Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The endocrine system is responsible for the synthesis and secretion of chemical messengers known as hormones . Hormones may be disseminated throughout the body by the bloodstream, where they may act on specific target organs or affect a wide range of organs and tissues. Other hormones act locally, often arriving at their site of action by way of a specialised microcirculation. In conjunction with the nervous system, hormones coordinate and integrate the functions of all the physiological systems.
As a general rule, endocrine glands are composed of islands of secretory epithelial cells with intervening supporting tissue, rich in blood and lymphatic capillaries. The secretory cells discharge hormone into the interstitial spaces and it is rapidly absorbed into the circulatory system.
Reflecting their active hormone synthesis, cells of the endocrine system have prominent nuclei and abundant mitochondria, endoplasmic reticulum, Golgi bodies and secretory vesicles. The nature of the secretory vesicles varies according to the hormone secreted. There are four main groups of chemicals which can act as hormones:
Protein and glycoprotein molecules, e.g. insulin, growth hormone, parathyroid hormone (PTH).
Small peptide molecules, e.g. vasopressin, products of enteroendocrine cells, e.g. glucagon-like peptide 1 (GLP-1).
Amino acid derivatives, e.g. thyroxine, adrenaline (epinephrine) and noradrenaline (norepinephrine).
Steroids derived from cholesterol, e.g. adrenal cortical hormones, ovarian and testicular hormones.
Endocrine cells which produce hormones based on amino acids, peptides and proteins often have characteristic membrane-bound secretory vacuoles with electron-dense central cores ( dense core granules ).
The endocrine system can be divided into three parts:
The major endocrine organs in which the sole or major function of the organ is the synthesis, storage and secretion of hormones (e.g. thyroid and adrenal glands)
Endocrine components within other solid organs , for example, the endocrine components of the pancreas, ovary, testis and kidney, in the form of clusters of endocrine cells within other tissues
The diffuse endocrine system , scattered individual hormone cells (or small clumps), usually within an extensive epithelium (e.g. gastrointestinal and respiratory tracts). The major function of these cells is probably paracrine (i.e. acting on adjacent non-endocrine cells, rather than entering the bloodstream and producing systemic effects).
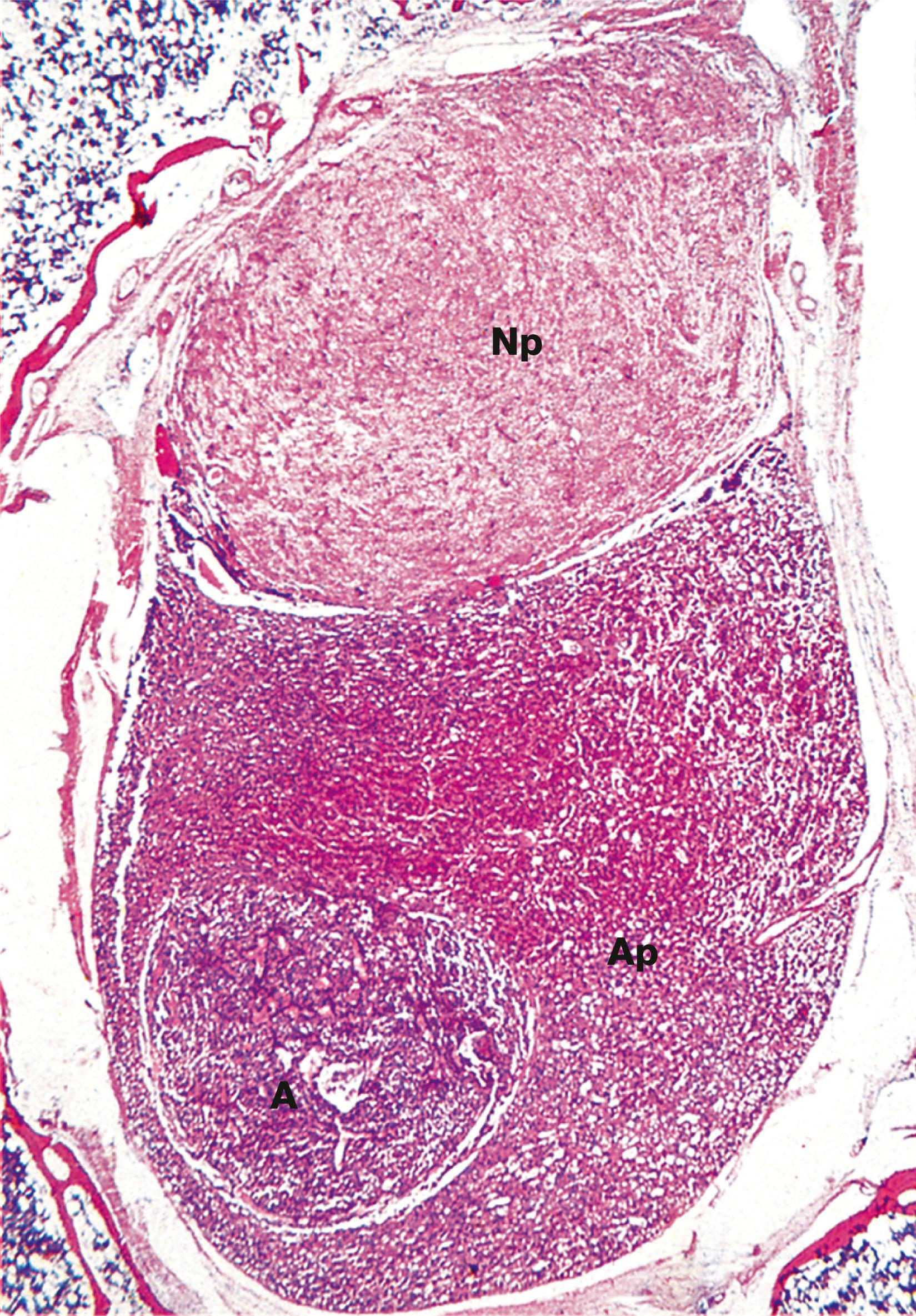
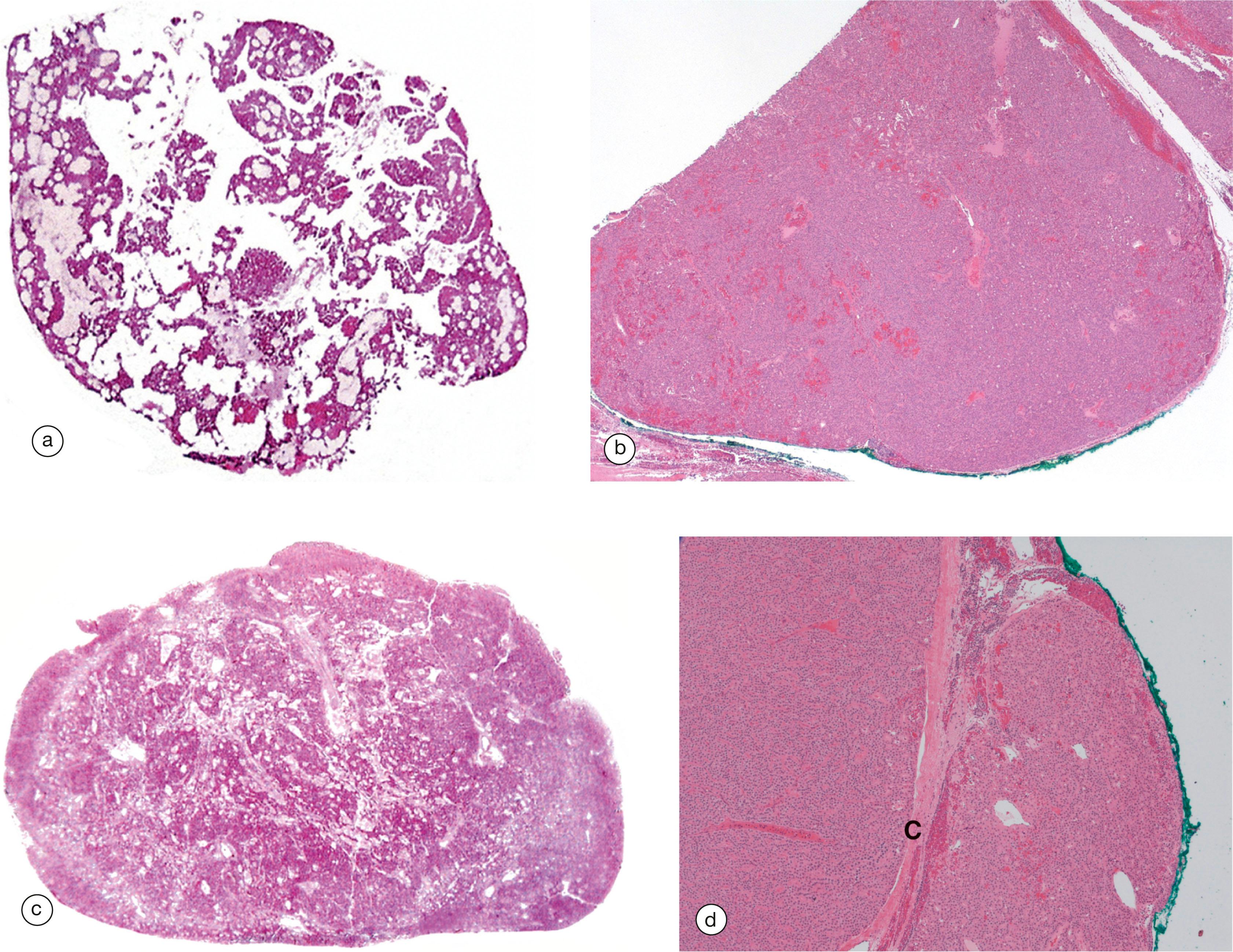
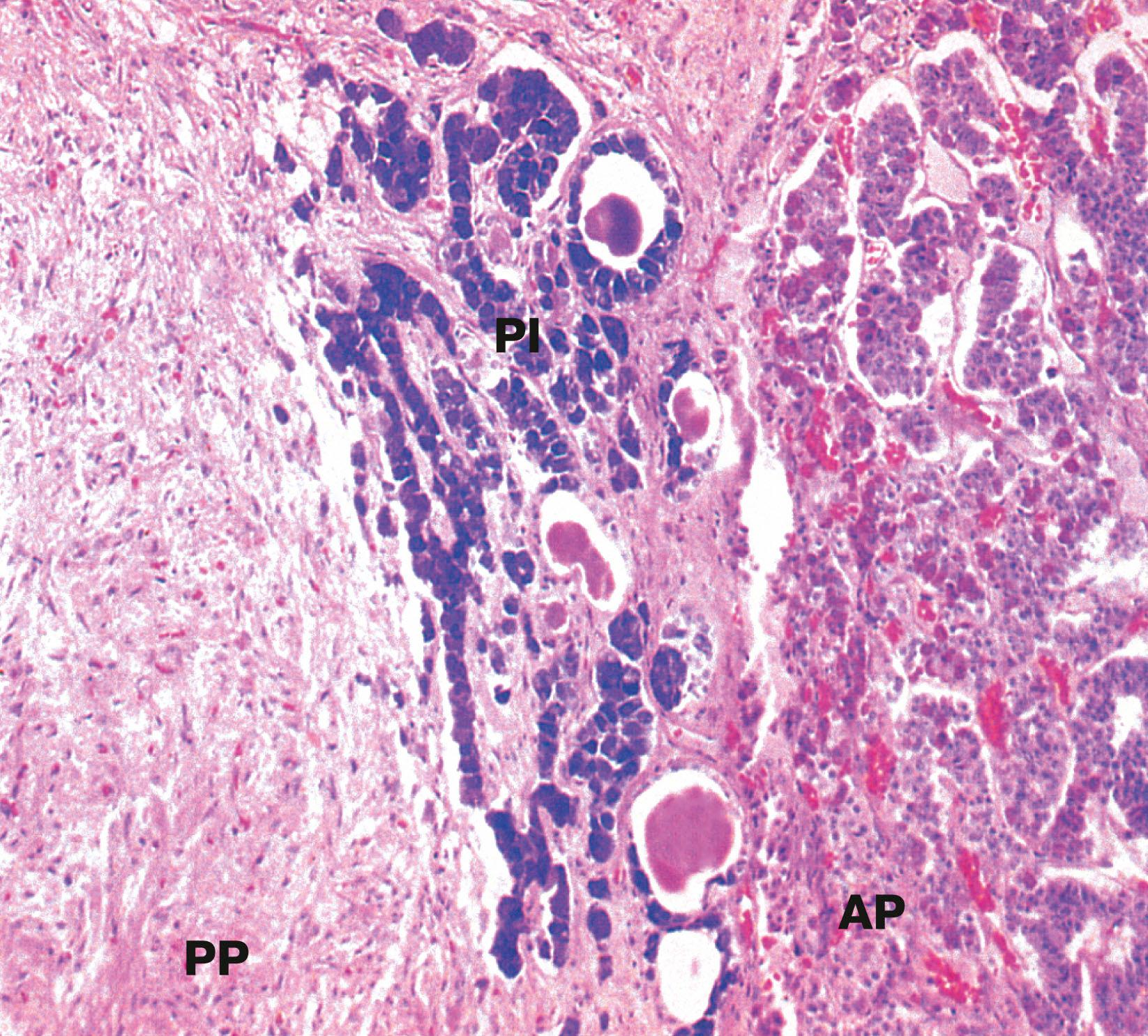
The pituitary gland ( hypophysis ) is a small bean-shaped gland, about 1 cm diameter, at the base of the brain beneath the third ventricle, sitting in a bony cavity in the base of the skull (the sella turcica ). The gland is divided into anterior and posterior parts which have different embryological origins, functions and control mechanisms.
The secretion of all major pituitary hormones is controlled by the hypothalamus, which itself is under the influence of nervous stimuli from higher centres in the brain. Control is mainly by feedback from the levels of circulating hormones produced by pituitary-dependent endocrine tissues.
The pituitary hormones fall into two functional groups:
Hormones which act directly on non-endocrine tissues: growth hormone (GH), prolactin, antidiuretic hormone (ADH, vasopressin), oxytocin and melanocyte stimulating hormone (MSH).
Hormones which modulate the secretory activity of other endocrine glands (trophic hormones): thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH) and the gonadotrophic hormones, follicle stimulating hormone (FSH) and luteinising hormone (LH).
Thus the thyroid gland, adrenal cortex and gonads may be described as pituitary-dependent endocrine glands .
A acidophil AP anterior pituitary B basophil C corticotroph Ca capillary Cp chromophobe F fenestration G gonadotroph H hypothalamus O optic chiasma P pituitary stalk PP posterior pituitary S somatotroph T thyrotroph V third ventricle
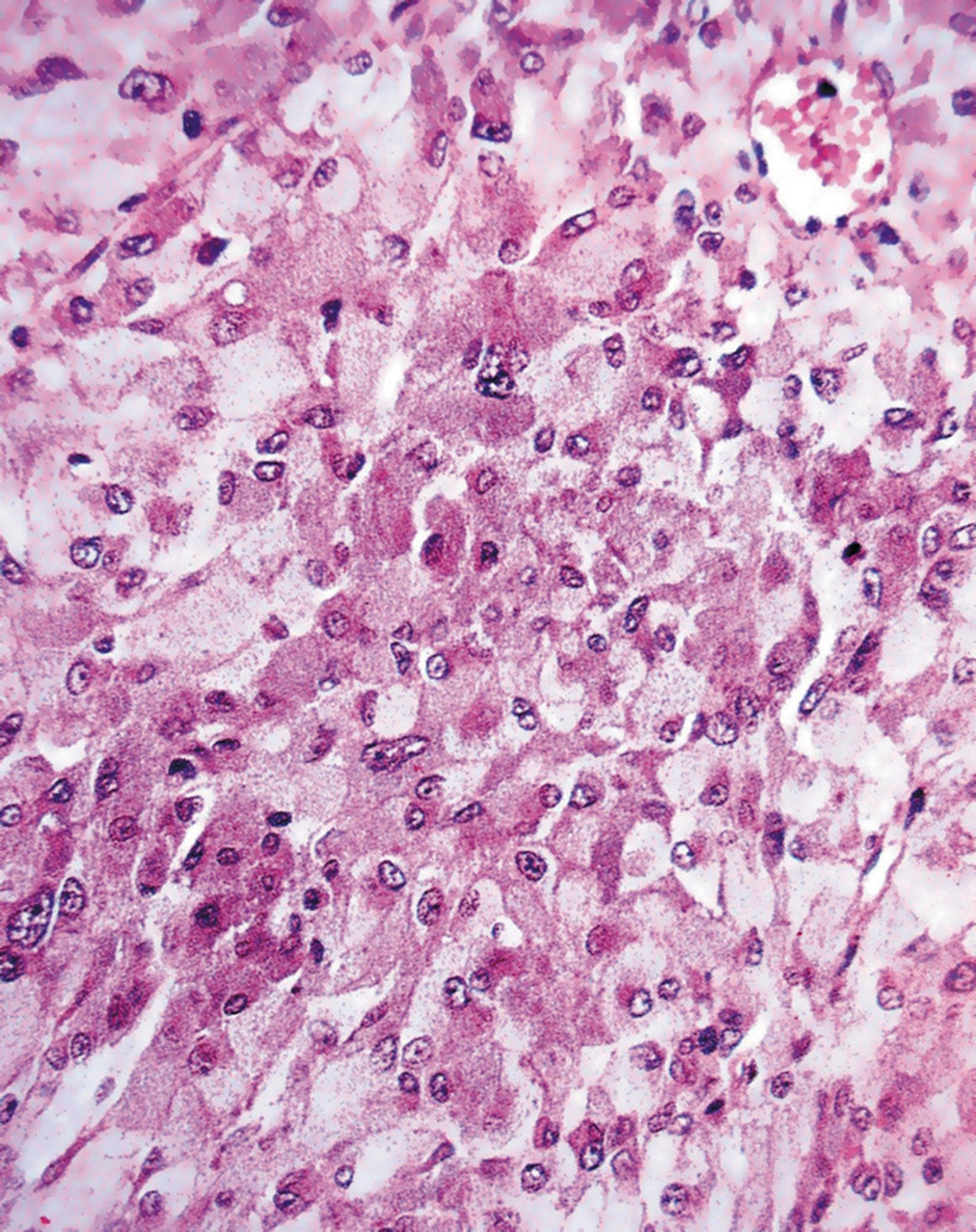
The most common disease of the pituitary is a pituitary adenoma. These tumours are classified as benign because they do not invade adjacent tissues. However, they may have serious or even fatal consequences. They cause their effects by the excessive, continuous production of hormone, uncontrolled by any feedback mechanisms. Thus a tumour of corticotrophs secretes excess ACTH, stimulating the adrenals to produce large quantities of corticosteroid, leading to Cushing’s disease. Tumours of somatotrophs produce excess growth hormone, causing gigantism in children or acromegaly in adults. Some pituitary adenomas produce no hormones but grow locally so large that they grow upwards out of the sella turcica to compress and damage the overlying optic chiasma and nerves, leading to visual disturbance and eventual blindness.
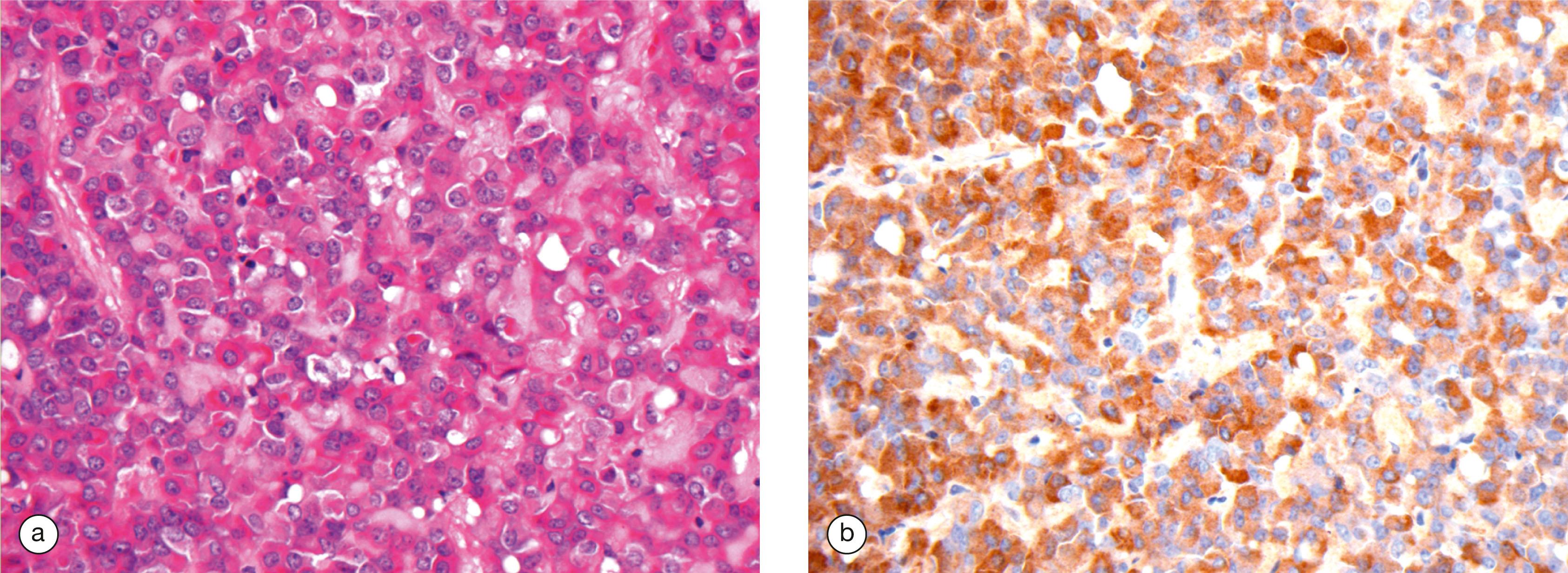
The pituitary gland can rarely be destroyed by disease compromising its blood supply, leading to necrosis of the cells and failure of hormone output (panhypopituitarism).
The thyroid gland is a butterfly-shaped endocrine gland lying in the neck in front of the upper part of the trachea. The thyroid gland produces hormones of two types:
Iodine-containing hormones tri-iodothyronine ( T 3 ), and thyroxine ( tetra-iodothyronine , T 4 ); T 4 is converted to T 3 in the general circulation by removal of one iodothyronine unit, although a small amount of T 3 is secreted directly. T 3 is much more potent than T 4 and appears to be the metabolically active form of the hormone. Thyroid hormone regulates the basal metabolic rate and has an important influence on growth and maturation, particularly of nerve tissue. The secretion of these hormones is regulated by TSH secreted by the anterior pituitary.
The polypeptide hormone calcitonin ; this hormone regulates blood calcium levels in conjunction with parathyroid hormone. Calcitonin lowers blood calcium levels by inhibiting the rate of decalcification of bone by osteoclastic resorption and by stimulating osteoblastic activity. Control of calcitonin secretion is dependent only on blood calcium levels and is independent of pituitary and parathyroid hormone levels.
The thyroid gland is unique among the human endocrine glands in that it stores large amounts of hormone in an inactive form within extracellular compartments in the centre of follicles; in contrast, other endocrine glands store only small quantities of hormones in intracellular sites.
The main bulk of the gland develops from an epithelial downgrowth from the fetal tongue, whereas the calcitonin-secreting cells are derived from the ultimobranchial element of the fourth branchial pouch.
AP anterior pituitary F thyroid follicle H Herring body PI pars intermedia PP posterior pituitary
An excess of circulating thyroid hormone causes clinical symptoms due to the systemic effects of thyroid hormone. This is called thyrotoxicosis . Clinical symptoms include palpitations, fatigue, weight loss and anxiety. Blood tests for thyroid stimulating hormone and thyroid hormones (T3 and T4) can help to determine the underlying cause of thyroid disease. If TSH is elevated, and T3 and T4 are reduced, then a primary cause in the thyroid gland is likely, e.g. Graves disease. If TSH is elevated and T3/T4 are elevated, then a secondary cause, e.g. a pituitary adenoma that secretes TSH, could be the cause. A lack of production of thyroid hormone leads to symptoms of thyroid hormone deficiency, termed hypothyroidism . These symptoms include tiredness, weight gain and poor tolerance to cold weather. The most common cause of hypothyroidism is the auto-immune condition Hashimoto’s thyroiditis .
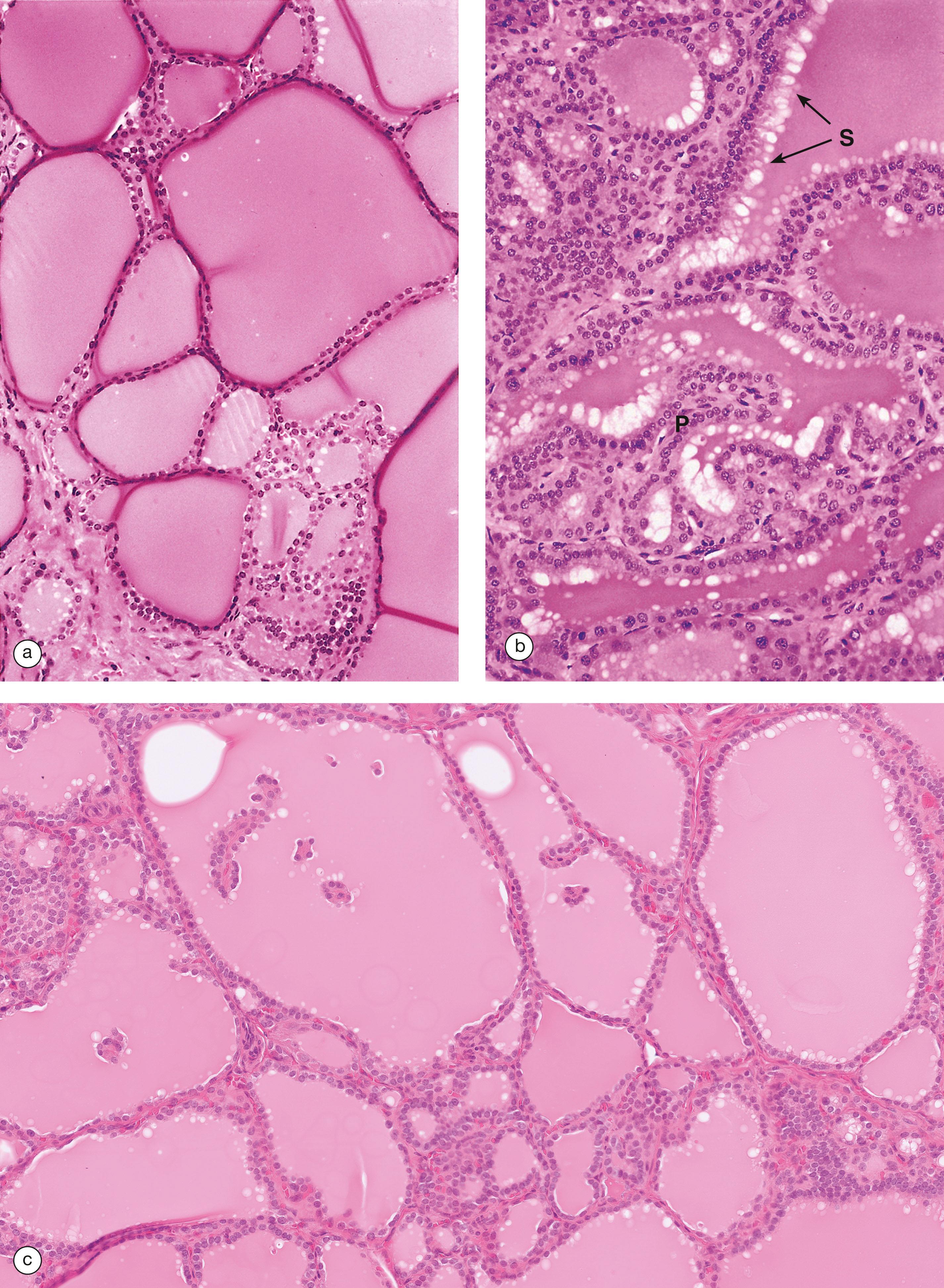
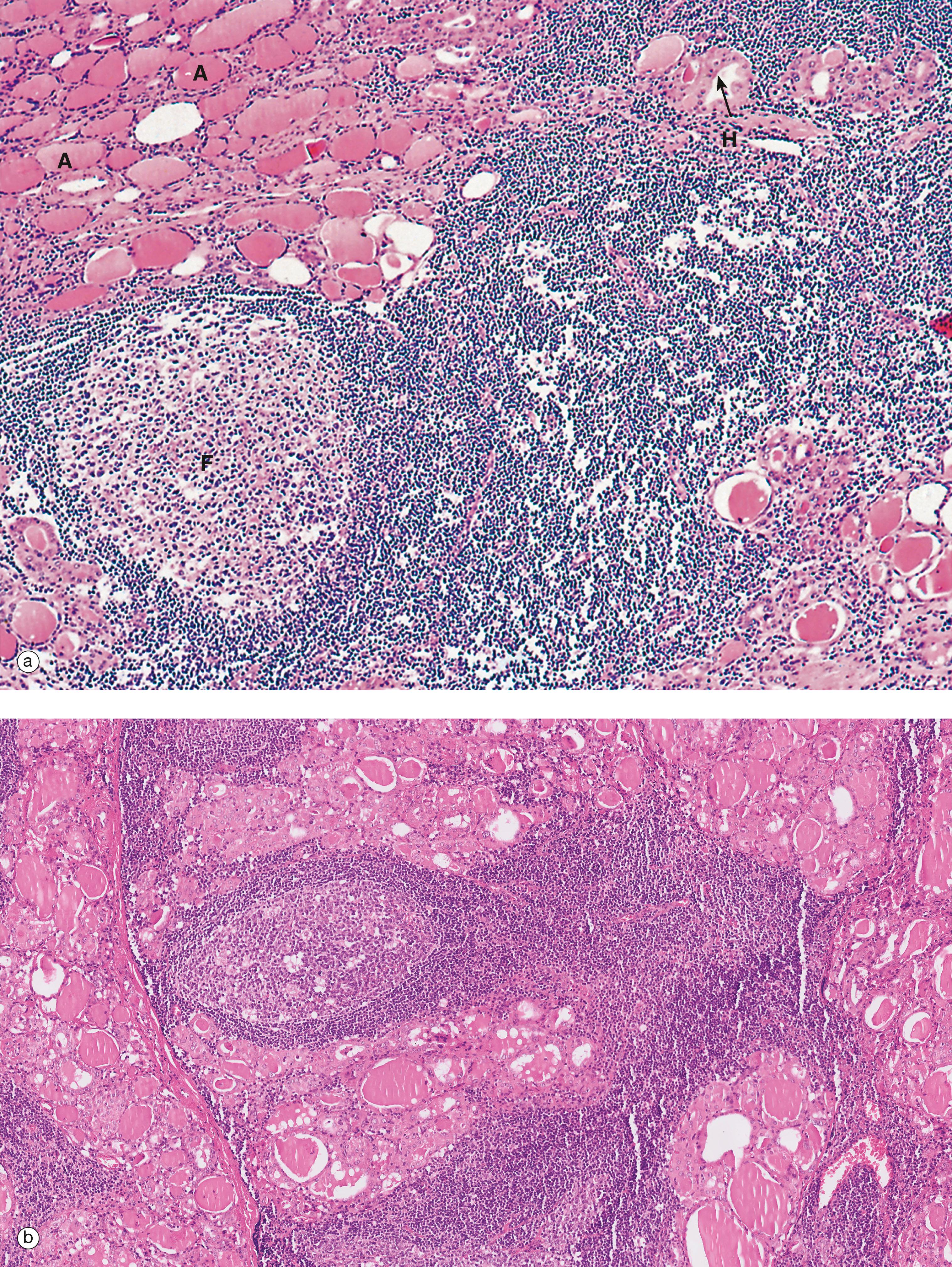
BM basement membrane C C cell Cap capillary Co colloid F follicular cell G Golgi complex L lysosome Mi mitochondrion Mv microvilli rER rough endoplasmic reticulum V vacuole
The parathyroid glands are small, oval endocrine glands which are closely associated with the thyroid gland. In mammals, there are usually two pairs of glands, one pair situated on the posterior surface of the thyroid gland on each side, although occasional individuals possess five or even six parathyroids. The embryological origins of the parathyroid glands are from the third and fourth branchial (pharyngeal) pouches. The parathyroid glands regulate serum calcium and phosphate levels via parathyroid hormone ( PTH ).
Parathyroid hormone raises serum calcium levels in three ways:
Direct action on bone, increasing the rate of osteoclastic resorption and promoting breakdown of the bone matrix
Direct action on the kidney, increasing the renal tubular reabsorption of calcium ions and inhibiting the reabsorption of phosphate ions from the glomerular filtrate
Promotion of the absorption of calcium from the small intestine; this effect involves vitamin D.
Secretion of parathyroid hormone is stimulated by a decrease in blood calcium levels. In conjunction with calcitonin secreted by the C cells of the thyroid gland, blood calcium levels are maintained within narrow limits. Parathyroid hormone is the most important regulator of blood calcium levels and is essential to life, whereas calcitonin appears to provide a complementary mechanism for fine adjustment and is not essential to life.
A adipose tissue C capillary G glandular elements O oxyphil cells P principal or chief cells PT parathyroid gland T thyroid gland
The parathyroid glands may either overwork, producing excessive PTH ( hyperparathyroidism ) or underwork, producing little or no hormone ( hypoparathyroidism ).
The commonest cause of hyperparathyroidism is a benign tumour of one of the parathyroid glands (parathyroid adenoma) which constantly produces excessive PTH, unresponsive to normal feedback mechanisms related to the blood calcium levels. The excess parathormone stimulates excessive osteoclastic erosion of bone (see Fig. 10.6 ), with the release of bone calcium into the blood to produce hypercalcaemia . The results include bone pain with X-ray abnormalities and an increased risk of kidney stones. This pattern is called primary hyperparathyroidism. Secondary hyperparathyroidism is a secondary response of all the parathyroid glands to a persistent low serum calcium level in patients with kidney failure who are constantly losing calcium in their urine. The feedback mechanism is triggered and all of the parathyroids become enlarged ( parathyroid hyperplasia ) and secrete excess PTH in an attempt to bring the serum calcium level back to normal.
Tertiary hyperparathyroidism occurs when the hyperplastic glands of secondary hyperparathyroidism cease to respond to serum calcium levels. The glands secrete high levels of PTH autonomously.
Hypoparathyroidism is rare and is usually due to inadvertent surgical removal of all parathyroid glands during total thyroidectomy.
The adrenal ( suprarenal ) glands are small, flattened endocrine glands which are closely applied to the upper pole of each kidney. In mammals, the adrenal gland contains two functionally different types of endocrine tissue which have distinctly different embryological origins; in some lower animals, these two components exist as separate endocrine glands. The two components of the adrenal gland are the adrenal cortex and adrenal medulla .
The adrenal cortex has a similar embryological origin to the gonads and, like them, secretes a variety of steroid hormones , all structurally related to their common precursor cholesterol . The adrenal steroids may be divided into three functional classes, mineralocorticoids , glucocorticoids and sex hormones . The mineralocorticoids are concerned with electrolyte and fluid homeostasis. The glucocorticoids have a wide range of effects on carbohydrate, protein and lipid metabolism. Small quantities of sex hormones are secreted by the adrenal cortex and supplement gonadal sex hormone secretion.
Embryologically, the adrenal medulla has a similar origin to that of the sympathetic nervous system and may be considered as a highly specialised adjunct of this system. The adrenal medulla secretes the catecholamine hormones, adrenaline ( epinephrine ) and noradrenaline ( norepinephrine ).
The control of hormone secretion differs markedly between the cortex and medulla. Glucocorticoid secretion is mainly regulated by the pituitary trophic hormone ACTH, while mineralocorticoid secretion is under the control of the renin–angiotensin–aldosterone system (see Ch. 16 ). In contrast, the secretion of adrenal medullary catecholamines is directly controlled by the sympathetic nervous system. The function of the adrenal medulla is to reinforce the action of the sympathetic nervous system under conditions of stress, the direct nervous control of adrenal medullary secretion permitting a rapid response.
Around 10% of adrenal tumours are associated with familial cancer syndromes. Phaeochromocytoma is a tumour of the adrenal medulla (see Fig. 17.4 ). A diagnosis of phaeochromocytoma necessitates investigations for familial cancer syndromes. An example is succinic dehydrogenase B deficiency (SDH-B deficiency). Adrenal cortical carcinomas are malignant tumours of the adrenal cortex. These tumours can be seen in association with Li Fraumeni syndrome (caused by a mutation in TP53, a tumour suppressor gene) and Beckwith–Weidemann syndrome (caused by a mutation in chromosome 11p15).
C cortex Cap capsule F zona fasciculata G zona glomerulosa M medulla R zona reticularis T trabecula V vein
Destruction of both adrenals (e.g. by autoimmune adrenalitis or, in former years, by tuberculosis) leads to failure of secretion of all adrenal cortical hormones ( hypoadrenalism ), leading to the clinical syndrome called Addison’s disease (weakness, tiredness, skin pigmentation, postural hypotension, hypovolaemia and low blood sodium). More common is hyperadrenalism where there is excess secretion of one or more of the cortical hormones, mainly glucocorticoids (producing Cushing’s syndrome) or mineralocorticoids (producing Conn’s syndrome). The excess hormone may be produced by a benign tumour (adrenal cortical adenoma) or a malignant tumour (adrenal cortical carcinoma) or by diffuse hyperplasia of the adrenal cortex. In adrenal cortical carcinoma, the excessive output may affect all three types of cortical hormone, including androgens, and hirsutism or virilisation are sometimes present.
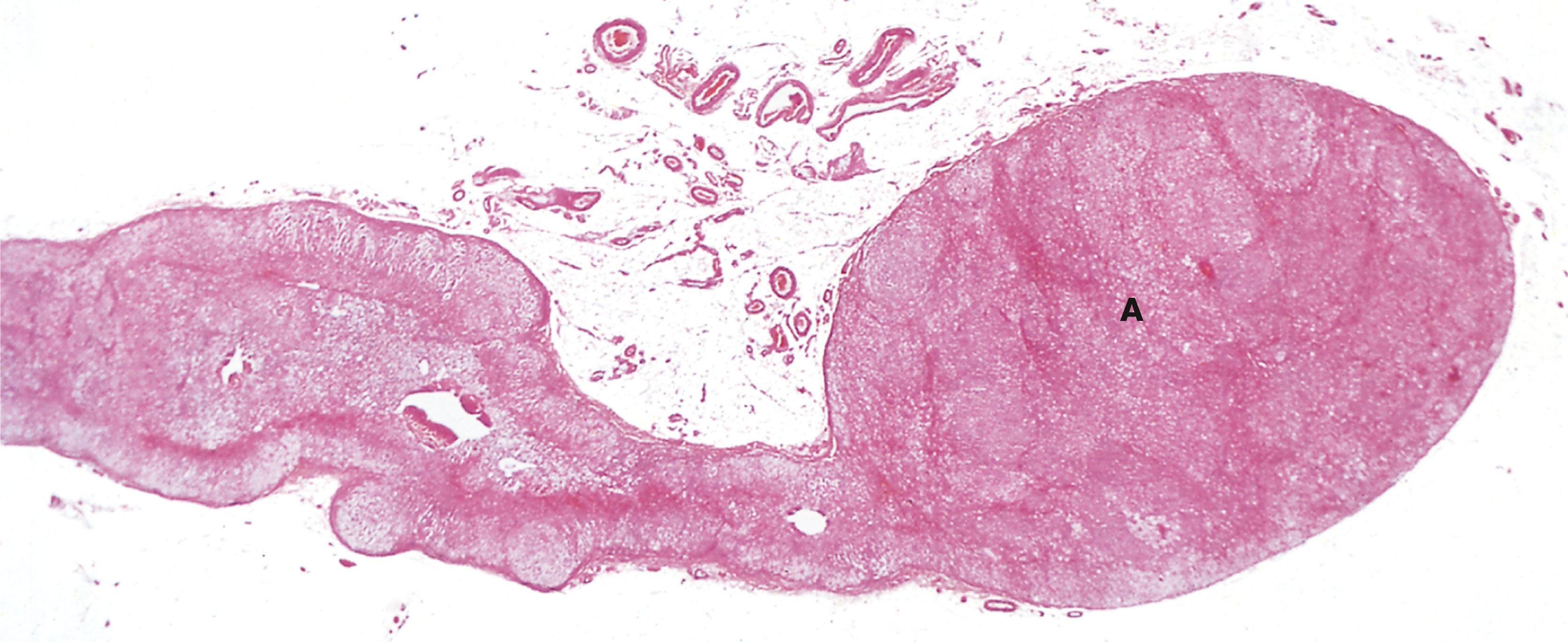
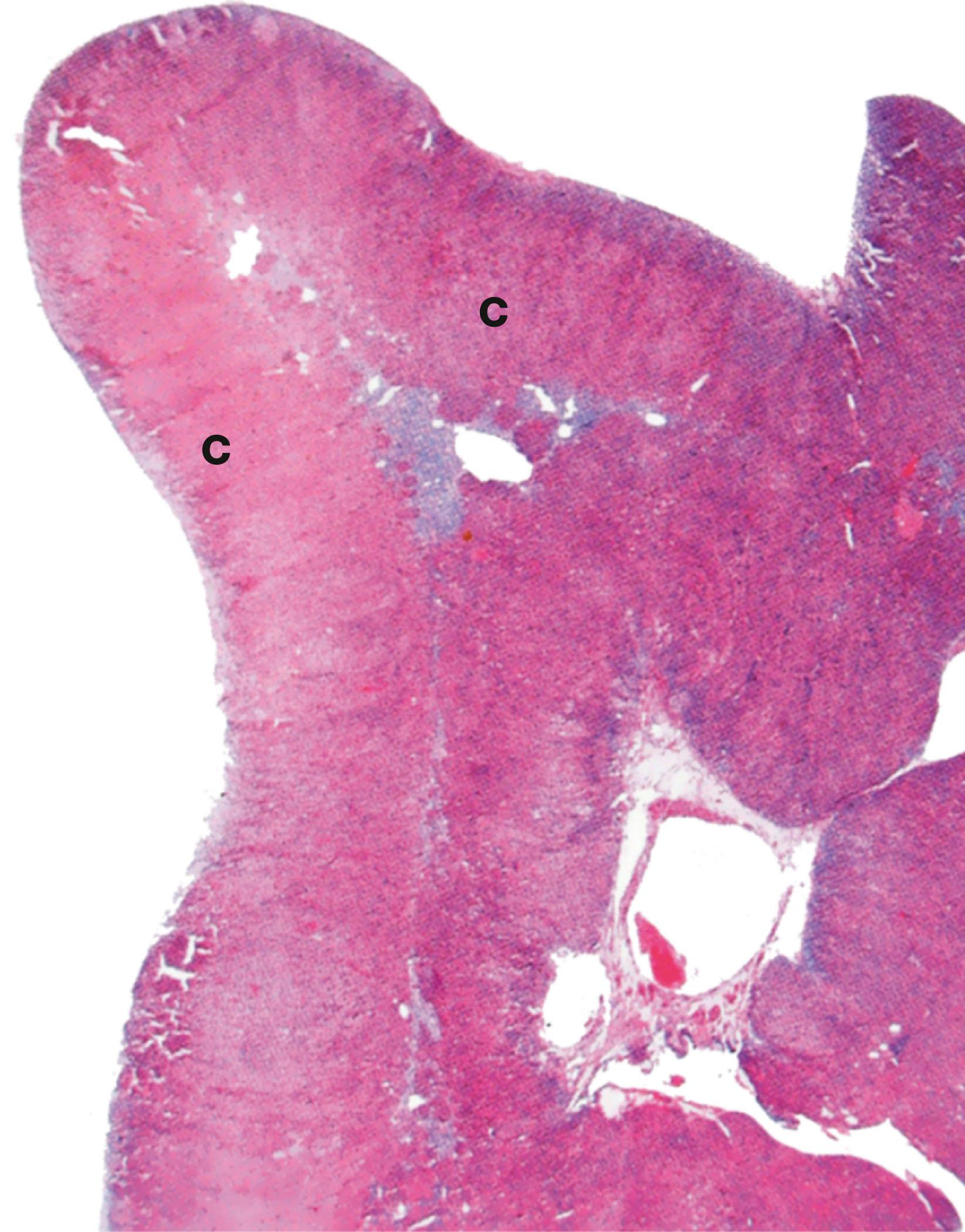
Ectopic ACTH syndrome occurs when some types of tumour elsewhere in the body (e.g. neuroendocrine carcinomas in the lung) secrete excessive amounts of an ACTH-like substance which stimulates the zona fasciculata to produce excess glucocorticoids.
A adrenaline-secreting cell C adrenal cortex Cap capillary Cr cristae F zona fasciculata G zona glomerulosa Go Golgi apparatus L lipid droplet M medulla Mi mitochondrion Na noradrenaline-secreting cell Nu nucleolus R zona reticularis sER smooth endoplasmic reticulum V vein
The pancreas is not only a major exocrine gland (see Ch. 15 ) but also has important endocrine functions.
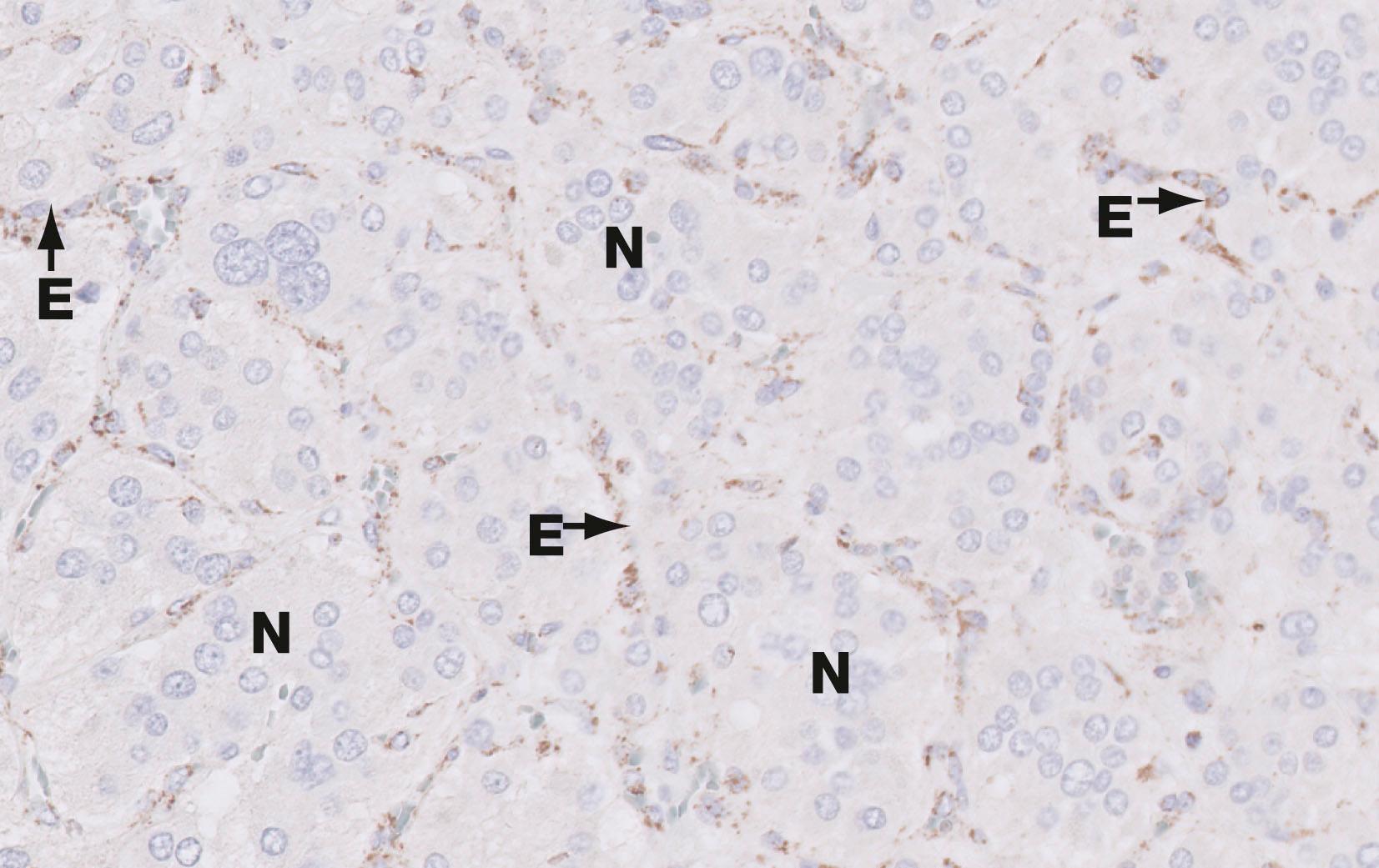
The embryonic epithelium of the pancreatic ducts consists of both potential exocrine and endocrine cells. During development, the endocrine cells migrate from the duct system and aggregate around capillaries to form isolated clusters of cells known as islets of Langerhans , scattered throughout the exocrine glandular tissue. The islets vary in size and are most numerous in the tail of the pancreas. The islets contain a variety of cell types, each responsible for secretion of one type of polypeptide hormone.
The main secretory products of the endocrine pancreas are insulin and glucagon , polypeptide hormones which play an important role in carbohydrate metabolism. Insulin promotes the uptake of glucose by most cells, particularly those of the liver, skeletal muscle and adipose tissue, thus lowering plasma glucose concentration. In general, glucagon has metabolic effects that oppose the actions of insulin. Apart from their role in carbohydrate metabolism, these hormones have a wide variety of other effects on energy metabolism, growth and development.
At least five other types of endocrine cells are present in the islets or else scattered singly or in small groups between the exocrine acini and along the ducts. Their secretory products include somatostatin (which has a wide variety of effects on gastrointestinal function and may also inhibit insulin and glucagon secretion), and pancreatic polypeptide ( PP ). Another cell type, the enterochromaffin ( EC ) cell, appears to secrete several different peptides including motilin , 5-hydroxytryptamine (5-HT, serotonin) and substance P .
The islets of Langerhans contain endocrine cells which produce a range of hormones, but the most important disease is that associated with the production and function of insulin.
Diabetes mellitus
Diabetes mellitus is a common and important disease of insulin metabolism, resulting in hyperglycemia. There are two main types:
Type 1 diabetes usually begins in childhood or adolescence and is the result of loss of endocrine cells in the pancreatic islets, including those which secrete insulin. The islet cell destruction is thought to be due to an abnormal autoimmune response, possibly triggered by a viral infection, and results in insulin deficiency. This has widespread metabolic effects on carbohydrate, protein and fat metabolism, leading to complex metabolic and structural effects.
Type 2 diabetes is the result of the resistance of target cells to the effect of insulin, rather than a failure of insulin production by the pancreatic islets. It is becoming more common due to the increasing incidence of obesity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here