Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human body is an astonishingly well-coordinated organism. Made up of several billion cells, the body depends on communication systems that are precisely regulated and that reach every one of those cells. There are two main communication systems in the human body: the nervous system and the endocrine system . In Chapter 8 , we saw how the nervous system is constructed and how it processes information and controls a range of functions, from regulation of blood pressure to movement of our limbs. In this chapter, we shall look at the endocrine system and how it interacts with the nervous system to control the body's homeostatic and reproductive systems ( Information box 10.1 ).
Both systems use chemical signals ( hormones and neurotransmitters ). The nervous system is hard-wired with the signalling molecule delivered precisely to the point where it is needed. This means that only a very few different signalling molecules are required as they do not affect any cells except at their site of delivery. The endocrine system is not hard-wired. All the cells in the body are exposed to hormones, and so there is a wide range of signalling molecules needed.
Both systems need receptors for their chemical signals. In the nervous system, specificity is conferred by hard-wiring: the neurotransmitter is delivered directly and specifically to the cell that has the receptor and responds to the transmitter. In the endocrine system, where hormones are delivered to all cells, specificity comes only from the expression of the receptor for the hormone. Only cells with receptors for a hormone can respond to that particular hormonal signal.
The endocrine, or hormonal, system is responsible for regulating most of the systems of the body. You will find descriptions of hormones and their effects throughout this book: on energy metabolism ( Ch. 3 ), bone ( Ch. 9 ), the cardiovascular system ( Ch. 11 ), bone marrow and haemopoiesis ( Ch. 12 ), the renal system ( Ch. 14 ), the gastrointestinal system ( Ch. 15 ) and the regulation of food intake ( Ch. 16 ). It is also responsible for regulating reproductive processes.
The endocrine system is made up of a number of hormone-producing glands. These are called endocrine glands ( Fig. 10.1 ) . Examples of endocrine glands include the pituitary and the thyroid glands. These glands do not have secretory ducts like the exocrine glands (e.g. salivary glands), but instead they release hormones directly into the bloodstream. In addition to these discrete endocrine glands, there are various different endocrine cells found in other tissues of the body, such as the G cells, located in the antrum of the stomach, which secrete gastrin. Isolated endocrine cells such as the G cells are often collectively referred to as the ‘ diffuse endocrine system ’.
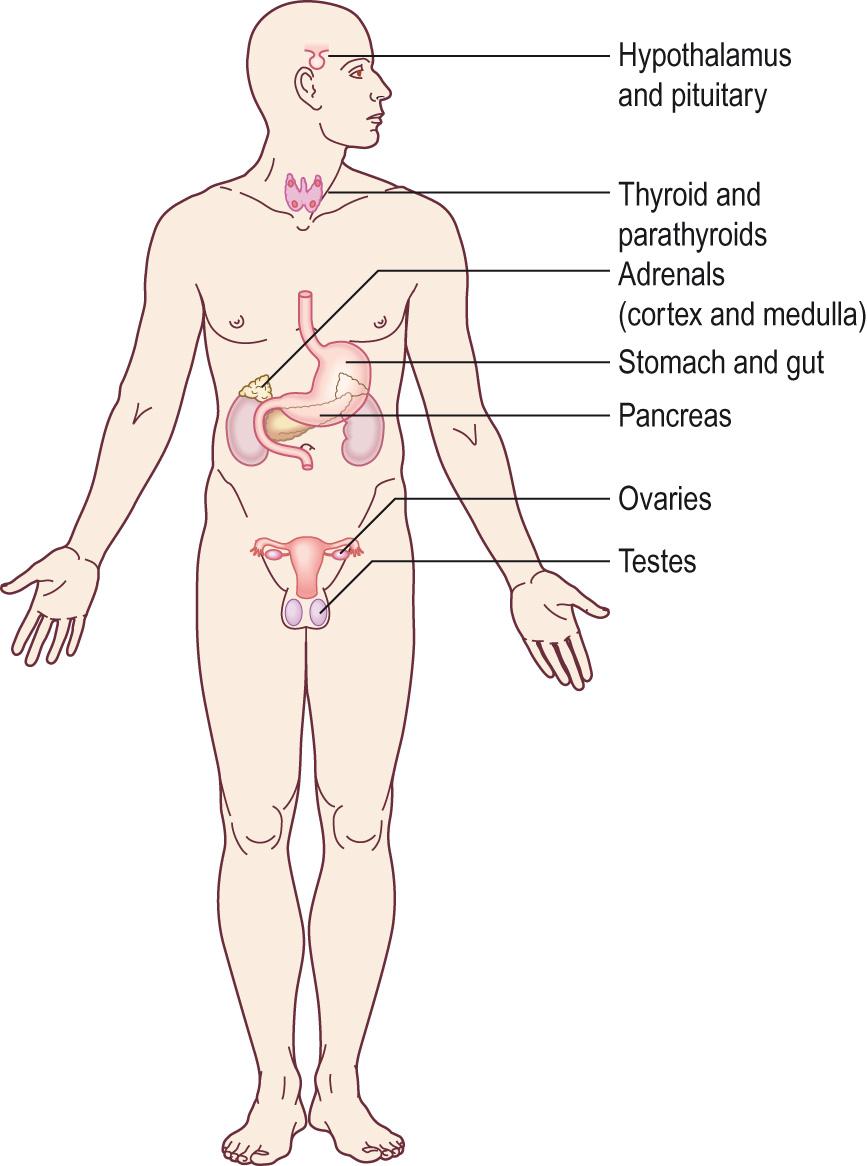
Hormones released by endocrine glands and cells circulate in the blood and, therefore, all cells in the body, by virtue of having a blood supply, are exposed to all hormones. However, only the cells that have a specific receptor for a hormone can ‘see’ the hormone and respond to the hormonal signal. These cells are called ‘ target cells ’ for the hormone. Hormone receptors have a very high affinity for their hormones, which means that even very low circulating concentrations of hormones are biologically effective. Most hormones circulate in picomolar or nanomolar concentrations (10 −12 to 10 −9 mol/L).
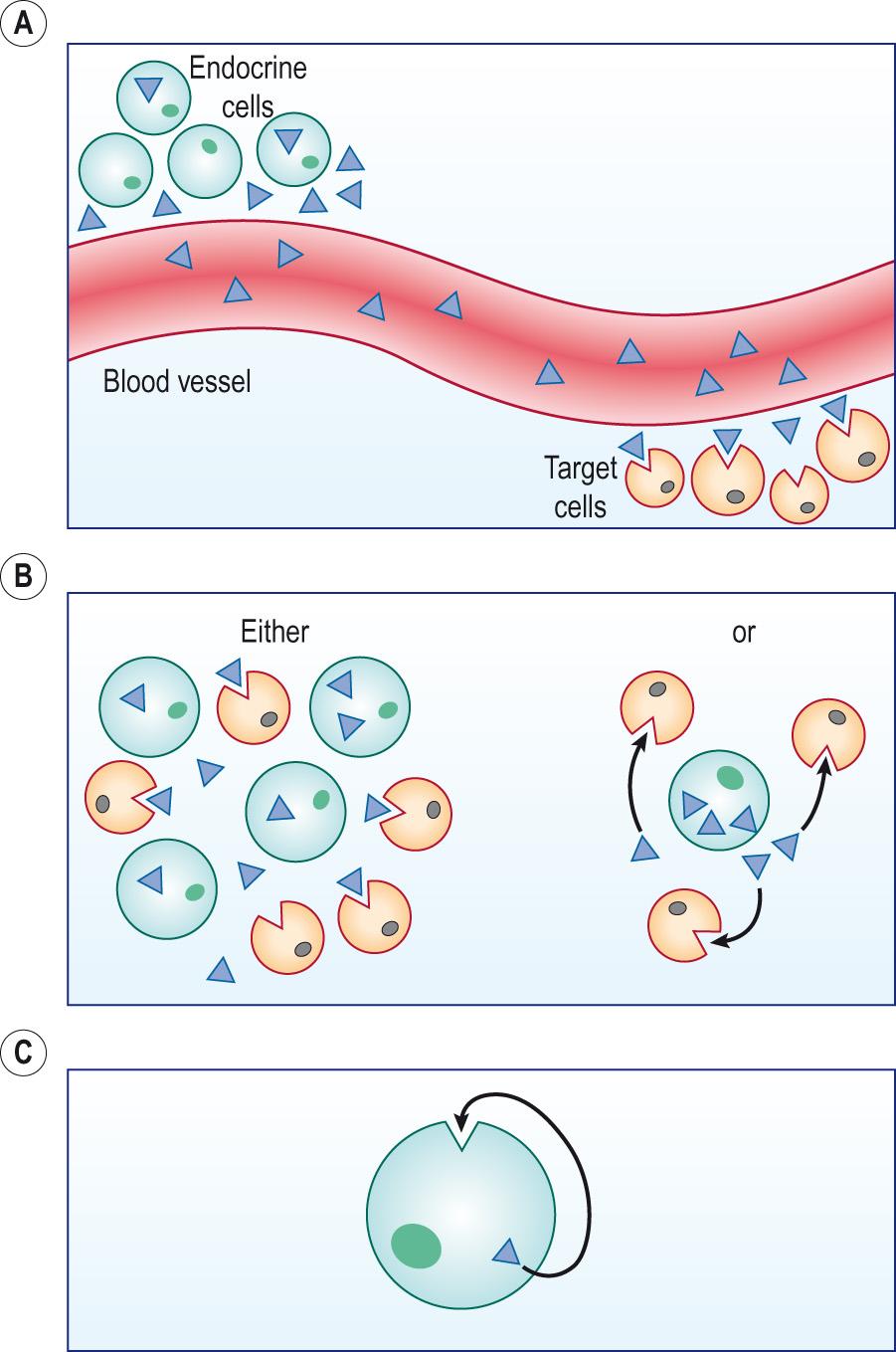
Classically, hormones are released into the bloodstream and act on tissues that are distant from the site of hormone production, an endocrine effect. However, some hormones act locally within the tissue where they are produced. These are called ‘local hormones’ or ‘ paracrine ’ effects. Some hormones have both local and systemic effects and therefore act in a paracrine and endocrine manner ( Fig. 10.2 ) . An example is testosterone, which has local actions in the testes and hormonal effects on muscle. Some hormones, particularly growth factors, exert their actions on the cells that secrete them. These are called ‘ autocrine effects’ (see Ch. 2 ).
There are three broad groups of hormones: peptides , steroids and amino acid derivatives ( Table 10.1 ).
| Chemical class | Hormone |
|---|---|
| Amino acid derivatives | Epinephrine (adrenaline) Thyroid hormones (T 3 , T 4 ) |
| Steroids | Oestrogens (e.g. oestradiol) Androgens (e.g. testosterone) Progesterone Cortisol Aldosterone |
| Peptides | Thyrotropin-releasing hormone (TRH) Gonadotropin-releasing hormone (GnRH) Vasopressin Oxytocin (OT) Vasoactive intestinal peptide (VIP) Glucagon Adrenocorticotropic hormone (ACTH) Somatostatin |
| Proteins | Insulin Insulin-like growth factors (IGFs) Growth hormone (GH) Prolactin (PRL) Placental lactogen (PL) Parathyroid hormone (PTH) |
| Glycoproteins | Thyroid-stimulating hormone (TSH) Follicle-stimulating hormone (FSH) Luteinising hormone (LH) Chorionic gonadotropin (CG) |
The largest group of hormones, and the most diverse, is the peptide hormones. These vary in size from just three amino acids, such as thyrotropin-releasing hormone (TRH) , to 30-kDa glycosylated proteins, such as thyroid-stimulating hormone ( TSH , also called thyrotropin ). Peptide hormones are made in a similar way to all secreted proteins. Most peptide hormones are the product of a single gene that encodes a larger protein, called a pre-prohormone . After production of the protein in the rough endoplasmic reticulum of the endocrine cell, the protein is processed in the Golgi apparatus and the secretory granules. The initial N-terminal signal sequence is cleaved, leaving the prohormone . The action of other proteolytic enzymes produces the mature hormone with associated peptide fragments, which are secreted together with the hormone. Peptide hormones are usually stored in secretory granules within the cell until they are released in response to an appropriate signal. Secretion is by non-constitutive exocytosis, which depends upon increases in intracellular calcium acting as a second messenger (see Ch. 2 ).
There are several distinct ‘families’ of peptide hormones. These are groups of structurally similar hormones that may have arisen through duplication of an ancestral gene. However, not all peptide hormones are members of a family.
Peptide hormones are water soluble and thus are readily transported in blood without needing specific carrier proteins. The half-life in blood of most peptide hormones is short, a matter of a few minutes only, and they are inactivated and degraded by proteolysis.
Peptide hormones all bind to receptors located on the plasma membrane of their target cells and exert their effects on the cell either by the direct activation of a protein kinase or by the generation of a second messenger and the indirect activation of a protein kinase (see Ch. 2 for more details). In either case, the net result is phosphorylation of target proteins within the cell and altered cell function.
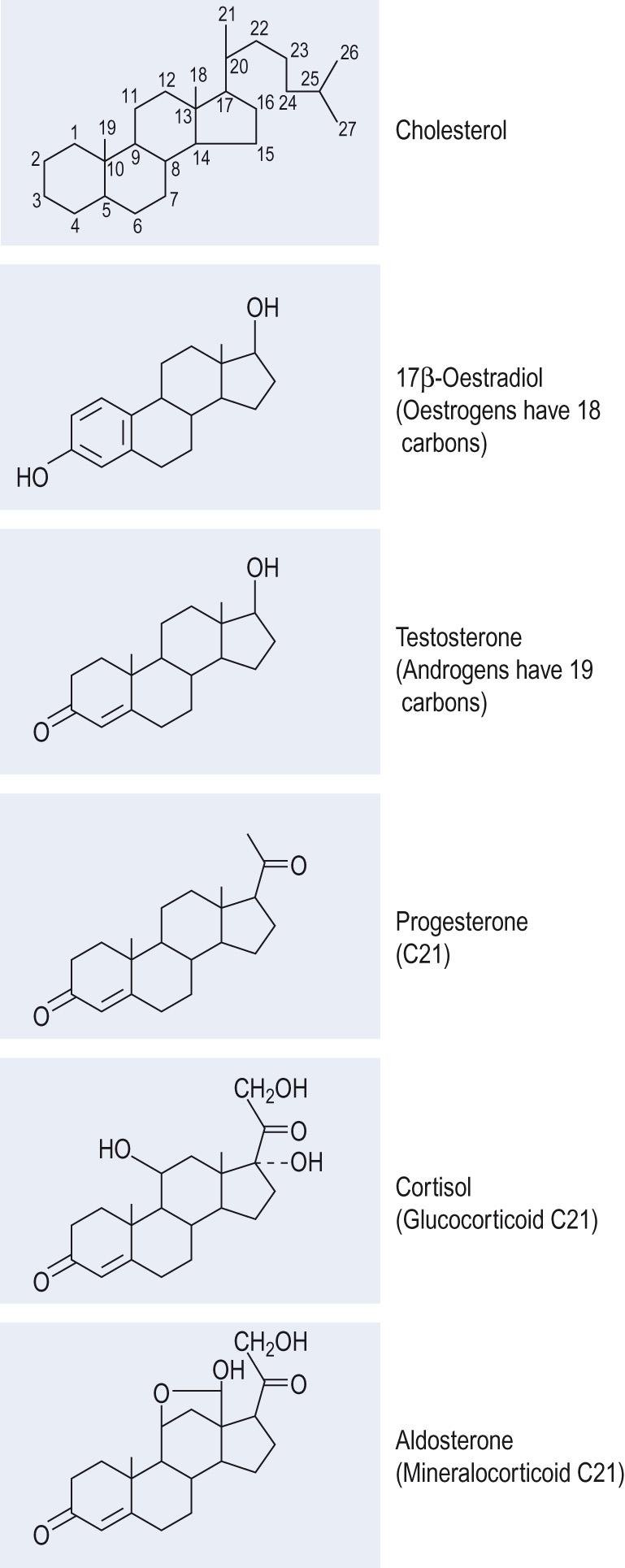
There are five major classes of steroid hormone ( Fig. 10.3 ) , although calcitriol , the active form of vitamin D 3 , is often counted as a sixth class. The steroid hormones are all synthesised from cholesterol , which is either taken up by the cell from circulating lipoproteins by receptor-mediated endocytosis or made de novo, from acetate, within the cell. In contrast to the peptide-secreting cells, those that secrete steroids do not store the final hormonal product, but instead store cholesterol, the precursor for steroid synthesis. The cholesterol is stored in an esterified form in lipid droplets within the cell. Steroid synthesis takes place in the mitochondria and smooth endoplasmic reticulum, and the abundance of these organelles, together with the lipid droplets, gives steroid-secreting cells a characteristic appearance under electron microscopy.
The pathway of steroid synthesis is shown in Fig. 10.4 . Most of the enzymes involved belong to the CYP gene family, encoding the cytochrome P450 steroid hydroxylases . The initial step, the conversion of cholesterol to pregnenolone , is common to the synthesis of all the steroid hormones and is the rate-limiting step of steroidogenesis. Unusually, it is not the activity of the enzyme cytochrome P450 scc , which cleaves the side chain from cholesterol to form pregnenolone, which limits the rate of steroidogenesis ( Information box 10.2 ), but rather the rate of delivery of cholesterol into the mitochondrion.
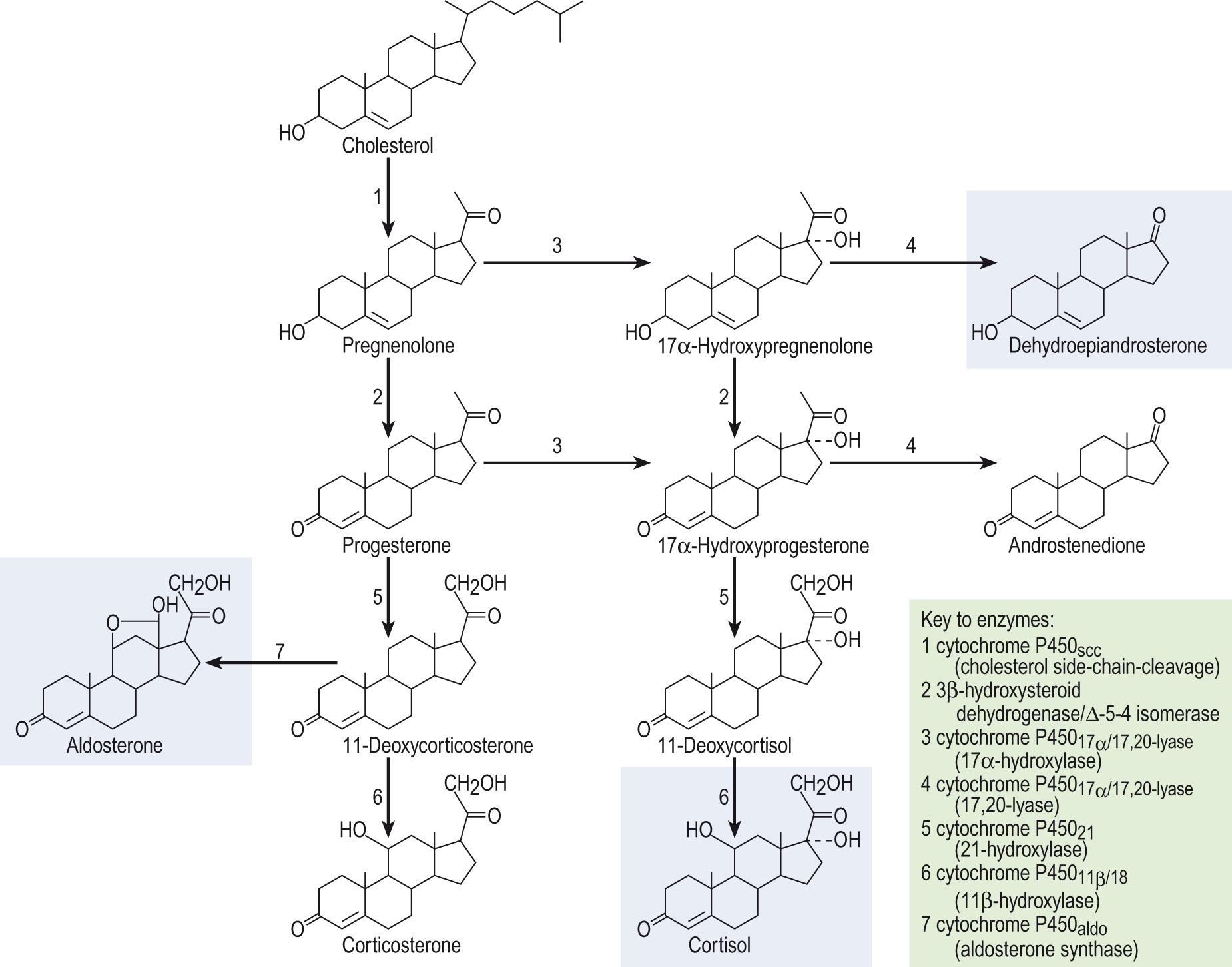
In order to reach the enzyme that catalyses the first reaction of steroid biosynthesis, cholesterol has to travel from its storage site in lipid droplets to the inner mitochondrial membrane. The protein that facilitates this transfer is called steroidogenesis acute regulatory protein (StAR) and is found in all the cells of steroid synthesis in the adrenal glands and the gonads. Only very low levels of steroid synthesis can occur in the absence of functional StAR protein. Mutations in the gene encoding the StAR protein result in a disorder termed ‘ lipoid congenital adrenal hyperplasia ’, which is characterised by a failure of appropriate sexual differentiation during foetal development and by severe glucocorticoid deficiency early in life. The affected adrenal cells have a characteristic lipid-rich appearance under microscopy.
Steroid synthesis is a multistep process that involves the movement of steroid hormone precursors between the mitochondria and the smooth endoplasmic reticulum. This process is not well understood, but it is clear that there is a mechanism for holding the steroid within the cell until the final hormonal steroid has been made. Unlike peptide hormones, there is no specific secretory mechanism for steroids; they are thought to simply diffuse out of the cell down a concentration gradient. What prevents the intermediate products from escaping the cell is, as yet, unknown.
The steroid hormones are lipophilic and do not readily dissolve in blood. They mostly rely on specific transport proteins, a group of steroid-binding globulins, to carry them in blood. As a result, steroid hormones have a half-life in plasma of an hour or longer. They are metabolised in the liver into less active compounds. The liver enzymes that metabolise steroids belong to the same two families as the main enzymes of steroid synthesis: the hydroxysteroid dehydrogenases and the CYP family of hydroxylases. In addition, steroids are conjugated in the liver to produce glucuronides and sulphates, which increases their solubility and allows them to be excreted in bile or urine. Not all steroid metabolism results in deactivation, however. For some hormones, such as testosterone, peripheral metabolism in adipose tissue or muscle produces either dihydrotestosterone , the more potent androgen, or oestradiol , the main female sex steroid. In the case of vitamin D 3 , metabolism in the liver and kidney is essential to produce the active hormone.
Steroid hormones are small and lipophilic and readily cross the plasma membrane of cells. They act by binding to intracellular receptors, which function as transcription factors, in turn binding to response elements in the promoter regions of certain genes and thus modifying gene transcription (see Ch. 2 ). In some tissues, steroids also appear to act by binding to cell-surface receptors, but this mode of action is unusual.
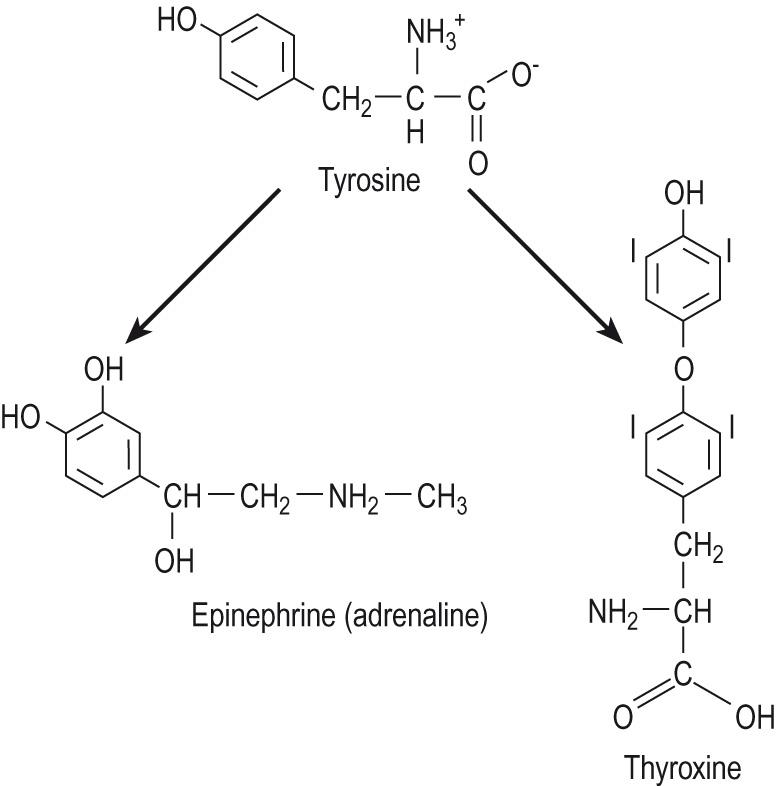
The final group of hormones comprises those derived from the amino acid tyrosine (see Table 10.1 ). These are the adrenal catecholamines, epinephrine ( adrenaline ) and norepinephrine ( noradrenaline ), and the thyroid hormones ( Fig. 10.5 ) . Despite their common precursor, these are very different hormones. The catecholamines are synthesised by stepwise modification of free tyrosine residues and then stored in secretory vesicles and released in response to an action potential. The thyroid hormones, thyroxine ( T 4 ) and tri-iodothyronine ( T 3 ), on the other hand, are formed by the iodination of pairs of tyrosine residues, held within a large precursor molecule called thyroglobulin . The thyroid hormones generally behave like steroid hormones, binding to intracellular receptors and altering gene transcription, whereas the catecholamines bind to cell surface receptors and act by the generation of second messengers . One difference between the thyroid hormones and steroids is the requirement for thyroid hormones to have a specific mechanism for crossing the plasma membrane. They also have very different half-lives in blood. Thyroid hormones have the longest half-life of hormones, circulating in blood for days before being de-iodinated and conjugated in the liver. Catecholamines have the shortest half-life of all hormones, lasting only a few seconds before they are degraded by monoamine oxidase , an enzyme found in most tissues.
There is a degree of interaction between the nervous system and the endocrine system. Several hormones are released from neurons, in response to an action potential, in exactly the same way that neurotransmitters are released. These are called neurohormones . However, instead of being released into a synaptic cleft, neurohormones are released into the blood, where they circulate and exert their effects on target tissues ( Information box 10.3 ). The major neuroendocrine tissues are the hypothalamus, the posterior pituitary and the adrenal medulla.
In general, peptide hormones are hydrophilic and readily soluble in blood and therefore circulate in blood in a free, unbound form. Plasma albumin does bind small peptide hormones, but this is a loose association and does not have a regulatory function. One peptide hormone, insulin-like growth factor 1 ( IGF-1 ), does have a specific plasma binding protein, but this is unusual.
Steroid and thyroid hormones, however, are lipophilic and poorly soluble in blood. These are usually transported bound to a specific binding globulin:
Thyroid hormone-binding globulin (THBG) , binds thyroxine
Sex hormone-binding globulin (SHBG) , binds oestradiol and testosterone
Cortisol-binding globulin (CBG) , is also called transcortin
Vitamin D -binding protein.
Aldosterone is usually transported like small peptides, loosely bound to plasma albumin.
These hormone-binding globulins have several functions:
They increase the solubility of steroid hormone and thyroxine.
They increase the plasma half-life of these hormones by protecting them from renal filtration and peripheral metabolism. Cortisol, bound to CBG, has a half-life in plasma of approximately 90 minutes, whereas aldosterone, which does not have a specific binding protein, has a half-life of approximately 15 minutes.
They may act as an accessible store of hormone in blood.
They may serve to deliver hormones to specific target tissues.
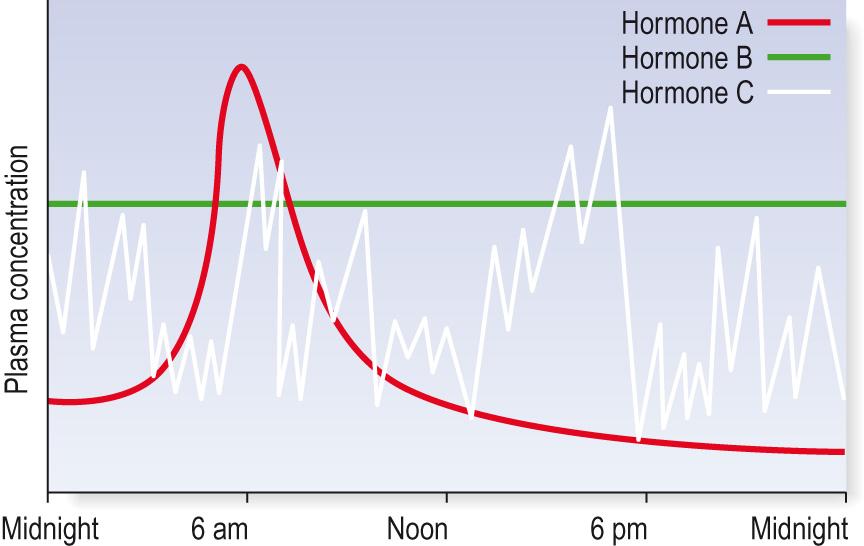
Most hormones are secreted in response to a specific stimulus. In some cases, this means that their secretion is episodic. Insulin , for example, is secreted in response to high blood glucose, and therefore, it is secreted in an episodic manner, whenever blood glucose reaches the appropriate threshold ( Fig. 10.6 , see also Fig. 1.2 ). At some times the plasma level of insulin may be very high, whereas at other times it is undetectable. Other hormones, such as T 4 , which is needed to maintain physiological functions all the time, are secreted in a manner that allows a constant plasma concentration of the hormone over a period of days or weeks. This is a form of ‘set point’ regulation of a hormone: levels fluctuate only slightly around a constant set point. In other cases, particularly the adrenal corticosteroid cortisol, secretion is subject to diurnal variation, which means that there is a fairly predictable pattern of hormone secretion over a 24-hour period. The diurnal variation in hormone secretion is controlled by the suprachiasmatic nucleus in the hypothalamus. Most hormones that are subject to diurnal variation also have some episodic secretion superimposed on this daily variation, so that there is some response to immediate physiological demand as well as to the underlying pattern ( Information box 10.4 ).
The hypothalamus and pituitary gland have a role in orchestrating the activity of several other parts of the endocrine system. They achieve this through a number of endocrine axes. The hypothalamic–pituitary–thyroid axis is illustrated here ( Fig. 10.7 ) . The hypothalamus releases thyrotropin-releasing hormone (TRH) in response to a stimulus such as cold. TRH acts on the anterior pituitary to stimulate the release of thyroid-stimulating hormone (TSH), which acts on the thyroid gland to increase the release of thyroxine. Thyroxine, being small and lipophilic, crosses the blood–brain barrier and is able to exert a negative feedback inhibition on the hypothalamus, preventing further release of TRH. Thyroxine also acts on the anterior pituitary, inhibiting further TSH release.
There is also a hypothalamic–pituitary–adrenal axis , a hypothalamic–pituitary–gonadal axis and a hypothalamic–pituitary–IGF axis .
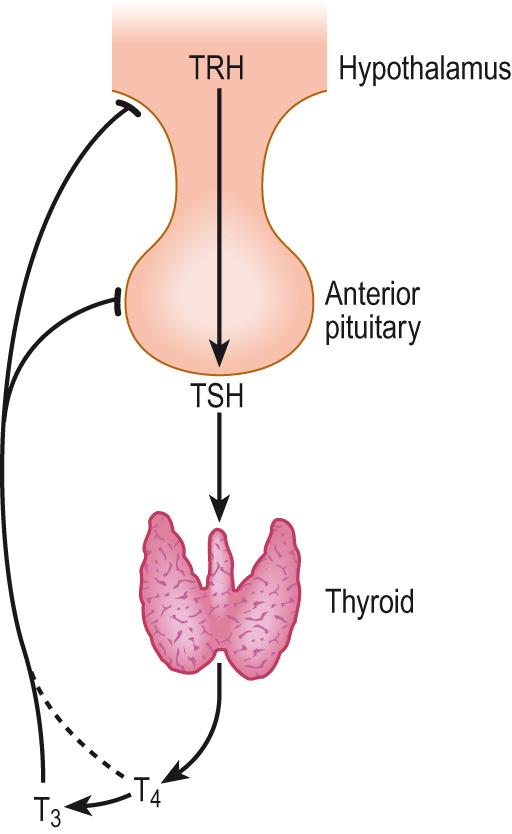
The concept of negative feedback regulation is fundamental in understanding homeostatic mechanisms, including the endocrine system (see Fig. 1.1 ). The body has some fairly basic mechanisms to protect it from excess of any kind, and this includes protection against hormonal excesses. The simplest form of negative feedback regulation is illustrated by the control of parathyroid hormone (PTH) secretion (see later). The main action of PTH is to increase plasma calcium, and when this rises above a set point, further production of PTH is inhibited. In an endocrine axis, the final hormonal product of the axis exerts feedback inhibition on the other elements higher up the axis. An example of negative feedback is seen in the hypothalamic–pituitary–thyroid axis ( Fig. 10.7 ) .
The endocrine system involves finely controlled patterns of hormone secretion. When these patterns are disrupted, disease occurs. In terms of clinical medicine, endocrinology is very simple: there can be either too much or too little of a hormone. An imbalance either way causes disease. Most endocrine diseases are caused by autoimmune disorders. These usually result in loss of endocrine function, such as the autoimmune destruction of the endocrine pancreas, which results in type 1 diabetes , or the autoimmune loss of adrenal function causing Addison disease ( Clinical box 10.1 ). However, in some cases, autoantibodies cause stimulation of an endocrine gland with an increase in hormone secretion. The commonest example of this is Graves disease of the thyroid ( Clinical box 10.2 ).
Failure of the adrenal cortex to secrete aldosterone and cortisol is most commonly due to autoimmune disorder or tuberculosis. Other autoimmune disorders (e.g. type 1 diabetes mellitus, Hashimoto thyroiditis, Graves disease, vitiligo, etc.) are commonly seen in patients with autoimmune Addison disease. The signs and symptoms are predictable from the actions of the missing hormones ( Table 10.2 ). Pigmentation of the skin, due to the excess adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary released from its glucocorticoid negative feedback, is often the first manifestation and distinguishes it from secondary adrenocortical insufficiency.
Loss of ACTH secretion from the anterior pituitary, either because of hypopituitarism or because of atrophy of corticotropes induced by long-term glucocorticoid treatment, results in a failure of the zona fasciculata and zona reticularis to secrete glucocorticoids and androgens. However, the output of aldosterone by the zona glomerulosa is normal. Secondary adrenal insufficiency is easily distinguished from primary:
There is no pigmentation
ACTH concentrations are low
ACTH injection will stimulate cortisol secretion (note that, in very long-standing cases of secondary adrenocortical insufficiency, the response will be slow, such that a 3-day test, at the least, will be needed)
No effects attributable to lack of aldosterone are seen
Other evidence of pituitary failure (hypogonadism, hypothyroidism) except when the insufficiency follows withdrawal of long-term glucocorticoid therapy.
| Loss of aldosterone | Loss of cortisol |
|---|---|
| Na + loss : | reduced gluconeogenesis, increased glucose transport and utilisation by muscle and fat : |
| hyponatremia | hypoglycaemia |
| hypovolemic shock | loss of enhanced catecholamine actions: |
| raised plasma renin | postural hypotension, hypotension |
| impaired excretion of K + and H + : | loss of appetite stimulation and gastrointestinal trophic effects: |
| hyperkalaemia | anorexia, weakness, fatigue, nausea, vomiting |
| metabolic acidosis | increased ACTH from loss of negative feedback: |
| hyperpigmentation | |
| decreased secretion of adrenal androgens: | |
| loss of pubic and axillary hair in women |
Hyperthyroidism is overactivity of the thyroid gland. Many of the consequences of exposure to excess thyroid hormone – thyrotoxicosis – are related to the physiological effects of the hormones.
The commonest form of hyperthyroidism is Graves disease , an autoimmune disorder in which naïve T helper lymphocytes (Th2 cells) are inappropriately primed to recognise thyroid-stimulating hormone (TSH) receptors on thyroid follicle cells. The activated Th2 cells stimulate cognate B lymphocytes to produce antibodies to the TSH receptors. These are termed thyroid-stimulating immunoglobulins because, in effect, they act as agonists of the TSH receptor and the consequence is unregulated thyroid hormone production. The high thyroid hormone concentration clamps TSH at low levels by intense negative feedback.
Retro-orbital connective tissues suffer autoimmune attack by activated Th2 cells because they express TSH receptors:
Fibroblasts secrete glycosaminoglycans, generating oedema, which pushes the eyes forward (proptosis) and may produce visual impairment by compression of the optic nerve.
Cytokines from activated lymphocytes stimulate abnormal growth of the extraocular eye muscles, causing disturbance to gaze and vision.
Overactivity of sympathetic transmission (an effect of elevated thyroid hormone levels) retracts the eyelids; inability to cover the eyes results in conjunctival oedema and corneal damage, with scarring.
Total thyroidectomy and treatment with radioactive iodine ( 131 I) are the most effective treatments for ophthalmopathy because they remove the source of self-antigens. Less drastic management includes corticosteroids to suppress the autoimmune inflammatory response.
Graves disease can occur in neonates owing to placental transfer of thyroid-stimulating immunoglobulins from an afflicted mother. The disorder resolves over 4–12 weeks as the infant clears the offending antibodies.
Anti-thyroid drugs inhibit thyroid peroxidase, blocking iodide organification and coupling reactions. The main ones are the thioamides ( carbimazole or its metabolite methimazole , and propylthiouracil ). Because of the long half-life of T 4 , it can take 3–4 weeks for clinical improvement.
Iodides (e.g. potassium iodide) at high concentrations suppress organification and thyroid hormone synthesis via the Wolff–Chaikoff effect. Because iodide administration produces improvement within 2–7 days, it is used (along with β-blockers, which antagonise the T 4 -mediated increase in β-adrenoceptors, and other measures) in thyrotoxic crisis (or ‘thyroid storm’ ), a rare but rapid, life-threatening, worsening of hyperthyroidism that is usually triggered by stress.
Iodine radioisotope : 131 I is rapidly absorbed by the gut and concentrated in the thyroid by iodide trapping. It decays by emitting electrons (β − particles) and has a biological half-life of 5 days. The electrons have sufficient energy to penetrate 0.4–2.0 mm and kill large numbers of thyroid cells. Because 131 I crosses the placenta and is secreted into milk, it is not used in pregnant or lactating women. Early fears that the radiation would lead to increased incidence of leukaemias or thyroid cancers have not been borne out. 131 I treatment restores normal thyroid hormone levels in most patients, but hyperthyroidism often re-occurs.
Subtotal thyroidectomy : care must be taken not to remove all the parathyroid glands and to avoid damaging the recurrent laryngeal nerve.
Hormone-secreting tumours, called adenomas , are relatively uncommon but result in significant disease. Adenomas have usually lost the normal endocrine regulation, and therefore the tumour produces hormone in an uncontrolled manner. The commonest endocrine tumour is a prolactinoma , a tumour of the anterior pituitary that secretes prolactin ( Clinical box 10.3 ). The hormone secreted by the tumour presents one set of problems, but in the pituitary gland where space is limited, the presence of a tumour displaces other endocrine cells, resulting in loss of other hormones and the symptoms of hormone deficiencies.
Hyperprolactinaemia is the overproduction of prolactin and may be physiological, e.g. during pregnancy and lactation. Pathological causes include the following:
Prolactin-secreting tumours, which account for over 70% of all anterior pituitary tumours (commonly a microadenoma)
Lesions of the hypothalamus or pituitary stalk
Dopamine (D 2 ) receptor antagonist drugs, which interfere with dopaminergic inhibition of prolactin secretion, e.g. metoclopramide, phenothiazines, etc.
Hyperprolactinaemia causes infertility in both sexes by suppressing the following:
The secretion of gonadotropin-releasing hormone (GnRH)
The gonadotroph response to GnRH
The response of the gonads to luteinising hormone.
Chromosomal abnormalities are responsible for most of the disorders of sexual differentiation, but endocrine disorders can also result from defects of the hormone receptor, such as androgen insensitivity syndrome and, of course, the insulin resistance seen in type 2 diabetes . Finally, it is important to be aware that some endocrine diseases are iatrogenic; for example, the Cushing syndrome , which is commonly caused by prolonged therapeutic use of high levels of glucocorticoids ( Clinical box 10.4 ).
Long-term therapeutic use of pharmacological doses of synthetic glucocorticoids is the commonest cause of Cushing syndrome . The condition could also be secondary to an adrenocorticotropic hormone (ACTH)-secreting adenoma of the pituitary or ectopic ACTH sources (e.g. small cell carcinoma of the lung). Primary Cushing disease is uncommon and usually due to an adrenal tumour. The major features of Cushing syndrome are as follows:
Skin pigmentation, which is infrequent in the secondary syndrome, but is seen particularly in the case of ectopic ACTH-secreting tumours. It is not seen in the primary disease because, here, ACTH concentrations are low
Hyperglycaemia, which results because of the diabetogenic effect of glucocorticoids, i.e. they raise blood glucose concentration
Increased appetite, with truncal obesity adding to insulin resistance
Increased proteolysis, which reduces muscle mass, with weakness and wasting, especially of the proximal limb muscles
Enhanced actions of catecholamines, resulting in hypertension
Bone demineralisation and hence osteoporosis , which often leads to pathological fractures
Decreased collagen synthesis, which increases wound healing time and is the cause of abdominal striae
Immune suppression, which increases the risks of infection
Increased erythropoietin production, which increases red cell number.
When endocrine disorders are suspected, it is usual to carry out a range of clinical tests. In most cases, hormone concentrations are measured directly in blood samples . However, there are many factors that need to be taken into consideration when endocrine tests are carried out. A key factor is the pattern of hormone secretion. It is obviously easiest to take a blood sample and measure hormone levels when the patient happens to be in a clinic. This is entirely appropriate for some hormones, notably calcitriol and thyroid hormones. Both these hormones have fairly constant levels in plasma, as they are maintained at a set point. It therefore does not matter when the hormone is measured. For hormones with a pronounced diurnal rhythm, this can be used to judge the most appropriate time for sampling. In the case of cortisol, which peaks in the morning and has a low point around midnight, the morning peak can be used to determine whether there is sufficient cortisol secretion. Determining whether there is over-secretion of a hormone is more difficult. For growth hormone, which is often undetectable during the day, but which exhibits episodic secretion, there is no good time to take a blood sample. One way of getting around these problems is to use dynamic testing of endocrine systems ( Clinical box 10.5 ).
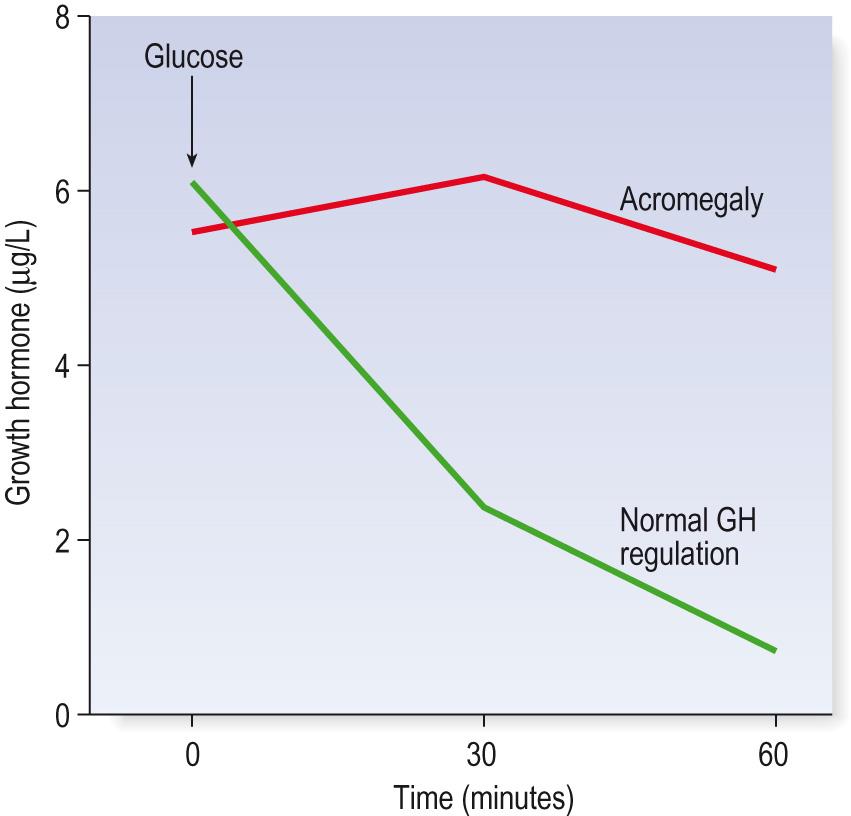
Growth hormone is secreted in an episodic manner. It is very often completely undetectable during the day, but there are peaks of secretion. A patient with excess growth hormone secretion has plasma growth hormone that is always detectable, but this is difficult to differentiate from a normal secretory peak.
In order to be certain of the diagnosis, one needs to perform a dynamic test. This uses the negative feedback effect of high blood glucose on growth hormone. One of the effects of growth hormone is to increase blood glucose; therefore, a rise in blood glucose concentration acts on the hypothalamus and pituitary to suppress further growth hormone secretion. In a person with normal secretion, a rise in blood glucose leads to a fall in blood growth hormone, making it undetectable within 60 minutes. In a patient with abnormal secretion (e.g. acromegaly ), growth hormone suppression by glucose will not occur. Therefore, a patient is fasted overnight and then given 50 g of glucose in a drink. Growth hormone is measured after 60 minutes. If it is still detectable, then this confirms growth hormone excess ( Fig. 10.8 ) .
The principles of dynamic testing are very simple: a challenge is applied to the endocrine system, and the hormonal response is measured. If a clinician suspects that there is a hormone deficiency, then the challenge used is designed to stimulate hormone secretion. If there is a suspected hormone excess, then the challenge is designed to inhibit hormone secretion.
In order to investigate endocrine disease, it is important to be able to measure circulating hormone levels. The fact that hormones circulate in such low concentrations makes this measurement more challenging than most clinical assays. In the early days of endocrinology, hormone assays relied on measuring the responses of a biological system to different hormone concentrations. These bioassays were expensive, relatively insensitive and highly variable. Now, it is possible to purchase kits to reliably and reproducibly measure every hormone. The modern kits use immunological methods to detect small concentrations of a hormone ( Information box 10.5 ).
As hormones circulate in such low concentrations, usually in the nanomolar range, measuring hormones in blood presents a significant challenge. The earliest hormone measurements used a biological response to a hormone, i.e. bioassays. Pregnancy tests, for example, used an animal that would be injected with the urine from a patient. A positive test would be seen if the animal ovulated. This type of test was unreliable and raised significant ethical issues. Modern testing uses immunological methods for measuring hormone concentrations, relying on specific anti-hormone antibodies. The most common methods are immunometric assays and immunoassays. Immunometric assays are more sensitive and more readily automated than immunoassays and therefore more widely used. These tests are simple to perform, very reliable and rapid. It is now possible to buy assay kits that measure all the known hormones at the concentrations found in human blood.
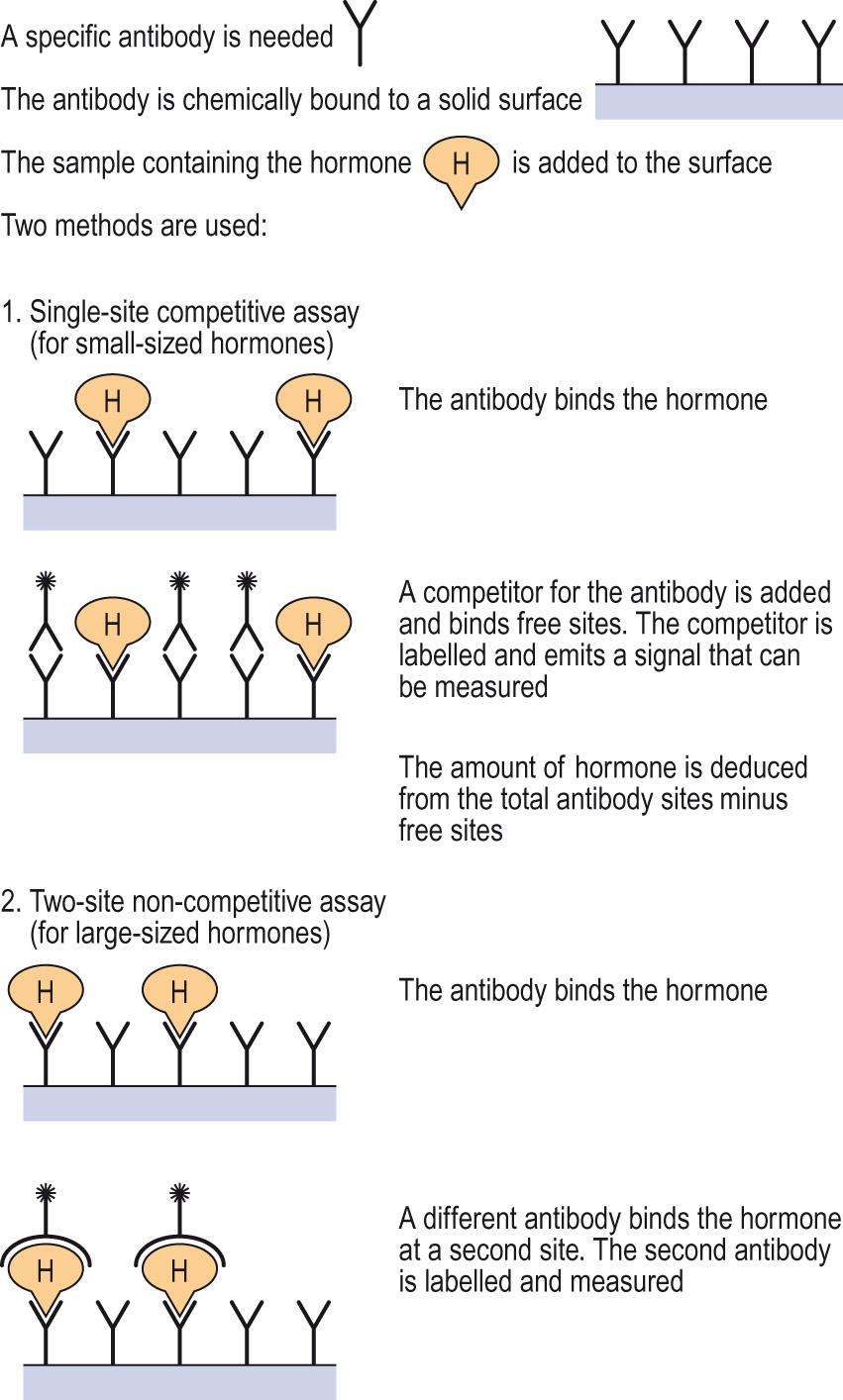
The principle of immunoassay ( RI ) is that an unknown amount of hormone and a known amount of labelled hormone compete for a limited number of binding sites on anti-hormone antibodies.
Known amounts of labelled hormone and antibody are added to samples containing an unknown hormone concentration. The label may be a radioactive tracer ( radioimmunoassay , RIA ), a fluorescent molecule or some other marker.
The lower the unknown concentration of hormone in the samples, the greater the amount of labelled hormone that binds the antibody.
A variety of methods are then used to separate antibody-bound and free hormone, and the ratio of bound to free labelled hormone (B/F) is measured.
The B/F ratio is a measure of the competition between labelled and non-labelled hormone, and the unknown hormone concentration is found by comparison with a standard curve prepared from a range of known concentrations of unlabelled hormone.
This method uses two antibodies that bind to different epitopes on the hormone.
One antibody is adsorbed onto the surface of a multi-well plate. This serves to anchor the unknown amount of hormone present in the samples added to the wells. The antibody must be present in large excess to ensure all the hormone is captured.
The second reporter antibody is now added, and the amount of this antibody that binds is in direct proportion to the amount of anchored hormone. The reporter antibody is so called because it bears an easily measured marker such as a fluorescent tag or an enzyme.
When the marker is an enzyme, the assay is called an enzyme-linked immunosorbent assay ( ELISA ).
The hypothalamus and pituitary are important coordinators of the endocrine system. The hypothalamus is part of the brain and integrates a variety of different internal and external inputs to produce a range of neural and hormonal outputs that control various homeostatic functions. The role of the hypothalamus in the regulation of appetite is covered in Chapter 16 , whereas its role in the control of blood pressure and volume is covered in Chapter 11 . The hypothalamic nuclei produce an array of regulatory hormones that control the hormonal output of the anterior pituitary gland. These pituitary hormones, in turn, regulate the activity of the thyroid gland, the adrenals and the gonads, as well as control growth.
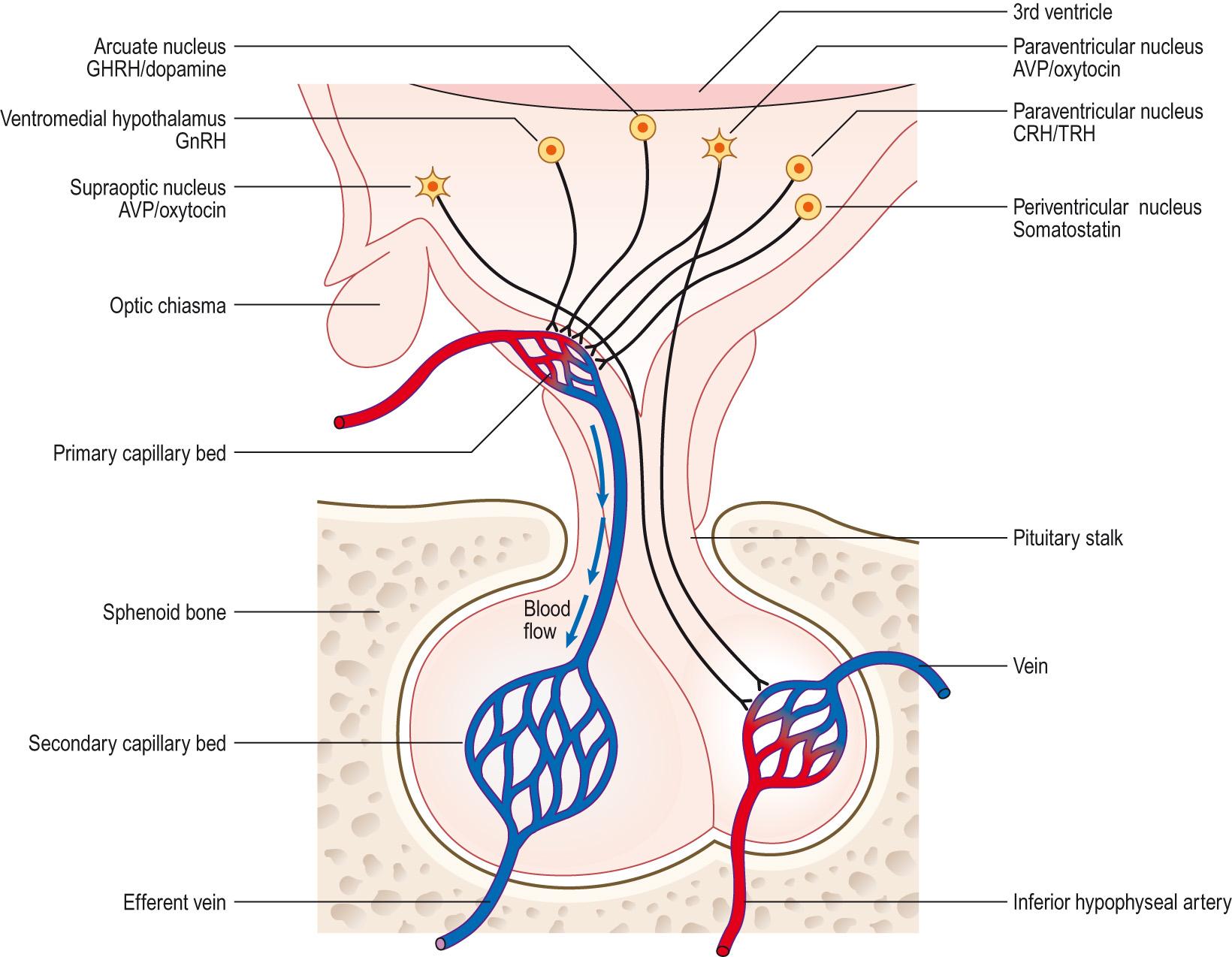
The hypothalamus is located at the base of the brain, adjacent to the third ventricle ( Fig. 10.10 ) . Like all brain areas, it is composed of a collection of nuclei, several of which are neurosecretory. The hypothalamus is connected to the pituitary by the pituitary stalk, which contains both neural and vascular elements ( Fig. 10.11 ) . The pituitary gland is formed from two quite distinct tissues. The posterior pituitary is derived from neural ectoderm and is connected to the hypothalamus by the axons of secretory neurons. These neurons have their cell bodies in the hypothalamus, but have their nerve endings in the posterior pituitary. The anterior pituitary is derived from an outgrowth of the oral ectoderm, called the Rathke pouch, which grows to meet the neural ectoderm of the posterior pituitary and finally separates from the buccal cavity. The anterior pituitary is joined to the hypothalamus by a portal vascular system, which has its primary capillary bed in the hypothalamus and the secondary capillary bed in the anterior pituitary. Hormones secreted by the hypothalamic neurons into the portal system act on the cells of the anterior pituitary ( Information box 10.6 ).
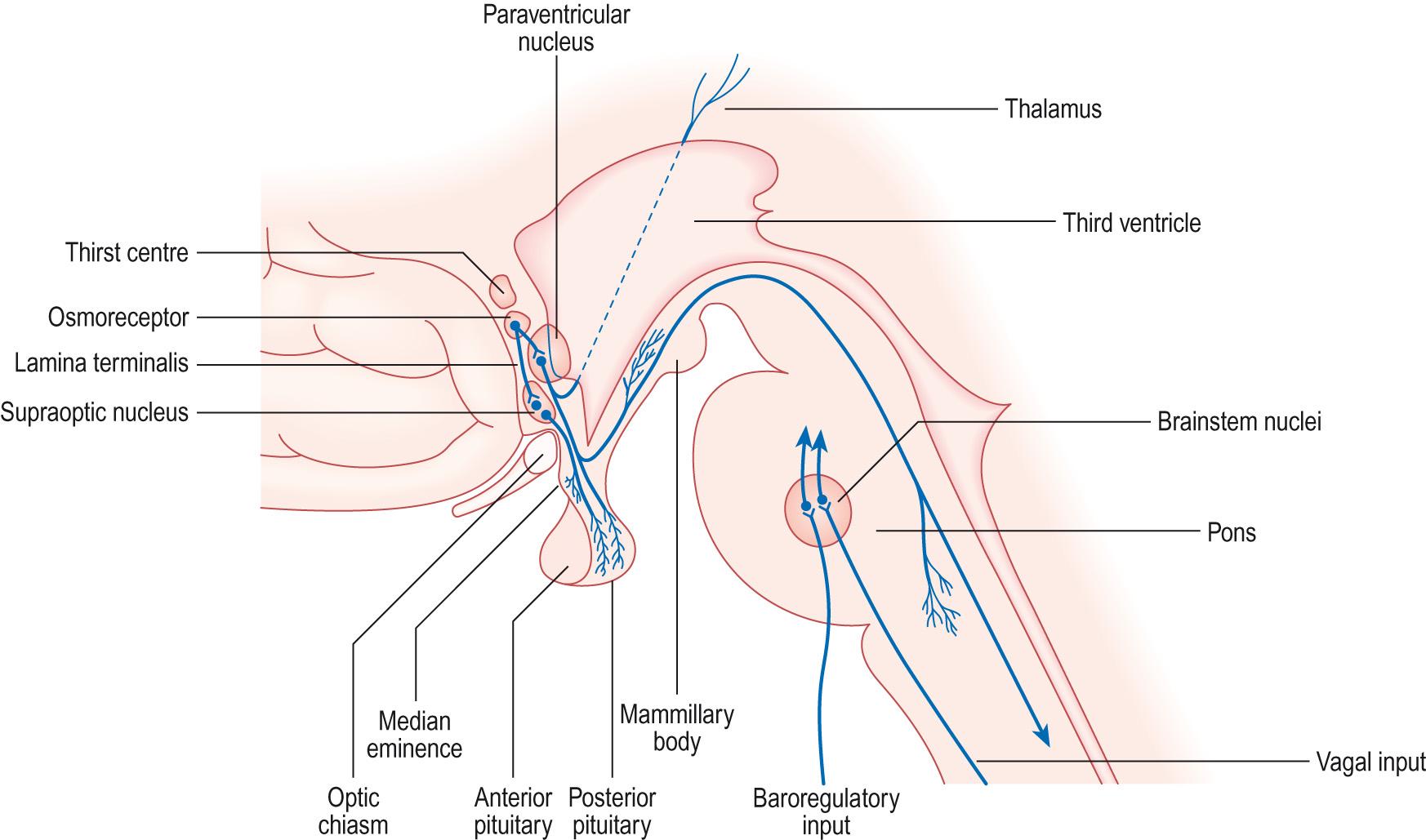
The posterior pituitary used to be called the neurohypophysis and the anterior pituitary the adenohypophysis (with ‘hypophysis’ pronounced as ‘high-poff-iss-iss’). These terms have largely gone out of fashion, and the reference to the pituitary as the ‘hypophysis’ is now used only when describing the removal of the pituitary, which is called ‘hypophysectomy’.
The pituitary gland is located in a hollow in the sphenoid bone and is almost completely surrounded by bone. A tumour or other increase in size of the pituitary results in pressure upwards, towards the hypothalamus. The proximity of the optic chiasma to the hypothalamus means that a pituitary tumour causes pressure on this structure, resulting in visual disturbances. These are important diagnostic features of a pituitary tumour (see Clinical box 10.6 for hypopituitary states).
Total loss of pituitary function is rare, but can occur as a result of accident, injury or surgery, or in the presence of a space-occupying tumour of the pituitary gland. One cause of loss of pituitary function, which is fortunately now rare in the developed world, is Sheehan syndrome , where the pituitary suffers a collapse of its blood supply (infarction) as a result of severe blood loss during childbirth.
If there is a loss of all pituitary hormones ( panhypopituitarism ), then it is important to treat the patient with hormone replacement. The pituitary hormones are all peptides and would have to be administered by injection; therefore, more commonly a patient is given the replacement end products of the axes controlled by the pituitary, thyroxine, cortisol and sex steroids, which can all be administered orally. It is now recognised that growth hormone has beneficial effects in adults, and, therefore, this is also administered, but has to be injected.
The posterior pituitary secretes two peptide hormones, oxytocin and arginine vasopressin, which used to be called anti-diuretic hormone. The secretion and actions of this hormone are covered in Chapter 14 . Both oxytocin and vasopressin are short peptides (eight amino acids), synthesised from a large precursor called neurophysin in the axons of nerves from the supraoptic and paraventricular nuclei, which have their nerve endings in the posterior pituitary (see Fig. 10.11 ). The hormones are contained within secretory granules and released into the circulation in response to an action potential. The secretion of these hormones is part of a neuroendocrine reflex. Both hormones act on smooth muscle, vasopressin in the cardiovascular system and oxytocin in the uterus and breast ducts, causing contraction of the muscle. Vasopressin, however, has its major effects on the kidney (see Ch. 14 ). Although oxytocin has its classical effects in parturition and lactation (see pp. 678 – 681 ), it is now also recognised that this hormone has profound effects on the brain. The oxytocin released during lactation, for example, strengthens the mother–child bond. Popularly known as the ‘love hormone’ or ‘cuddle hormone’, oxytocin is also released in response to hugging and during orgasm, and acts to increase trust and partner bonding. This has caused a resurgence of interest in oxytocin, particularly as it seems to have such a significant part to play in complex human behaviours. For example, it appears to have a prominent role in the development of trust. In terms of therapeutic applications, the most promising appears to be in childhood autism, where there is increasing evidence that the administration of oxytocin can improve social interaction.
The hypothalamus secretes a range of ‘releasing hormones’ that bind to receptors on the anterior pituitary cells and modify their function. The main effect of these releasing hormones is to stimulate the release of hormones from the anterior pituitary. The anterior pituitary hormones then act on other endocrine tissues and thus form the central part of an endocrine axis.
The anterior pituitary secretes six hormones, which are all regulated by hypothalamic hormones. Five of these hormones are regulated by stimulatory hormones from the hypothalamus:
Adrenocorticotropin hormone ( ACTH ) is released by pituitary corticotrope cells in response to corticotropin-releasing hormone ( CRH ), a 41-amino acid peptide from the hypothalamic paraventricular nucleus. ACTH acts on the adrenal to stimulate the release of cortisol.
Luteinising hormone ( LH ) and follicle-stimulating hormone ( FSH ) are collectively known as the gonadotropins. They are released from gonadotropic cells in the pituitary when stimulated by gonadotropin-releasing hormone ( GnRH ), a 10-amino acid peptide that is mainly made in the pre-optic area of the hypothalamus. The gonadotropins cause the gonads to secrete sex steroids.
TSH , also called thyrotropin , is released from pituitary thyrotrope cells when stimulated by TRH, a 3-amino acid peptide produced in the medial neurons of the hypothalamic paraventricular nucleus. TSH causes the thyroid to release T 4 . In each of these cases, the anterior pituitary hormone causes the final endocrine gland, adrenals, gonads or thyroid, to produce a hormone that exerts a negative feedback inhibition of the hypothalamus, preventing further release of the ‘releasing hormone’ and shutting down the axis.
The fifth pituitary hormone is growth hormone (also known as somatotropin ), which is partly regulated by growth hormone-releasing hormone ( GHRH ), a 44-amino acid peptide produced by the arcuate nucleus of the hypothalamus. The other factors involved in the complex regulation of growth hormone secretion are discussed later.
The hypothalamic releasing hormones are all peptides. However, the hypothalamus also produces two release-inhibiting hormones: prolactin from the arcuate nucleus and somatostatin from the periventricular nucleus.
Prolactin is unique in endocrinology. It is the only hormone whose secretion is under tonic inhibitory regulation. If the connection between the hypothalamus and the pituitary is cut, then the secretion of all the other anterior pituitary hormones falls dramatically. The exception is prolactin, which needs the influence of the hypothalamus to keep its secretion in check. Without the hypothalamus, prolactin secretion increases several-fold ( Clinical box 10.7 ). In order to achieve this effect, the hypothalamus produces a release-inhibiting factor, dopamine , the only one of the hypothalamic hormones that is not a peptide. Dopamine is a catecholamine derived from tyrosine and is an intermediate in adrenaline production. It is normally considered to be a neurotransmitter, but in the control of prolactin secretion, it is a neurohormone, like all the other hypothalamic hormones. Dopamine acts on the lactotrope cells of the anterior pituitary and inhibits prolactin secretion.
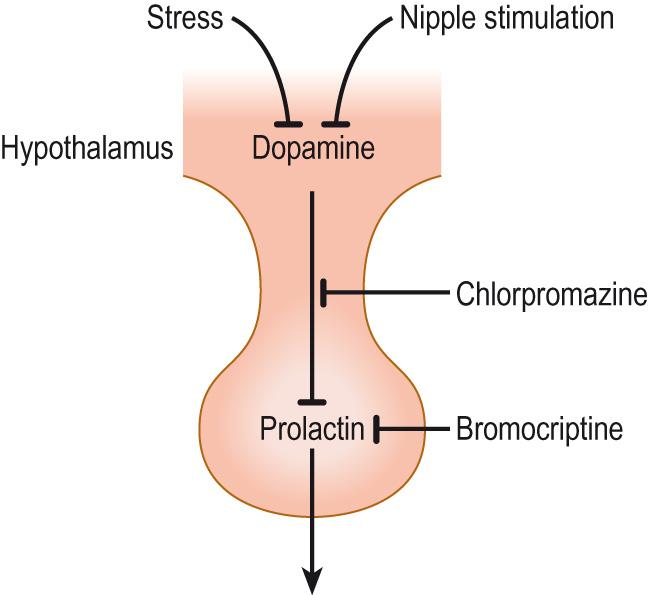
Hyperprolactinaemia is the over-production of prolactin. Pathological causes include the following:
Prolactin-secreting tumours, which account for over 70% of all anterior pituitary tumours (commonly a microadenoma)
Lesions of the hypothalamus or pituitary stalk
Dopamine (D 2 ) receptor antagonist drugs, which interfere with dopaminergic inhibition of prolactin secretion, e.g. metoclopramide, phenothiazines, etc.
Hyperprolactinaemia causes infertility in both sexes by suppressing the following:
The secretion of gonadotropin-releasing hormone (GnRH)
The gonadotroph response to GnRH
The response of the gonads to luteinising hormone.
Treatment of hyperprolactinaemia with a D 2 receptor agonist (e.g. bromocriptine) is usually effective (see Fig. 10.12 ). Surgery may be required for pituitary tumours.
Growth hormone is produced by the somatotrope cells of the anterior pituitary and is under dual regulation of a hypothalamic releasing hormone, GHRH, and an inhibitory hormone, somatostatin. We shall go on to consider the regulation of growth hormone secretion and its role in the regulation of growth.
Between birth and adolescence, children grow in a fairly predictable way. There are childhood growth charts that allow clinicians to compare the growth of their young patients with the published average, enabling them to determine whether growth is progressing as it should. These charts are gender specific and are constructed using both height and weight measures ( Fig. 10.13 ) . Body mass index ( BMI ), which is the weight in kilograms divided by the square of the height in metres, is not used as a clinical measure in children as this varies greatly through childhood. At puberty, there is a growth spurt that lasts approximately 4 years, during which a child can increase in height by approximately 10 cm each year.
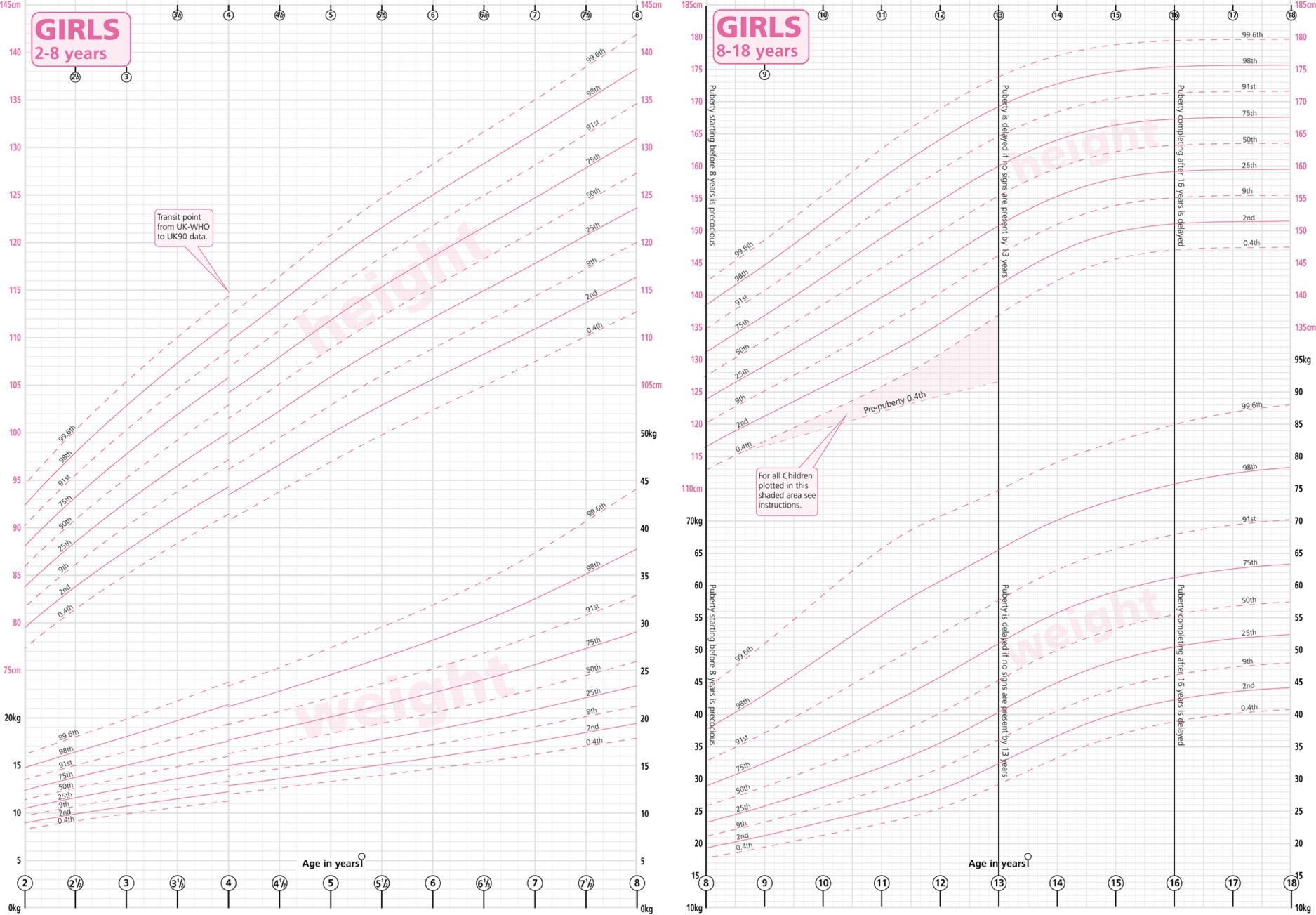
Growth hormone is the main driver of growth in children: the level of circulating growth hormone increases throughout childhood, peaking at the age of approximately 15 years, and then gradually declines through the rest of life. However, in order for childhood growth to proceed normally, the entire endocrine system needs to be working properly. Although appropriate growth hormone secretion is essential for normal growth, thyroid function is also important as T 4 has a significant permissive effect. Cortisol and insulin secretion are also required, along with appropriate hormonal regulation of calcium metabolism. There are several factors that can adversely affect childhood growth. These include chronic illness and psychosocial deprivation, as well as endocrine disease.
Growth hormone is a polypeptide hormone secreted by the anterior pituitary. It is a member of a family of hormones that includes prolactin and human chorionic somatomammotropin ( hCS , also known as human placental lactogen , hPL ). The gene encoding growth hormone ( GH1 ) is located on the long arm of chromosome 17, as part of a cluster of five highly conserved genes. The other genes in this cluster encode three different versions of hCS and a second form of growth hormone. GH1 encodes a 191-amino acid peptide (22 kDa), which is known as growth hormone and is mainly expressed in the pituitary somatotrope cells, whereas the other genes in this cluster are mainly expressed in the placenta.
The synthesis and secretion of growth hormone are regulated by the hypothalamus: a releasing hormone, GHRH, and an inhibitory hormone, somatostatin , act together to produce pulses of growth hormone secretion ( Fig. 10.14 ) . Somatostatin is also known as growth hormone inhibitory hormone and somatotropin release inhibitory factor . These names all refer to the same 14-amino acid hormone and are used interchangeably. The major stimuli to GHRH secretion are sleep, exercise, hypoglycaemia and high circulating concentrations of certain amino acids, particularly arginine. The satiety hormone produced by the gastric mucosa, ghrelin ( Growth Hormone RELease INducer ), is also a potent stimulus to GHRH secretion. Secretion of GHRH and growth hormone is subject to negative feedback inhibition by insulin-like growth factor 1 ( IGF-1 ) and glucose ( Clinical box 10.8 ).
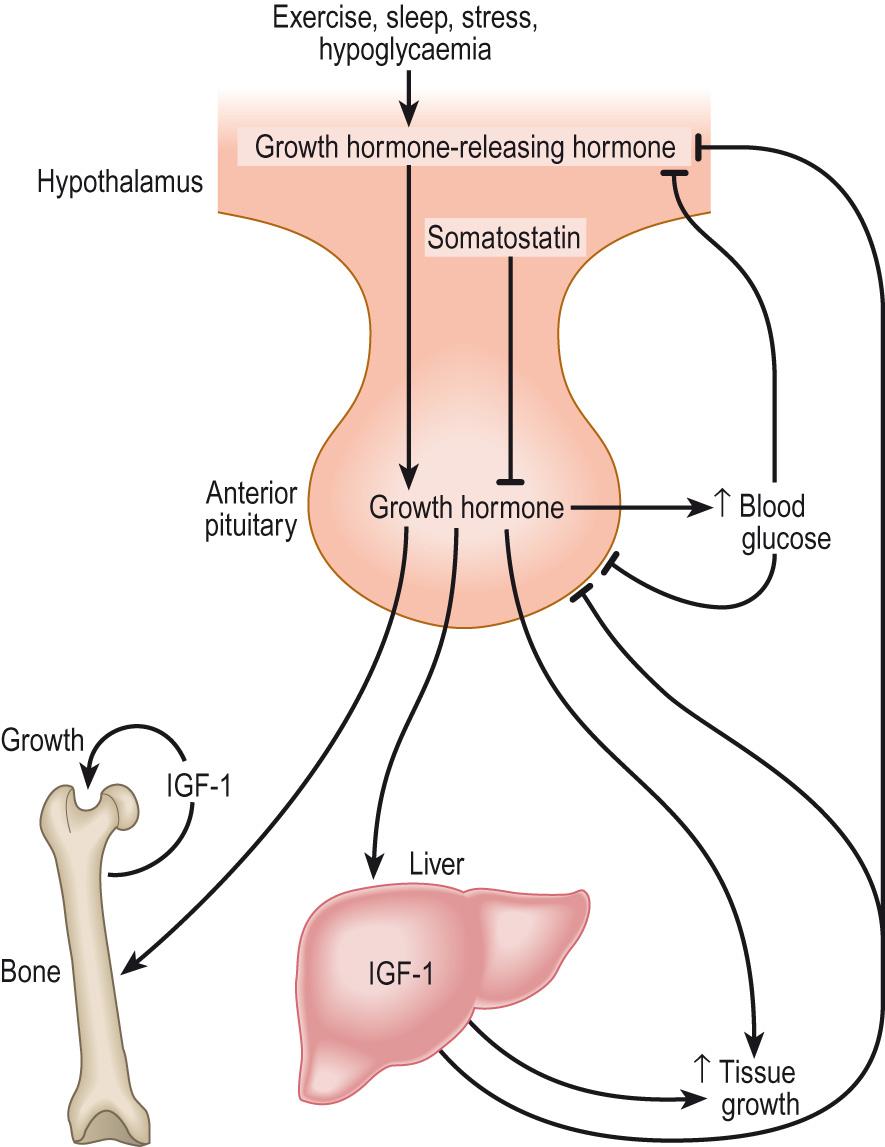
If a clinician suspects insufficient growth hormone secretion in a patient, tests that are designed to provoke its secretion can be used. Potentially, any of the known stimuli to growth hormone secretion could be used: sleep, exercise, etc. Historically, hypoglycaemia induced by an injection of insulin was used as a clinical test. It was simple and gave reproducible standardised results. However, over time, the dangers of insulin-induced hypoglycaemia became clearer and the current standard growth hormone stimulation test involves an intravenous infusion of arginine.
If a clinician suspects over-secretion of growth hormone, then the test is designed to suppress its secretion. The simplest test is to administer a glucose drink to the patient, who has fasted overnight. In most people, this will cause growth hormone secretion to be suppressed and blood levels of growth hormone to be undetectable after an hour. If the patient has a pituitary tumour secreting growth hormone, the most likely cause of high growth hormone levels, then it will not be suppressed by the glucose and after an hour blood growth hormone levels will still be measurable.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here