Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The uterus is a hollow muscular organ of the female reproductive system with a shape and size similar to that of an upside-down pear in women of reproductive age ( Table 31.1 ). It is located within the pelvic cavity along the body’s midline, posterior to the urinary bladder and anterior to the rectum. The gross anatomy of the uterus is divided into three main parts (fundus, body, and cervix), and it consists of different tissue layers:
Perimetrium or serosa: thin layer of simple squamous epithelium covering the uterus, which protects the uterus from friction by forming a smooth layer along its surface and by secreting watery serous fluid for lubrication.
Myometrium: many layers of visceral muscle tissue, allows the uterus to expand during pregnancy and then contracts the uterus during childbirth.
Uterine junctional zone (JZ):
First described by Hricak et al. in 1983 as a distinct low signal layer on magnetic resonance (MR) images in women of reproductive age, separating the endometrium in high signal intensity from the outer myometrium in intermediate signal.
Broadly represents the inner third of the myometrium with an average thickness under 12 mm.
Cannot be histologically distinguished from the outer myometrium on light microscopy.
Similar to the endometrium, it is derived from the embryonic paramesonephric ducts, whereas the outer myometrium has a non-Müllerian mesenchymal origin.
Enhanced immunostaining of the vascular endothelial marker CD31 in the JZ, reflecting either more vascularity or a higher level of endothelial activation.
Thickness and expression of estrogen and progesterone receptors exhibit a cyclical pattern, which parallels that of the endometrium.
Is the exclusive origin of propagated myometrial contractions in the nonpregnant uterus, the frequency, amplitude and orientation of which are dependent on the phase of the menstrual cycle and play a critical role in sperm transport, implantation, and menstrual shedding.
Endometrium: made of simple columnar epithelial tissue with many exocrine glands and a highly vascular connective tissue, responds to cyclic ovarian hormone changes and provides support to the developing embryo and fetus during pregnancy. In a woman of reproductive age, two layers of endometrium can be distinguished.
The functional layer (stratum compactum and stratum spongiosum): adjacent to the uterine cavity, is built up after the end of menstruation, proliferation is induced by estrogen during the follicular phase, and in the luteal phase progesterone when the corpus luteum takes over. It is completely shed during menstruation.
The basal layer (stratum basale): adjacent to the myometrium, provides the regenerative endometrium after menstrual loss of the functional layer, is not shed at any time during the menstrual cycle.
| Stage | Uterine Length (cm) | Fundal Width (cm) | Uterine Body-to-Cervix Ratio |
| Neonatal | 3.5 | 1.2 | 2÷1 |
| Pediatric | 1–3 | 0.4–1.0 | 1÷1 |
| Prepubertal | 3–4.5 | 0.8–2.1 | 1–1.5÷1 |
| Pubertal | 5–8 | 1.6–3.0 | 1.5–2÷1 |
| Reproductive | 8–9 | 3–5 | 2÷1 |
| Postmenopausal | 3.5–7.5 | 1.2–1.8 | 1–1.5÷1 |
Cycle-specific normal limits of endometrial thickness ( Box 31.1 ):
Menstrual, 2 to 3 mm.
Early proliferative, 5 ± 1 mm.
Periovulatory, 10 ± 1 mm.
Late secretory, up to 16 mm.
Postmenopausal, under 5 mm:
Vaginal bleeding, no tamoxifen: under 5 mm.
Risk of carcinoma around 7% if thickness greater than 5 mm.
Risk of carcinoma 0.07% if thickness less than 5 mm.
No vaginal bleeding: 8 to 11 mm.
Risk of carcinoma around 7% if thickness greater than 11 mm.
Risk of carcinoma 0.002% if thickness less than 11 mm.
On tamoxifen: under 5 mm.
Around 50% of these patients have a thickness greater than 8 mm.
Outer-to-outer margin on sagittal image:
Proliferative phase (days 6–14): up to 11 mm
Secretory phase (days 15–28): up to 16 mm
Postmenopausal women:up to 5 mm; may increase to 8 mm with hormone-replacement therapy or tamoxifen
Postmenopausal bleeding and endometrial stripe thickness:
<5 mm: biopsy yield is low (endometrial atrophy)
>5 mm: biopsy yield is higher (polyps, hyperplasia, or carcinoma)
Most often used for the initial assessment.
Endovaginal ultrasonography (US) offers higher-resolution imaging and is preferred to transabdominal US.
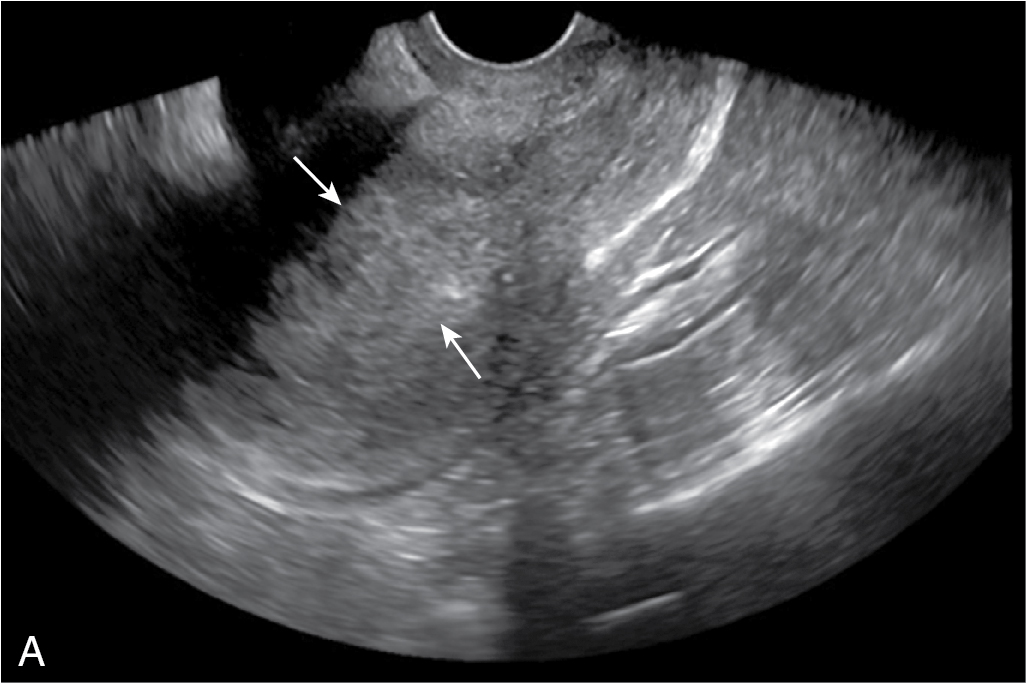
Endometrium appears echogenic, while JZ appears as a subendometrial hypoechoic halo on US ( Fig. 31.1 ).
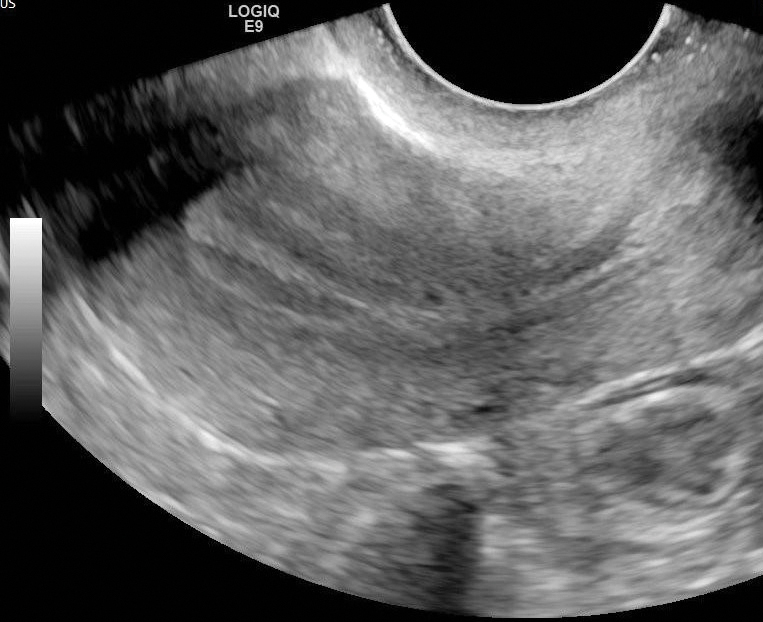
The appearance of the normal central endometrial echocomplex varies with the menstrual cycle and can be measured in the sagittal plane.
Early proliferative phase: the endometrium appears as a single echogenic line.
Late proliferative (periovulatory) phase: three hyperechoic longitudinal lines separated by the hypoechoic endometrium. The outer echogenic lines represent the interface between the endometrium and myometrium, and the central echogenic line denotes the endometrial cavity and the line of contact between the inner endometrial surfaces (see Fig. 31.1 ).
Secretory phase: the trilaminar appearance disappears within 48 hours of ovulation. The secretory endometrium is typically echogenic, with maximal thickness in the mid-secretory phase.
Endometrial thickness measurement: the distance between the outer margins of the endometrial echocomplex on midline longitudinal images.
Multiplanar images with a large field of view and excellent soft-tissue contrast.
An excellent method for imaging evaluation when US is not feasible or the findings at US are inconclusive.
Uterus:
Premenarchal: tends to have low to medium signal intensity with indistinct zonal anatomy.
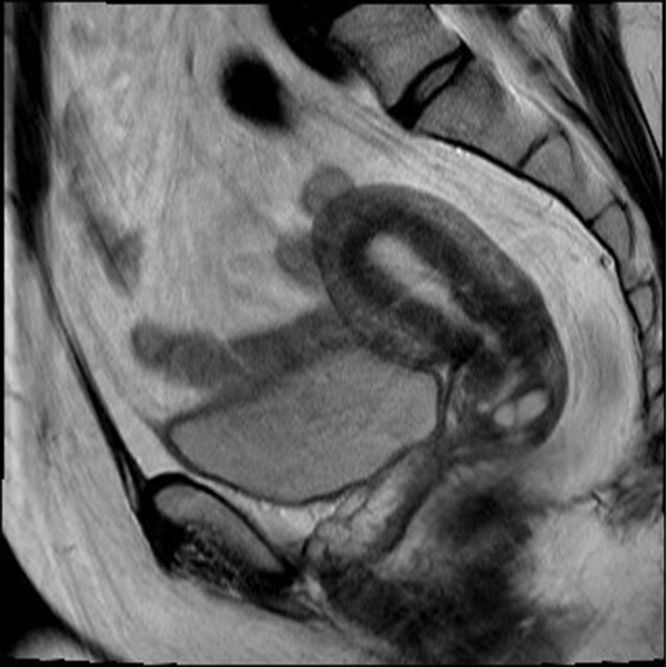
Pubertal adolescents: the zonal anatomy can be appreciated on T2-weighted magnetic resonance (MR) images ( Fig. 31.2 ):
Endometrium: high T2-weighted signal intensity.
JZ: low T2-weighted signal intensity, normally less than 12 mm thick.
Myometrium: intermediate T2-weighted signal intensity.
Not a primary imaging modality for endometrial or JZ thickening.
Commonly performed in patients with acute symptoms.
Normal uterus has the attenuation of soft tissue. The cervix demonstrates lower attenuation because of its fibrous stroma.
Identification of the uterus may be challenging at computed tomography (CT) in prepubertal females
Dynamic contrast enhanced CT:
Appearances of the myometrium and endometrium depend on the interval between intravenous contrast material administration and scanning, as well as the age of the patient.
In women of reproductive age, the endometrium exhibits hypoattenuation relative to the inner myometrium during most phases of contrast enhancement (could be misinterpreted as fluid within the uterine cavity).
Endovaginal US is preferred over transabdominal US, unless:
In infants, children, and adolescents.
Patient preference.
Visualization of the uterus at endovaginal US is compromised by large myomas, a high position and fixation because of adhesions, or postpartum enlargement.
Nonemergent ultrasounds are best performed in the early phase of the menstrual cycle to reduce the wide variation in endometrial thickness.
The thickest portion of the endometrium should be measured.
If fluid is present in the uterine cavity, it should be excluded from the measurement.
The use of three-dimensional volume-rendered US further enhances imaging.
On axial CT and MR images, the appearance of the uterus is influenced by the organ’s orientation and by the degree of distention of the urinary bladder.
Sagittal T2-weighted imaging without fat saturation is the critical pulse sequence on magnetic resonance imaging (MRI).
Abnormal myometrial echogenicity (mostly hypoechoic).
Heterogeneous myometrial echotexture.
Myometrial cysts: contribute to “venetian blind sign” ( Fig. 31.3 ).
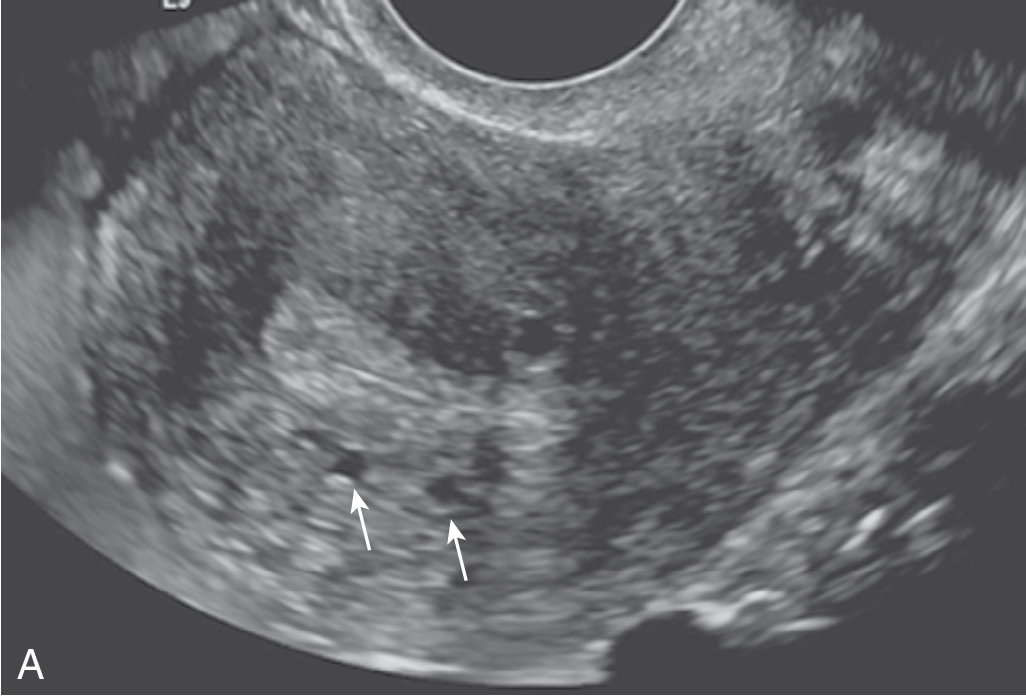
Echogenic nodules or linear striations.
Pseudowidening of endometrium.
Poor definition of JZ and lesion border.
Relative absence of mass effect.
Elliptical myometrial abnormality.
Low signal intensity on T2-weighted images.
JZ thickening:
JZ under 8 mm: unlikely to represent adenomyosis.
JZ over 12 mm: very likely represents adenomyosis ( Fig. 31.4 ).
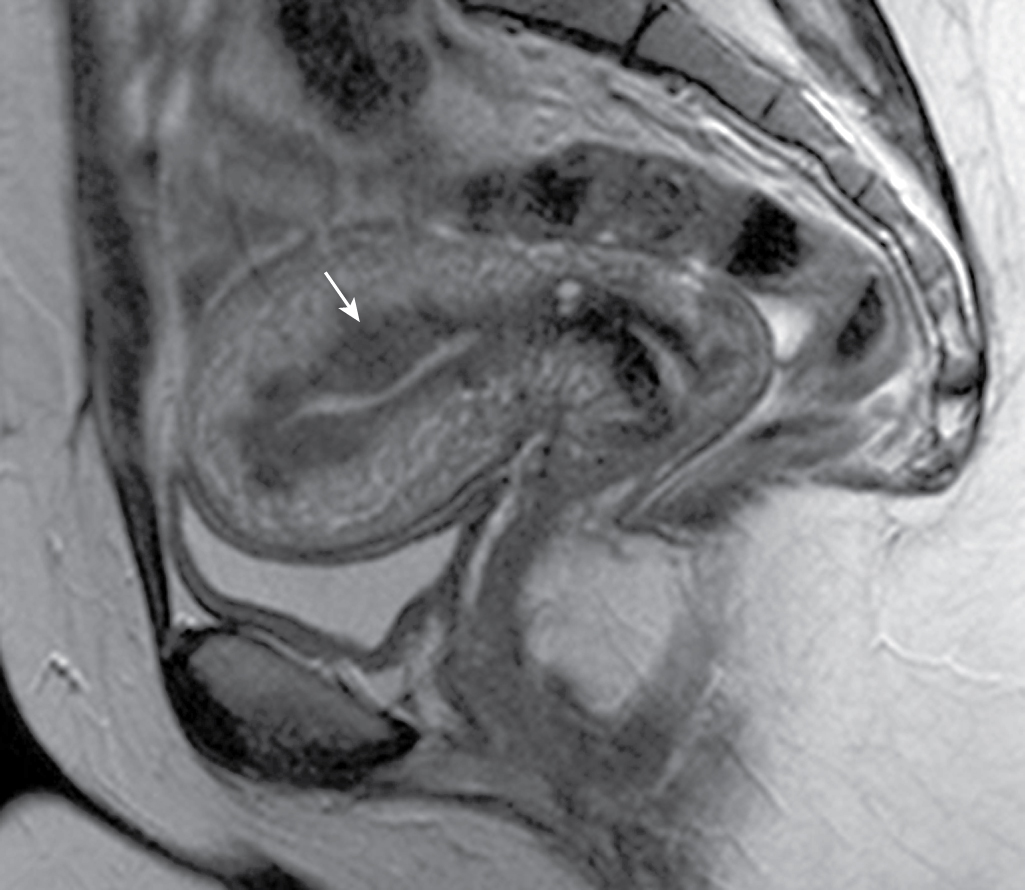
Myometrial foci or linear striations of high signal intensity on T2-weighted images ( Fig. 31.5 ).
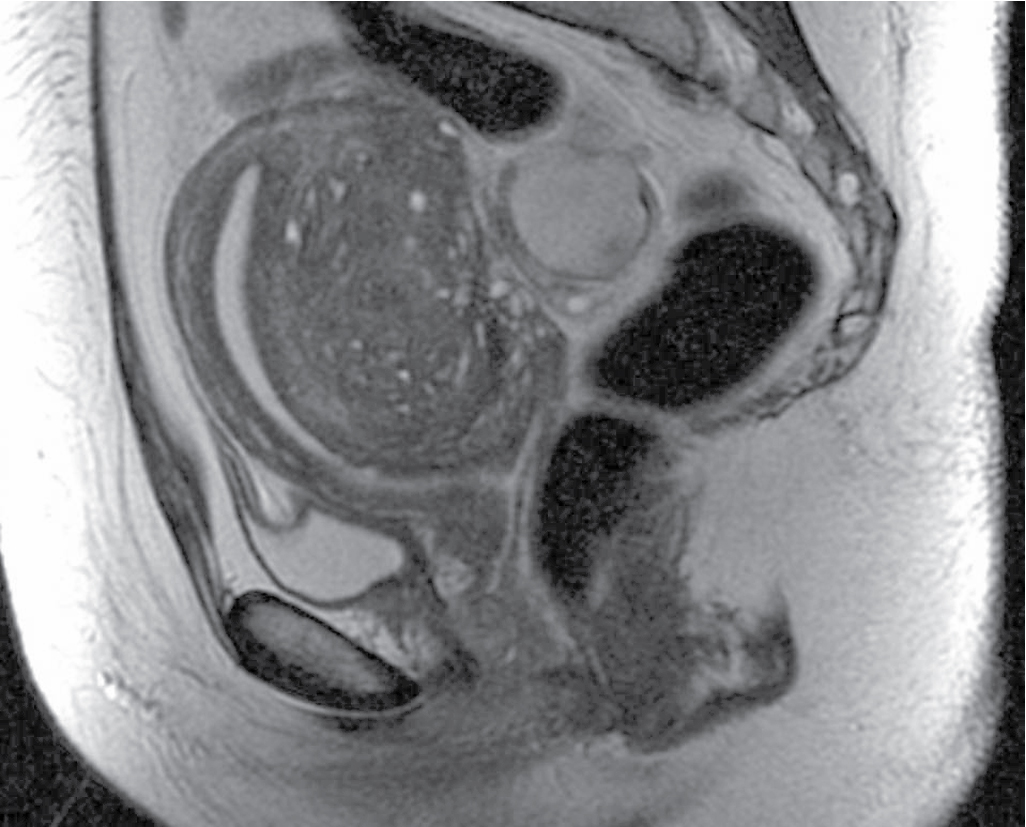
Pseudowidening of endometrium.
Relative absence of mass effect ( Fig. 31.6 ).
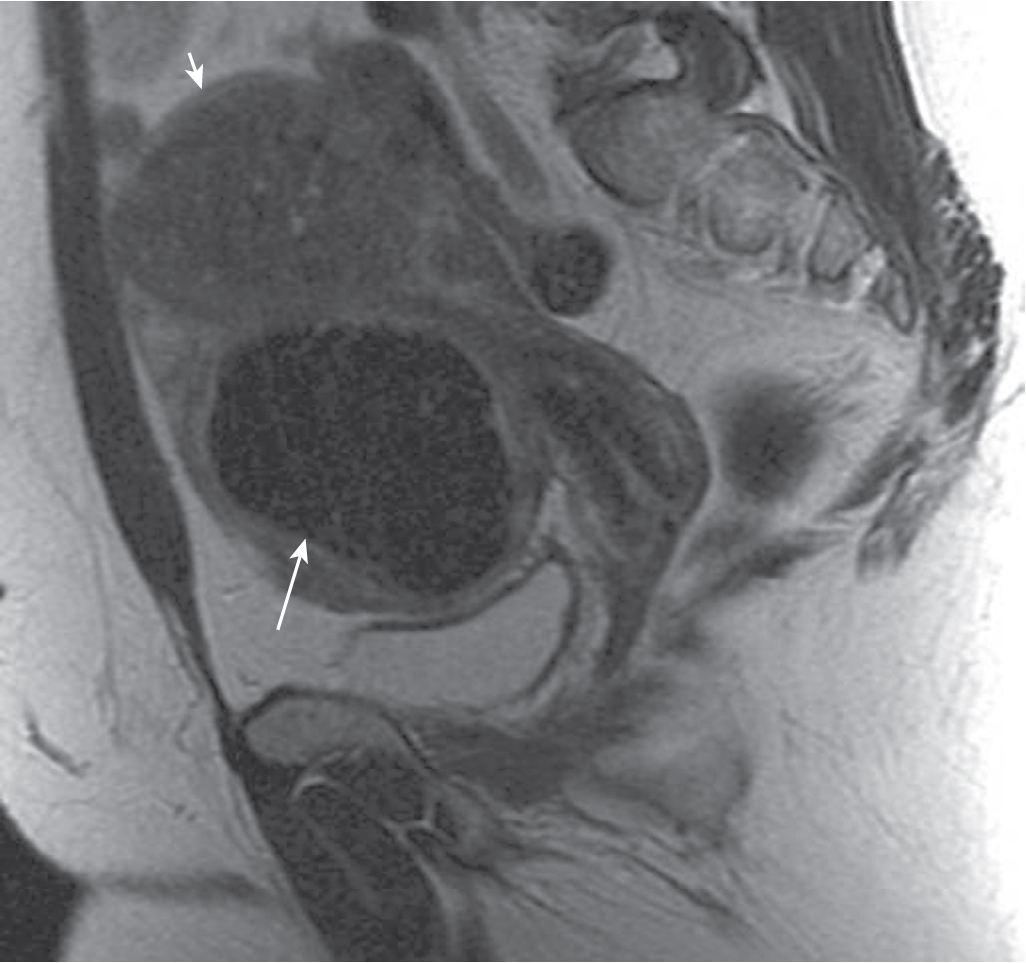
Elliptical myometrial abnormality.
Heterogeneous and irregular endometrial thickening ( Fig. 31.7 ).
Intrauterine fluid collection.
Polypoid mass lesion.
Myometrial invasion (disruption of subendometrial halo).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here