum if possible; use anesthetic at the site if needed.
Minimize cervical dilation. Use the scope to advance under vision.
Dilate the distal cervix if stenosis is encountered. Place the scope into the cervical opening and attempt to use the scope to advance through the endocervix under vision with fluid running to decrease the risk of perforation.
Preoperative nonsteroidal medication has been shown to decrease postoperative discomfort after office hysteroscopy, and can be taken by the patient in advance of the appointment. Ingestion of a light meal is encouraged because it might also ward off any tendency to a vasovagal reaction and can help decrease any NSAID-associated gastrointestinal upset. Engagement with the patient is helpful, and this can include live observation of the procedure on the video monitor, as well as conversation, humor, and music.
There has been a trend toward smaller-diameter hysteroscopes with improved optics to facilitate the performance of high-quality operative procedures in the office setting, often without the need for a speculum, cervical dilation, anesthesia, or sedation. A thinner scope is ideal for an office-based procedure because the smaller the hysteroscope caliber, the more likely the scope can be placed into the cervical os and advanced into the endometrial cavity under direct vision, without having to dilate the endocervical canal or use anterior countertraction on the cervix. This direct entry approach, limiting the use of a tenaculum and instrumental cervical dilation and minimizing speculum use, is associated with less patient discomfort. This can facilitate a more simple, safe, convenient office-based procedure allowing for completion of the intended task without need for sedation and in certain cases without need for a local anesthetic.
A paracervical block can be administered to lessen discomfort associated with passage of instruments through the cervix and with the operative procedure in general. A well-placed, high-volume paracervical instillation of anesthetic can be helpful even when performing hysteroscopy under IV sedation in the operating room because it can facilitate avoidance of general anesthesia. Diffusion of a large volume of anesthetic to the hypogastric nerves lateral to the uterosacral ligaments is accomplished by a very superficial paracervical injection at five and seven o’clock, just barely into the vaginal epithelium, creating a large “wheal” with at least 10 mL at each site, for a total of at least 20 mL of anesthetic. This more medial, superficial instillation will lessen the risk of an intravascular injection into a descending branch of the uterine artery and is the same paracervical block that can be used in the operating room with longer-acting bupivacaine as an adjunct for postoperative pain control in laparoscopic hysterectomy and myomectomy procedures. Intravascular administration can cause a transient anesthesia reaction. A small-bore 22G spinal needle is recommended, and a control syringe is optimal for safety. One should pause to let the anesthetic take effect before proceeding with instrumentation. Additional anesthetic can be applied superficially at the anterior cervix if a tenaculum is to be used.
There is considerable positive experience with “vaginoscopy,” by which the hysteroscope introduced into the vagina and through the cervix without use of a speculum or paracervical block. One randomized trial comparing vaginoscopy with a standard hysteroscopic approach showed vaginoscopy to be faster and less painful, with the conclusion it should be the technique of choice for outpatient hysteroscopy ( ). There appears to be no increased risk for infection in a study of nearly 43,000 office hysteroscopies; antibiotic prophylaxis is not routinely prescribed ( ).
An alternative to vaginoscopy is to use an open-sided speculum to place any necessary paracervical block, then set the hysteroscope tip into the cervical os and remove the speculum. The hysteroscope can then be advanced under vision without the interference of the rigid speculum. Removal of the speculum is also often appreciated by the patient. Should a tenaculum be required, local anesthetic can first be superficially instilled into the anterior cervix.
Should cervical stenosis be encountered, the insertion of the hysteroscope will be more challenging, requiring anterior traction and use of graduated cervical dilators . Minicervical dilators can be used for this purpose. When cervical dilation is required, it is possible to only dilate the outer aspect of the cervical canal, place the hysteroscope into the cervical opening and under flow with direct vision, then advance the rest of the way into the cavity. Advancing the hysteroscope under direct vision might decrease the risk of uterine perforation, a complication that occurs more commonly in the setting of cervical stenosis.
Uterine perforation is usually not clinically consequential but could conceivably be of serious consequence if the perforation is lateral into the uterine vasculature or involves the active use of an energy device, in which case damage can occur to adjacent structures such as bowel, bladder, or blood vessels. There is conflicting evidence about the use of preoperative misoprostol as a cervical ripening agent before gynecologic transcervical procedures to reduce the risk of difficult insertion and associated cervical trauma and uterine perforation. The effect of preoperative administration of misoprostol as a cervical ripening agent before hysteroscopy is uncertain, and evidence does not support its routine use for operative hysteroscopy ( ).
Endometrial polyps can be isolated or multiple and are best resected from the uterine wall under hysteroscopic visualization . The simplest, most cost-effective method is with use of a reusable 5-French hysteroscopic forceps focused at the attachment site. Specimen removal from the endometrial cavity is often accomplished by maintaining a grasp on the polyp while withdrawing the hysteroscope. If this is not successful, extraction of the resected lesion does not necessarily require hysteroscopic visualization and can be accomplished with careful use of handheld endometrial polyp forceps. Reinsertion of the hysteroscope can confirm all pathologic tissue has been removed. An endometrial biopsy specimen can then be obtained as a global endometrial screen if desired.
Mechanical morcellation and resectoscopic techniques intended for submucosal fibroid removal are increasingly used for hysteroscopic polypectomy, despite the fact that polyp removal can usually be accomplished without energy. Endometrial polyps can be resected using electrosurgical energy with a bipolar (or monopolar) resectoscope. Care should be taken not to thermally damage the endometrial base in women planning future pregnancies. The electromechanical morcellator can remove endometrial polyps, and newer reusable systems are now available that could benefit cost effectiveness.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here