Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
First identified over 400 years ago by Greek anatomists, the pancreas is located in the retroperitoneum with the head of the pancreas lying in the C loop of the duodenum ( Fig. 39.1A and B ). The pancreas has distinct hormonal (endocrine) and digestive (exocrine) functions. Endocrine cells are organized in discrete clusters throughout the pancreas. First described in 1869 by then medical student Paul Langerhans, these islets of Langerhans ( Fig. 39.1C ) secrete hormones directly into the bloodstream.
The primary physiologic function of the endocrine pancreas is glucose/insulin regulation through secretion of insulin and glucagon directly into the bloodstream in response to blood glucose levels. In 1889, through a landmark study in dogs, Minkowski and von Mering made the connection between diabetes and the pancreas. While studying fat absorption in dogs after total pancreatectomy, they noted that surgical removal of the pancreas led to glucosuria, ketonuria, coma, and eventual death. On the heels of this discovery, Frederick Banting and Charles Best identified the hormone insulin in 1922 in studies where atrophied pancreas in an iatrogenic diabetic dog was extracted, homogenized, and injected back into the animal, temporarily reversing the diabetic condition.
In this chapter, we will cover the histomorphology, embryology, physiology, and pathophysiology of the endocrine pancreas. We will focus on the diagnosis and management of diseases relevant to surgeons, including tumors of the endocrine pancreas, diabetes, and the endocrine complications of surgical therapy.
In the human fetus, pancreatic islets initially comprise approximately one third of the pancreatic mass. Pancreatic formation begins during the fifth week of gestation as separate dorsal and ventral endodermal pancreatic buds, which form at the junction of the foregut and the midgut. The dorsal and ventral buds are comprised of endoderm covered in splanchnic mesoderm. Both the acinar and islet cells differentiate from the endodermal cells found in the embryonic buds while the splanchnic mesoderm eventually develops into the dorsal and ventral mesentery. The first glucagon-producing cells (A cells) appear in 3-week-old embryos and the first organized islets appear at approximately 10 weeks. B cell formation primarily occurs before birth with a burst of proliferation up to the first 2 years of life. The B-to-A-cell ratio doubles neonatally, reflecting increased growth of B cells.
The adult pancreas consists of endocrine cells organized in islets of Langerhans ( Fig 39.1C ) and digestive acinar cells contained in clusters and draining into a centralized ductal system. Endocrine cells comprise less than 2% of the overall pancreatic mass in the adult pancreas. The adult pancreas contains approximately 1 million islets, with each islet containing approximately 3000 cells and ranging in diameter from 40 μm to 1 mm. Pancreatic islets have complex architecture and are composed of four cell types: A (alpha), B (beta), D, and F cells. The four cell types are not evenly distributed within the islets or throughout the pancreas. Table 39.1 describes the cell types, their hormonal products, and their location within the islet and the pancreas.
| Cell Type | % Islet Cell Mass | Location Within Islet | Location Within Pancreas | Major (Minor) Hormone Secreted | Associated Tumor Syndrome | Diagnostic Hormone Levels |
|---|---|---|---|---|---|---|
| A (alpha) | 10% | Peripheral | Evenly distributed | Glucagon (glicentin, TRH, CCK, endorphin, PP, pancreastatin) | Glucagonoma: necrolytic migratory erythema, diabetes, hypoaminoacidemia | Normal = <150 pg/mL Tumor = fasting glucagon >1000 pg/mL |
| B (beta) | 70% | Central | Body/tail | Insulin (TRH, CGRP, amylin, pancreastatin, prolactin) | Insulinoma: hypoglycemia and associated symptoms | >5 μU/mL in the face of hypoglycemia |
| D | 5% ∗ | Evenly distributed | Evenly distributed | Somatostatin (met-encephalon) | Somatostatinoma: diabetes, gallstones, steatorrhea | Normal = 10–25 pg/mL Tumor = >160 pg/mL |
| D 2 | 5% ∗ | Evenly distributed | Evenly distributed | VIP | VIPoma: high-volume secretory diarrhea, hypokalemia, metabolic acidosis, hypochlorhydria | Normal = <200 pg/mL Tumor = 225–2000 pg/mL |
| F | 15% | Peripheral | Head and uncinate process | PP | Treatment directed at presenting symptoms | NA |
| E C |
<1% | Evenly distributed | Evenly distributed | Substance P, serotonin | None | NA |
| G | Not present in normal physiologic state | Not applicable | Head, uncinate process, duodenum | Gastrin, ACTH-related peptides | Gastrinoma: acid hypersecretion, gastric/duodenal ulcers, diarrhea | Normal <100 pg/mL Suspicious = >1000 pg/mL with secretin test, an increase of >200 pg/mL diagnostic |
The A cells, located in the periphery, secrete glucagon and constitute approximately 10% of the islet cell mass. Islets largely (up to 70%) consist of B cells which secrete the hormone insulin and are located centrally within the islet. In comparison, F cells constitute approximately 15% of islet cell mass and secrete the hormone pancreatic polypeptide (PP). The D cells are evenly distributed throughout the islet and constitute approximately 5% of the islet cell mass. D cells secrete somatostatin and D2 cells secrete vasoactive intestinal peptide (VIP). Within the actual pancreas, B and D cells are concentrated in the body and tail of the pancreas, while F cells are heavily concentrated in the uncinate process, and A cells are evenly distributed throughout the gland.
The rich portal microcirculation of the pancreatic islets allows for the endocrine-to-endocrine cell signaling necessary for hormonal regulation. Afferent arterioles enter the islet on the periphery into the center of the islet, which consists of B cells. The order of islet cellular perfusion and interaction is from this B cell core outward to the mantle, first to A cells and then to the more distal/peripheral D cells. This allows B cells to inhibit A cell secretion and A cells to stimulate D cell secretion.
Pancreatic endocrine secretion regulates pancreatic exocrine secretion through the islet-acinar axis of the pancreas. Although islets constitute less than 2% of pancreatic volume, the arterial blood supply to the pancreas predominantly flows first to the islets and then via the islets to the exocrine portion of the gland. The distribution of blood flow is relevant to the potential physiologic interactions. The B cells’ insulin stimulates pancreatic exocrine secretion, amino acid transport, and synthesis of protein and enzymes. On the other hand, the islets A cells’ glucagon acts in a counterregulatory fashion, inhibiting the same processes.
The primary function of the endocrine pancreas is regulation of glucose homeostasis. In response to blood glucose levels, the secretion of insulin and glucagon is tightly regulated through a variety of feedback and regulatory mechanisms. Secreted by B cells within the islets of Langerhans, insulin functions to store energy by promoting glucose transport into cells, inhibiting glycogenolysis and fatty acid breakdown, and stimulating protein synthesis. Glucagon is the major counterregulatory hormone to insulin, increasing blood glucose levels through stimulation of glycogenolysis, lipolysis, and gluconeogenesis.
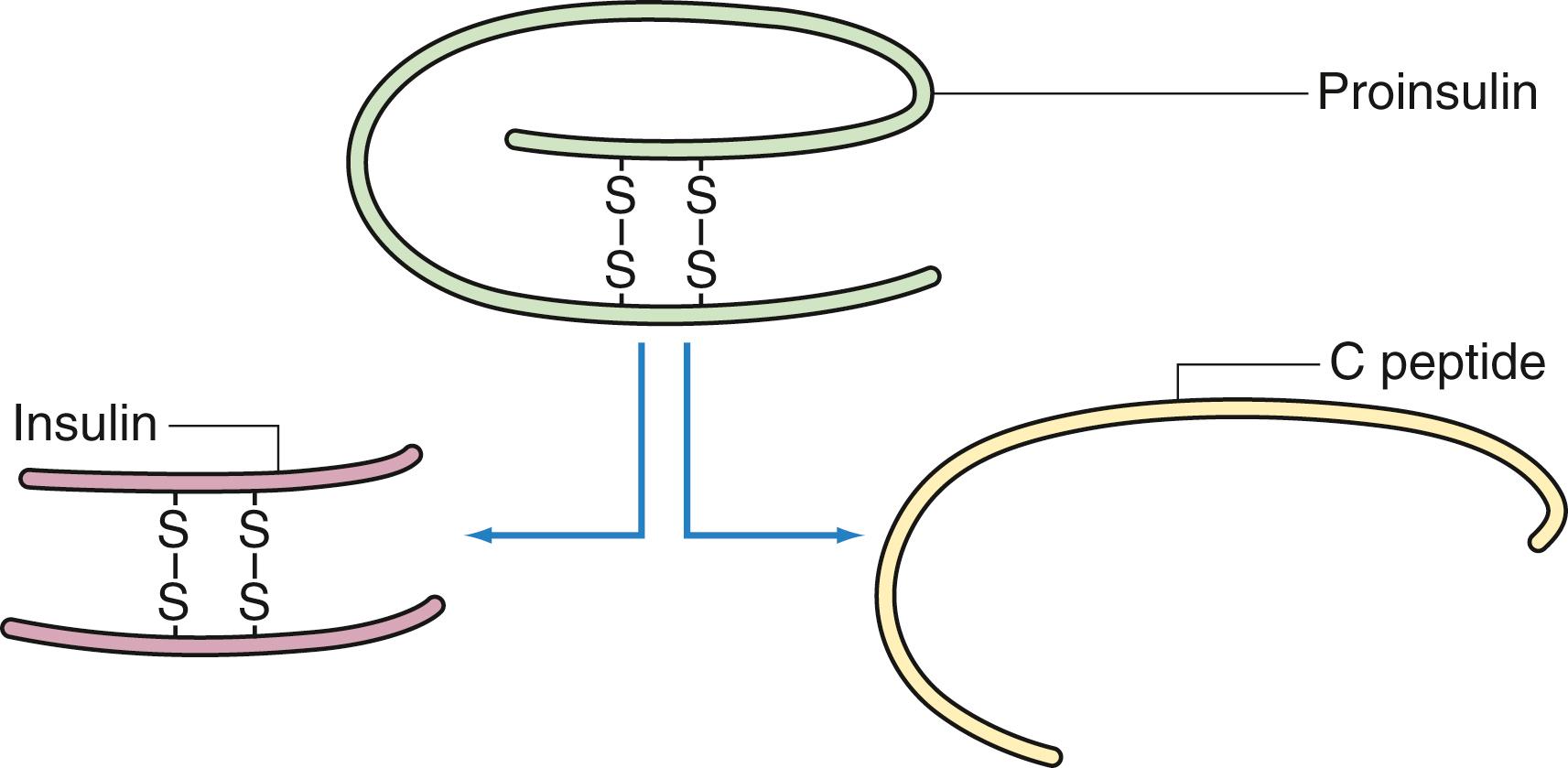
Insulin is an anabolic hormone that promotes glucose transport into all cells except B cells, hepatocytes, and central nervous system cells. Insulin is a 56–amino acid polypeptide with a molecular weight of 6 kDa, which is synthesized as proinsulin, its precursor peptide. In response to pancreatic B cell stimulation by glucose, proinsulin is synthesized in the endoplasmic reticulum and transported to the Golgi complex, where it is cleaved into insulin and the residual C-peptide ( Fig. 39.2 ). The resulting insulin molecule consists of two polypeptide chains (A and B) joined by two disulfide bridges. C-peptide and insulin are secreted in equimolar amounts. After cleavage of C-peptide, insulin is moved via microtubules into secretory granules, where it is released directly into the bloodstream via exocytosis.
The B cell is highly sensitive to changes in glucose concentration and is maximally stimulated at concentrations of 400 to 500 mg/dL. In response to glucose, the islets of Langerhans immediately react with a short burst of stored insulin (4–6 minutes), followed by a sustained secretion of insulin, which requires active synthesis of the hormone within the islet cell. Insulin binds to a specific 300-kDa glycoprotein cell surface receptor, and stimulation of this insulin receptor is dependent on insulin concentration. After insulin receptor stimulation, glucose is actively transported across cell membranes throughout the body by membrane-bound glucose transporters. There are several classes of glucose transporters, with varying affinities for glucose. Insulin resistance, present in type 2 diabetes, can be the result of decreased numbers of receptors or a decreased affinity of receptors for insulin.
Glucagon is a 29–amino acid, straight chain polypeptide with a molecular weight of 3.5 kDa. Secreted by A cells within islets, the primary function of glucagon is to elevate blood glucose levels through stimulation of glycogenolysis and gluconeogenesis in the hepatocytes. Islet A and B cells respond primarily to serum glucose concentration, but in a reciprocal fashion. Like epinephrine, cortisol, and growth hormone, glucagon is considered a stress hormone because it increases metabolic fuel in the form of glucose during stress. Glucose has a strong suppressive effect on glucagon secretion. Excess glucagon can lead to hyperglycemia, whereas insufficient glucagon can lead to profound hypoglycemia. Dysfunctional secretion of glucagon may play a role in the elevation of blood glucose levels in diabetes.
Enteric peptide hormones released from the proximal gastrointestinal tract also influence glucose homeostasis through the enteroinsular axis. Therefore, orally administered glucose has a greater effect on insulin secretion than an equivalent amount of glucose administered intravenously, even though blood glucose levels might be similar. Insulinotropic factors, called incretins, act directly on the B cells to stimulate insulin release. Incretins such as glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 are intestinal hormones that are released in response to ingestion of nutrients, particularly carbohydrates. They have a number of important biological effects, which include release of insulin, inhibition of glucagon and somatostatin, maintenance of beta-cell mass, delay of gastric emptying, and inhibition of feeding. In comparison to incretins’ stimulation of insulin, humoral inhibitors of insulin secretion include somatostatin, amylin, leptin, and pancreastatin.
Ghrelin is a 28–amino acid peptide hormone produced by ghrelin cells of the gastrointestinal tract. Discovered in 1999, ghrelin was found to exert a series of metabolic effects, including the regulation of glucose metabolism. Ghrelin primarily inhibits insulin release from the pancreas, increases hepatic glucose production, and prevents glucose disposal in muscle and adipose tissues, which collectively leads to hyperglycemia and impaired glucose tolerance. In diet-induced obesity, ghrelin exacerbates hyperglycemia; in starvation or severe calorie restriction, ghrelin increases blood glucose concentrations in order to maintain glucose homeostasis.
Leptin is a peptide hormone produced in adipose cells. Leptin is released into the circulatory system based on energy stores and functions as a feedback mechanism that signals to regulatory centers in the brain to inhibit food intake and to regulate body weight. In response to adequate fat stores, leptin inhibits insulin secretion. In obese humans, leptin levels are increased, exacerbating hyperglycemia. In the obese state, there is thought to be leptin resistance, with lack of inhibition of food intake.
Both insulin and glucagon secretions are also under neuronal control. Vagal (cholinergic) stimulation leads to the release of insulin. Insulin release is stimulated by the autonomic peptidergic nerve release of gastrin-releasing peptide, cholecystokinin (CCK), gastrin, enkephalin, and VIP, as well as β-sympathetic nerve stimulation. On the other hand, insulin release is inhibited by neurotensin, substance P, somatostatin, and α-sympathetic nerve stimulation. In comparison, glucagon secretion is stimulated by sympathetic neural transmitters, epinephrine, and the amino acids arginine and alanine.
Somatostatin is a 14–amino acid polypeptide weighing 1.6 kDa secreted by islet D cells. Although exogenous administration of somatostatin has been shown to inhibit the release of insulin, glucagon, and PP, and to inhibit gastric, pancreatic, and biliary secretion, endogenous somatostatin has not been proven to influence the secretion of other islet hormones directly. Both long- and short-acting synthetic octapeptides that mimic the pharmacologic action of somatostatin have been developed. These synthetic peptides have a longer half-life in the serum than endogenous somatostatin and are more potent inhibitors of growth hormone, glucagon, and insulin secretion than the natural hormone. The potent inhibitory effect of synthetic somatostatin analogues has been used to treat exocrine and endocrine disorders of the pancreas, including secretory diarrhea, bowel fistulas, pancreatic fistulas, and endocrine hypersecretory syndromes.
PP is a 36–amino acid, 4.2-kDa polypeptide secreted by the islet F cells. PP belongs to the peptide YY/neuropeptide Y family of polypeptides. Infusion of PP in humans caused loss of appetite and reduced food intake. As its true physiologic role remains unclear, PP’s clinical usefulness is limited to its role as a marker for other endocrine tumors of the pancreas. As cholinergic innervation predominantly regulates PP secretion, surgical vagotomy ablates the increased PP response normally observed after meals. In diabetes and normal aging, PP secretion is increased, resulting in increased circulating PP levels. Absence of PP may play a role in the diabetes observed after total pancreatectomy or after chronic atrophic pancreatitis.
In addition to the main hormones secreted by islets cells, other peptide hormones secreted includes VIP, amylin, galanin, and serotonin. VIP is a 28–amino acid, 3.3-kDa polypeptide that stimulates insulin release and inhibits gastric secretion at physiologic level. It is found not only throughout the gastrointestinal tract but also in the respiratory tract, where it causes vasodilatation and bronchodilation. Amylin, a 36–amino acid polypeptide, is secreted by B cells and inhibits the secretion and uptake of insulin. Amylin deposits in the pancreas of patients with type 2 diabetes have been implicated in the pathogenesis of the disease. Pancreastatin is part of a larger ubiquitous molecule, chromogranin A, which inhibits insulin secretion. Gastrin-producing cells are present in the fetal pancreas, but not in the normal adult pancreas. Many additional peptides, including thyrotropin-releasing hormone, glicentin, CCK, peptide YY, gastrin-releasing factor (GRF), calcitonin gene–related peptide, prolactin, adrenocorticotropic hormone (ACTH), parathyroid hormone–related protein, and ghrelin have been reported in normal islets and in islet cell tumors.
Pancreatic neuroendocrine tumors (PNETs) account for less than 3% of pancreatic neoplasms. The incidence of PNETs has increased more than sevenfold in the last two decades. In a study using the Surveillance, Epidemiology, and End Results database, the overall incidence of gastroenteropancreatic neuroendocrine tumors (NETs) was 1.00 case per 100,000 between 1973 and 1977 and increased to 3.65 cases per 100,000 between 2003 and 2007. PNETs comprised 7% of all gastroenteropancreatic NETs. The observed increase in incidence is likely multifactorial and includes increased awareness among physicians, more frequent use of computed tomography (CT) and magnetic resonance imaging (MRI), and improved sensitivity of immunohistochemical and radiologic diagnostic testing. PNETs are broadly classified as functional or nonfunctional. Secretion of hormones by functional tumors leads to the characteristic syndromes and physiologic derangements associated with these rare neoplasms ( Table 39.1 ). Immunostaining often identifies multiple hormone products, even in the absence of clinically relevant hormone secretion. Although multiple hormones may be secreted by a single tumor, the term “functional” should be reserved for tumors associated with clinical symptoms.
Nonfunctional tumors historically presented with local symptoms related to tumor growth including pain, mass effect, or biliary obstruction, similar to exocrine pancreatic cancer. However, more frequently, nonfunctional tumors are being identified incidentally on imaging done for other purposes and are asymptomatic at the time of diagnosis.
The incidence of malignancy in these tumors varies from approximately 10% in insulin-secreting PNETs (insulinoma) to almost 100% in glucagon- or somatostatin-secreting tumors ( Table 39.1 ). However, unlike most other solid tumors, which are classified as benign or malignant based on histopathology of the primary tumor, malignancy in PNETs can only definitely be determined by the presence of metastasis.
The 2010 World Health Organization (WHO) staging system is the most widely used staging system for NETs. It includes all NETs regardless of site of origin or functional hormone secretion and classifies based on differentiation and grade. The tumor grade for NETs is categorized as low grade (grade 1, G1), intermediate grade (grade 2, G2), or high grade (grade 3, G3) based on appearance, mitotic rates, invasion of other organs, angioinvasion, and the Ki-67 proliferative index ( Table 39.2 ). G1 and G2 tumors are considered well differentiated, and G3 tumors are poorly differentiated and is by far the most important prognostic indicator. High-grade/poorly differentiated PNETs are sometimes referred to as “neuroendocrine carcinoma” and account for fewer than 3% of PNETs. However, it is important to emphasize that well-differentiated tumors do still have malignant potential, but that the differences in behavior persist, even for patients with metastatic disease.
| Well Differentiated | Poorly Differentiated | ||
|---|---|---|---|
| Low Grade (G1) | Intermediate Grade (G2/G3) | High Grade (G3) | |
| Appearance | Homogeneous small, round cells with abundant expression of neuroendocrine markers | Pleomorphic cells with nuclear irregularity, necrosis | |
| Mitotic rate | <2 mitoses/10 HPF | G2 2–20 mitoses/10 HPF G3 >20 mitoses/10 HPF |
>20 mitoses/10 HPF |
| Ki-67 Index | <3% | G2 3%–20% G3 >20% |
>20% |
| Behavior | Indolent | Aggressive | |
The American Joint Committee on Cancer (AJCC) and European Neuroendocrine Tumor Society (ENETS) also have proposed staging schemes for PNETs. Neither system includes tumor grade, and both apply staging similar to that of exocrine pancreatic cancers to PNETs ( Table 39.3 ). In another study, a tumor, grade, metastases (TGM) staging system was proposed as a more accurate prognostic tool. In a recent study using the Surveillance, Epidemiology, and End Results databases, the 8th edition AJCC staging system exhibited good prognostic discrimination across stages in both resected and unresected patients.
| AJCC 8TH Edition | Enets | |
|---|---|---|
| Primary Tumor (T) | ||
| T1 | Maximum tumor diameter <2 cm | Tumor limited to the pancreas <2 cm |
| T2 | Maximum tumor diameter >2 but <4 cm | Tumor limited to the pancreas, 2–4 cm |
| T3 | Maximum tumor diameter >4 cm | Tumor limited to the pancreas, >4 cm, or invading the duodenum or common bile duct |
| T4 | Tumor involves the celiac axis or superior mesenteric artery | Tumor invades adjacent structures |
| Nodal Metastases (N) | ||
| N0 | No regional lymph node metastases | No regional lymph node metastasis |
| N1 | Metastasis in one to three regional lymph nodes | Regional lymph node metastasis |
| N2 | Metastasis in four or more regional lymph nodes | |
| Metastatic Disease (M) | ||
| M0 | No distant metastasis | No distant metastasis |
| M1 | Distant metastasis | Distant metastasis |
| Stage | ||
| I | T1, N0, M0 (Ia) T2, N0, M0 (Ib) |
T1, N0, M0 (Ia) T2, N0, M0 (Ib) |
| II | T3, N0, M0 (IIa) T1-3, N2, M0 (IIb) |
T3, N0, M0 (IIa) T1-3, N2, M0 (IIb) |
| III | Any T, N2, M0 T4, any N, M0 |
T4, any N, M0 |
| IV | Any T, any N, M1 | Any T, any N, M1 |
Although most PNETs occur sporadically, others can be associated with genetic syndromes. The most common genetic syndrome associated with PNETs is multiple endocrine neoplasia type 1 (MEN1), characterized by PNETs, parathyroid adenomas or hyperplasia, and pituitary adenomas. MEN1 is caused by mutations or allelic deletions in the tumor suppressor gene, menin, on chromosome 11q13 and is inherited in an autosomal dominant fashion. Menin is a component of the histone methyltransferase complex and is involved in control of G1 to S phase cell cycle progression. Mutation or allelic deletion causes loss of tumor suppressor function and predisposes patients to neoplastic growth in the parathyroid, pituitary, and pancreatic endocrine tissue.
Von Hippel-Lindau (VHL) syndrome is also associated with PNETs. Patients with inherited mutations of the VHL gene are at risk for the development of renal cell carcinoma, pheochromocytoma, benign tumors of the central nervous system, retina, epididymis, and inner ear and pancreatic lesions, including NETs, microcystic adenomas, and simple cysts. Similar to MEN1, the management of PNETs can be challenging, since they are often multifocal and associated with tumors in other locations. PNETs associated with VHL generally behave in an indolent fashion, and it has been suggested that these tumors can be observed until they reach at least 2 3 cm in size. However, specific germline mutations in exon 3 of the VHL gene may be associated with a more aggressive phenotype and warrant earlier treatment and closer surveillance.
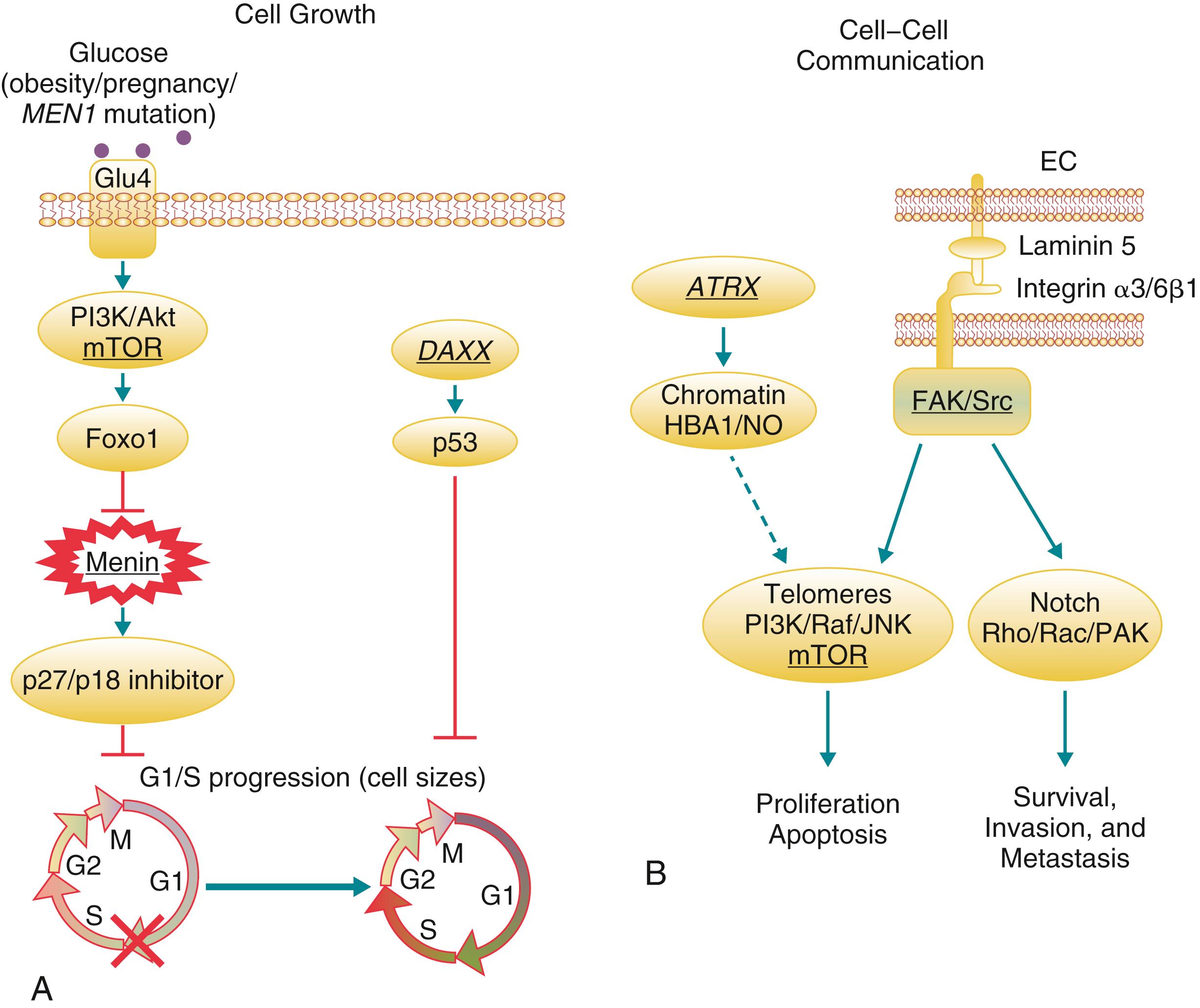
Most PNETs, however, are not associated with a known genetic syndrome and occur sporadically. Other than family history, risk factors for PNETs are not well defined. As in other neoplastic processes, tumorigenesis of PNETs involves an accumulation of a number of genetic events. The common genetic mutations and impacted signal transduction pathways in PNETs is shown in Fig. 39.3 . Complete exomic sequencing of a discovery set of 10 sporadic PNETs revealed mutations in 149 genes, of which 6 were selected for further analysis in a validation set of 58 PNETs. Inactivating mutations in MEN1 were seen in 44% of sporadic tumors. Mutations in death-domain associated protein (DAXX) and alpha thalassemia-mental retardation syndrome X-linked (ATRX), whose protein products are involved in p53-mediated DNA damage repair, were seen in 25% and 18%, respectively. Patients with mutations in MEN1 or DAXX/ATRX had prolonged survival compared to those who did not. Previous expression analyses had suggested dysregulation of the mammalian target of rapamycin (mTOR) pathway in a large proportion of tumors. , The mTOR protein is serine/threonine kinase and a key component of a cellular pathway playing an important role in the regulation of cell growth and proliferation. mTOR is upregulated in several tumors, including PNETs. This has potential clinical implications since the mTOR inhibitor everolimus has been U.S. Food and Drug Administration (FDA) approved for advanced NETs. Potentially, mutational testing will allow selection of patients most likely to benefit from this targeted therapy. Overall, the mutational analysis was most remarkable for how distinct the genetic abnormalities were from those observed in a similar study of pancreatic adenocarcinoma ( Table 39.4 ). Mutations in KRAS were not seen in PNETs and mutations in P53 were seen only rarely, at least not in these well-differentiated tumors.
| Genes | PNET | PDAC |
|---|---|---|
| MEN1 | 44% | 0% |
| DAXX, ATRX | 43% | 0% |
| Genes in mTOR pathway | 15% | 0.8% |
| TP53 | 3% | 85% |
| KRAS | 0% | 100% |
| CDKN2A | 0% | 25% |
| TGFBR1, SMAD3, SMAD4 | 0% | 38% |
In a separate study comparing well- and poorly differentiated PNETs, MEN1 and DAXX/ATRX expression by immunohistochemistry was abnormal in approximately half of well-differentiated tumors. In contrast, staining of DAXX/ATRX was normal in poorly differentiated tumors, but there was a high incidence of abnormal p53 and retinoblastoma expression as well as overexpression of the antiapoptotic protein Bcl2, implicating it as a target for therapy in these tumors.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here