Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Growth reflects a complex interaction of genetic, epigenetic, endocrine, and nutritional factors that regulate cell division, differentiation, and function (see Meler et al. for a recent compilation of genetic and epigenetic conditions leading to fetal growth restriction). Various phases of growth appear “programmed” to occur within specific time intervals. If missed, growth may not be fully recoverable. Ever-increasing evidence indicates that the pattern and rapidity of fetal and neonatal growth affect the endocrine environment in later life. Thus the role of endocrine factors in modulating growth during this critical epoch of development and the resulting long-term effects are herein reviewed.
The greatest growth rate occurs during fetal life; in fact, the development of a single fertilized cell into a 3.5-kg neonate entails a 5000-fold increase in length, an increase in surface area of 6 × 10 6 , and an increase in weight of 6 × 10 11 . The greatest postnatal growth rate occurs just after birth, followed by slower growth during mid-childhood, only to increase once more in puberty before the final cessation at epiphyseal fusion.
Both longitudinal and cross-sectional studies describe growth rates after birth. The Centers for Disease Control and Prevention (CDC) released the latest version of the commonly used growth charts from birth until the age of 18 years in 2001, using cross-sectional data from the United States. The charts demon strate growth between the 3rd to 97th percentiles of length during the first 3 years after birth; the growth charts of the World Health Organization (WHO), which are currently recommended for infants, display the 2nd to the 98th percentiles. Most iterations of the charts show a different color between the 3rd and 97th percentiles. Many versions of the growth charts demonstrate a color change below the lower line of demarcation on the chart. Even though the color change usually found at the 3rd percentile may cause concern in parents of an infant who falls below that line, it is important to recognize that 3% of normal children fall below these lower limits.
Analysis of the factors affecting various phases of growth has led to the development of the infant-childhood-pubertal growth charts, in which the amount of growth for each of three phases of growth (infancy, childhood, puberty) are considered and their sums plotted. Each component of this model can be described by specific mathematical functions ( Fig. 22.1 ).
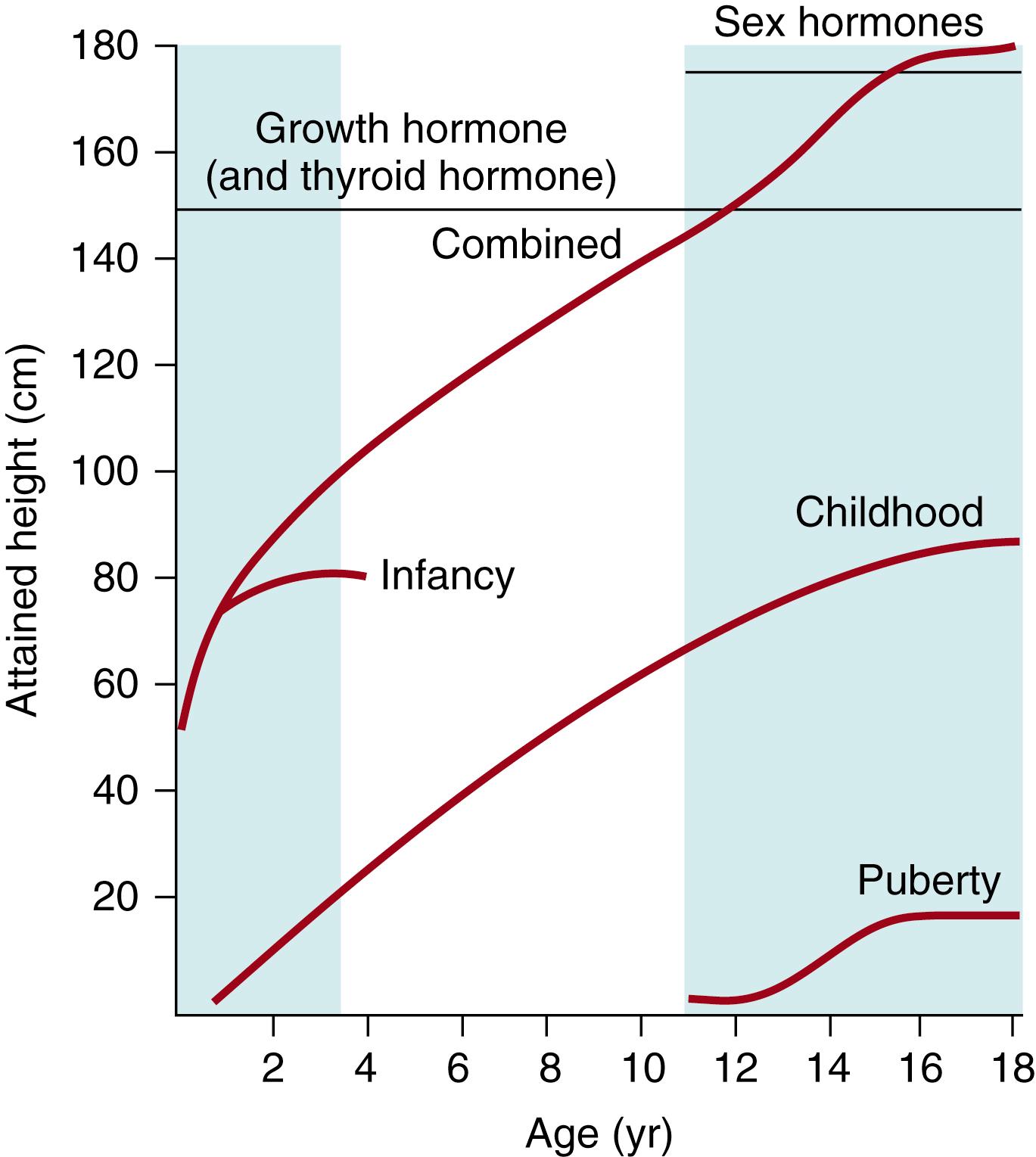
The slowly decelerating component in infancy occurs as the child has a decrease from the rapid fetal growth phase that starts before birth and falls off by age 3 to 4 years. An infancy–childhood growth spurt starts at 6 to 9 months of age, which originally defined the beginning of the childhood phase, as the infant phase decreases. An update to the original data, based upon a study of 2432 children in a longitudinal study, suggested that the average age of onset of the childhood phase is now 10 months in boys and 9 months in girls. Average total gain in height for Swedish boys (the population from which the plots were originally developed) is 79.0 cm (44.0% of final height) and for girls is 76.8 cm (46.2%) during the infancy period.
Infants undergo a minipuberty, which involves activation of the hypothalamic pituitary gonadotropin axis during the first 3 to 6 months of postnatal life. , The levels of sex steroids and gonadotropic hormones measured during this period in a longitudinal study of 17 males and 18 females exerted effects on linear growth in boys up to 6 years later. Subsequently, other investigations have been conducted with greater numbers of participants. Another longitudinal study of 45 males and 39 females demonstrated greater height velocity in males than in females up to 6 months of age; the greatest growth velocity difference between the sexes occurred at 1 month of age, which was the time when the highest levels of testosterone, equivalent to those of pubertal boys, were encountered.
Weight measurements are the standard method of evaluating neonatal growth. Scales used for this purpose may be accurate to within 10 g if calibrated regularly. For a growing 1-kg infant, errors of this magnitude may lead to errors in estimation of growth velocity of up to 2.9 g/kg/day when weights are obtained 1 week apart; with daily weighing, the error may be as much as 20 g/kg/day on any given day. Because the range of variation in measured weights is large relative to the true daily change, weight-gain velocities should be averaged over a period of 5 to 7 days to minimize “noise” in the data. Infants should be weighed naked, ideally disconnected from the ventilator or other respiratory support if feasible and safe, and measurements should be taken at approximately the same time each day. Weight should be plotted on an appropriate growth curve, depending on the infant’s age, and the z-score (number of standard deviations from the mean; provided in many growth curves embedded in electronic medical records) should be noted, as it is important to follow the z-score throughout hospitalization and after discharge as a measure of growth trajectory.
The accurate measurement of length at birth is as important as the measurement of weight, because abnormal changes in growth velocity after birth suggest a pathologic condition such as endocrine or genetic disorders. Additionally, length reflects skeletal growth and lean body mass. Unfortunately, accurate and reproducible measurements of neonates and infants are difficult to obtain in clinical practice, and measured lengths of term infants can change over the first 2 days after birth.
A study of the measurement of infants and inanimate dolls showed inaccuracies of up to 25% in length measurements, and weight measurement errors exceeded 10%. Length can be measured accurately if two individuals gently straighten the infant and hold him or her against a calipers-like device such as a Neo-Infantometer (Graham-Field, Inc., Hauppauge, NY) that expands and contracts to measure the linear distance from a perpendicular plate at the top of the head to a perpendicular plate at the bottom of the feet; staff can be trained to perform this with accuracy. Increased flexor tone in term and near-term infants and practitioners reluctant to fully extend the infant’s legs can limit accurate measurement. Length should be measured weekly to the nearest millimeter by two people, with one ideally being a parent. As technology becomes more advanced, techniques such as one based on stereoscopic vision may provide a more accurate and precise method for measuring length. Measurement of length is not just a matter of academic interest. Most analyses of infant deaths have been based on birth weight. However, careful analysis of the relationship between birth length and death after premature birth in the Swedish population showed the important effect of length in preterm infants. Careful, accurate measurement of length is critical, as many measures of body composition (such as weight for length ratios, body mass index [BMI], and ponderal index) rely on accurate length measurement, and errors in this measurement are magnified with these indices. Air displacement plethysmography studies are available for neonates and suggest a more direct method of evaluating body composition in neonates and infants.
Although low weight or ponderal index at birth have led to a greater risk of early death, those factors at 4 to 5 days of age no longer exerted such a strong effect; however, having a length less than −1 standard deviation (SD) at birth continued to increase the risk of long-term mortality.
Head circumference or occipitofrontal circumference (OFC) reflects brain growth and is an important growth measurement followed in the neonatal intensive care unit (NICU). OFC may decrease by approximately 0.5 cm in the first postnatal week owing to resolving edema and molding. OFCs outside of reference limits may be a variant of normal, and parental OFC should be measured and noted as a percentile or standard deviation from the mean for comparison. OFC should be measured weekly, to the nearest millimeter, using a nonstretchable measuring tape placed at the supraorbital ridges and occipital protuberance. The largest of three measurements should be recorded. Postdischarge head growth measured by OFC seems to better predict cognitive outcomes than does head growth during hospitalization.
Anthropometric measurements are important in the evaluation of neonatal growth and assessment of body composition and nutritional status; thus accurate measurement of weight, length, and head circumference is crucial and can be obtained with extreme care by trained staff.
An understanding of normal growth patterns enables the recognition of aberrations in growth that may represent underlying pathology. There are two types of growth charts, and they are to be used and interpreted differently: growth references and growth standards. Growth references are typically based on routinely collected data that may have been gathered decades earlier and often have limited quality control/standardization procedures. Growth references describe how subjects have grown at a specific place and time. Growth standards use rigorously collected anthropometric measurements that define how a population should grow under optimal conditions. Standards are thought to be universal and independent of time and place.
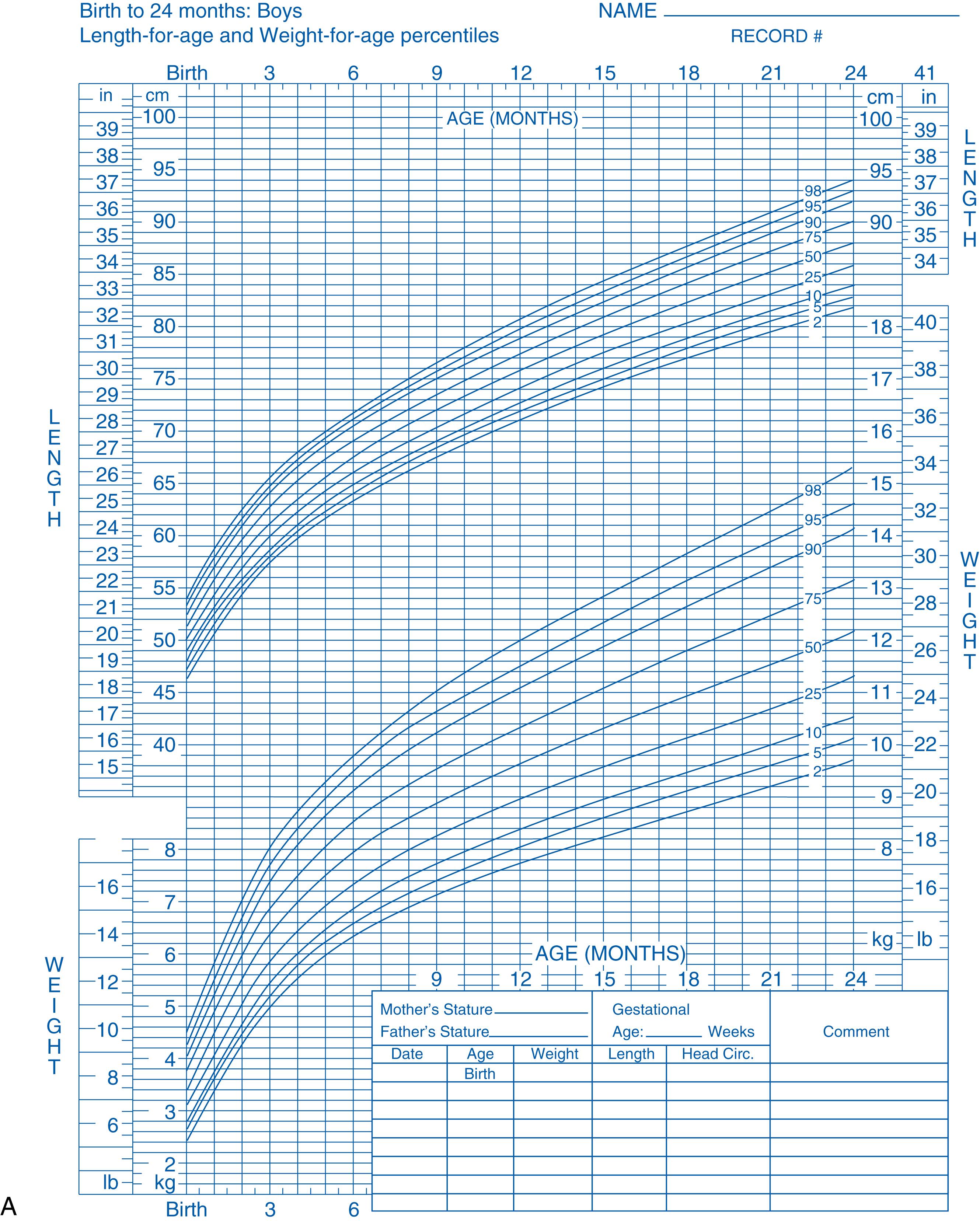
The CDC’s neonatal growth charts for term infants were revised and released in 2001 and are based on cross-sectional data for large numbers of infants; thus they can be assumed to be accurate. However, these are growth references that depict how infants grow but do not necessarily describe the ideal growth pattern of healthy infants. In 2006, the World Health Organization (WHO) released new growth standards for birth to 5 years derived from an international group of typically developing children raised in optimal environments that maximized growth and nutrition while eliminating confounding variables such as maternal smoking status, poor diet, and infection. The CDC recommends that healthcare providers use the WHO growth charts to monitor growth for infants and children ages 0 to 2 years of age ( Fig. 22.2 ) in the United States and CDC growth charts for children age 2 years and older, as after 2 years of age the charts are quite similar and the latter continue up to 19 years of age. Of note, there are several growth charts that were developed for certain populations; these include growth charts for trisomy 21, achondroplasia, Turner syndrome, Prader-Willi syndrome, and Williams syndrome (among others), which should be used when applicable.
Paralleling advancements in technology, the survival of preterm infants has improved dramatically over the past 2 decades. Unfortunately, the definition of “normal” extrauterine growth is still unknown; thus, growth standards for premature infants have been largely unavailable. The American Academy of Pediatrics and the Canadian Pediatric Society recommend that the growth of preterm infants should replicate that of a fetus of the same gestational age. This concept is highly debated among neonatal providers, given that the fetal environment and metabolic responses are very different than those of the preterm infant and many preterm infants experience growth faltering (falling down by one or two marked centile lines between birth and discharge). However, there are recent studies showing that, with proper nutrition, preterm infants may grow near their birth percentile.
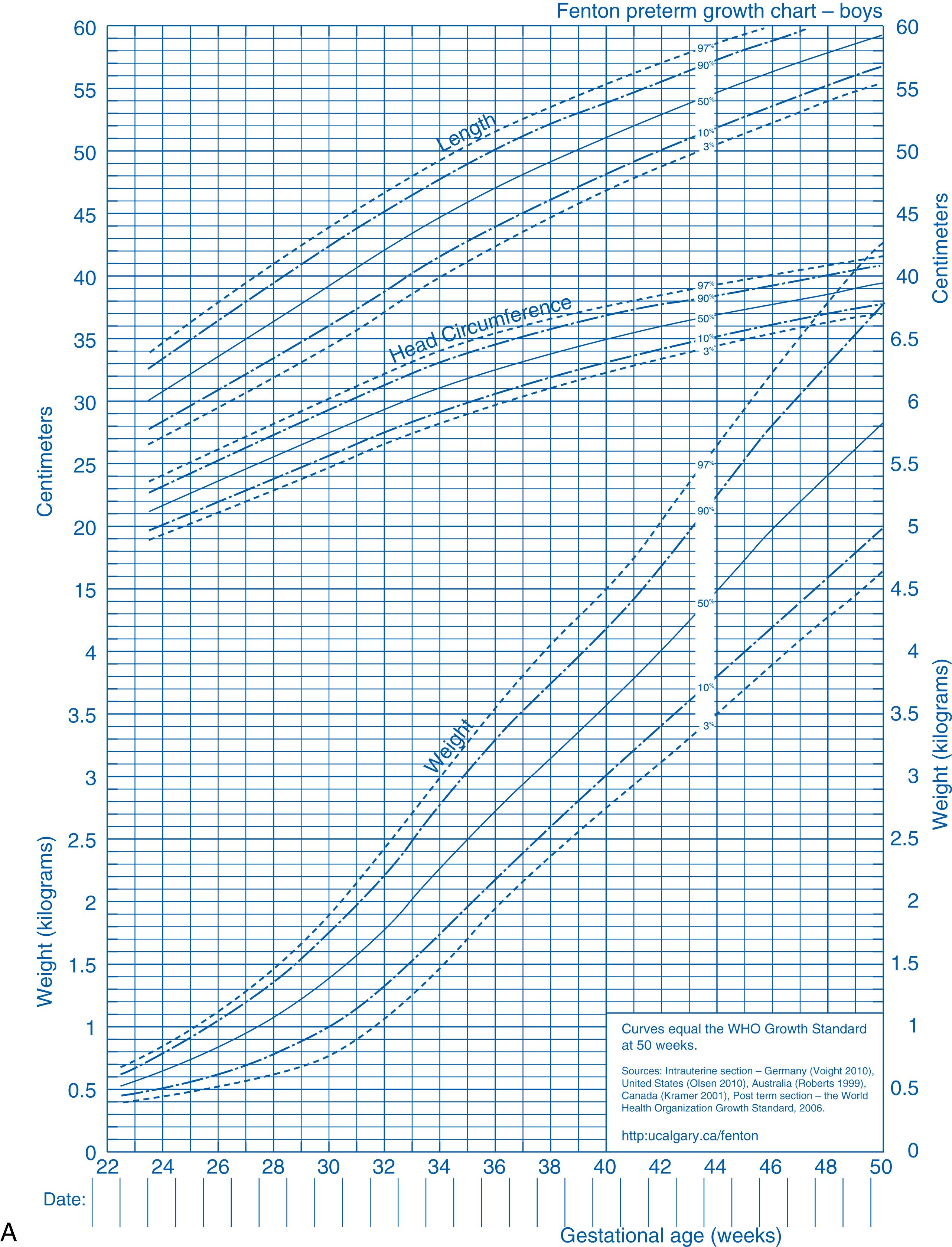
Despite the difficulties and limitations in establishing growth standards for preterm infants, growth references for height, weight, and head circumference adjusted for gestational age are available to determine the most likely appropriate weight gain, evaluate failure to thrive, and observe patterns of excessive weight gain in low-birth-weight infants. The most commonly used growth curves in neonatal units internationally are the Fenton growth curves ( Fig. 22.3 ), which were revised in 2013 to include the best available fetal and infant size data. They harmonize the preterm data with the WHO growth standards at 50 weeks of age. The revised sex-specific growth charts are based on data from nearly 4 million preterm births with confirmed or corrected gestational ages for infants born in developed countries, and they are also based on the recommended growth goal for the fetus, preterm infant, and term infant. The Fenton growth data are not accepted as a good method of assessing size at birth after 36 weeks’ gestation owing to concerns regarding discrepancy from intrauterine curves. BMI charts were recently developed for premature babies based on longitudinal data from 68,693 infants.
It has been estimated that up to 50% of preterm labor is associated with intrauterine growth restriction (IUGR); thus using growth data from this population to create a growth reference is problematic. Reference growth charts for preterm infants may underestimate the true prevalence of IUGR. One study examined the prevalence of small for gestational age (SGA) (<10th percentile) utilizing fetal growth standards and found higher rates of 26% to 30% compared with 10% when a neonatal reference chart was used, which is understandable considering that only infants born preterm contribute to the neonatal charts compared with presumably healthy fetuses contributing to the fetal growth charts. Likewise, feeding and postnatal nutritional supplementation practices vary widely between institutions, which further complicates the development of growth references and standards for preterm infants. Many believe that growth standards cannot be produced for preterm infants because babies born prematurely are inherently not normal, given the high proportion of placental insufficiency and other factors that contribute to preterm delivery. The monitoring of postnatal growth had traditionally been based on estimated fetal weight from ultrasounds, size at birth for gestational age, or data from preterm infants taken from longitudinal studies.
Alternate methods to improve the relevance of neonatal growth charts include the use of healthy population standards. A study from Burgundy determined a “healthy-population standard” by using birth weights for preterm infants born to healthy mothers only and compared this with an “entire-population standard” that included all preterm infants. Preterm infants determined to be SGA according to the “healthy-population standard” had a threefold increase in the risk of intraventricular hemorrhage, whereas this association was lost in infants who were deemed to be SGA using the “entire population standard.” Therefore growth standards based on healthy maternal populations may better indicate those infants at most risk of complications related to being SGA. Consensus is currently lacking regarding an optimal reference for the growth of premature infants. The INTERGROWTH-21st Project produced prospective, longitudinal, and prescriptive growth standards for fetal and postnatal growth of term and preterm infants up to 2 years of age. , The INTERGROWTH-21st postnatal growth standards serve as a robust tool for monitoring postnatal growth in infants born at 32 or more weeks gestation and can be used up to 6 months postterm. Despite the development of these growth standards, there are concerns that the relatively small sample size of very preterm infants (due in part to the recruitment of low-risk women) makes these growth standards less applicable to the general preterm population. Some argue that it may be useful to consider multiple growth charts and compare the percentiles and SD among the charts.
Longitudinal measurement of length of an infant helps predict childhood growth patterns and other factors in the evaluation of neonates. Although birth length appears to be the most important predictor for adult height, low birth weight is also associated with shorter adult height. The length of a term newborn is closely correlated with the maternal stature, weight, and parity. SGA babies who are born of short or thin mothers have lower risks of perinatal death than those born of primiparous mothers without these characteristics, suggesting that SGA infants born to short or thin mothers may be constitutionally small, whereas SGA infants born to other mothers may be pathologically small. Once free from the constraints of the uterus, the term infant will often adjust growth to reflect the midparental height, and the resulting growth channel should be followed for most of childhood. For children whose birth length percentile is less than their midparental height percentiles, an increase in percentiles occurs sooner after birth and the childhood percentile is reached by 11 to 12 months of age. For children whose birth length percentile is greater than their corrected midparental height percentile, a downward adjustment occurs after the first 3 to 6 months and will allow the child to reach the new lower percentile by a later mean age of 13 months. These changes are gradual, however, and show no abrupt change in growth velocity, which would indicate pathology. Between 3 years of age and the onset of puberty, normal healthy children will remain close to the same percentile, and a change in standard deviation score (SDS) of more than 0.25/yr is rarely seen. This tendency to maintain a narrow and predictable tract of growth is called “canalization.” Accurate data on weight and height velocity, as well as changes in body composition, are available for neonates.
Given the rapidity of growth in the neonatal period, this is a particularly vulnerable time for nutritional deficits, and preterm infants are at the greatest risk. Extrauterine growth restriction (EUGR) is common during the NICU stay, and poor growth postdischarge is also common. The Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition has a consensus statement for the identification of pediatric malnutrition (undernutrition), but these criteria do not apply to preterm neonates or less than 1 month of age. Utilizing the pediatric guidelines, a neonatal practice guideline was created to identify malnutrition in preterm neonates and infants less than 1 month of age. The assessment of change in z-scores for both weight and length is crucial in the assessment of neonatal malnutrition. One study showed that the contraction of extracellular water space that occurs in the first few days after birth causes a permanent change in postnatal growth trajectories, with z-score change of −0.8 from the intrauterine growth percentile. Thus the use of the weight z-scores for the determination of malnutrition takes this into account, in that a decline in weight z-score of 0.8 or more qualifies for malnutrition. Similarly, a decline in length z-score or 0.8 or more, when used in conjunction with another indicator, can signal malnutrition. Growth velocity should be monitored carefully, as this can be another reliable indicator of nutritional adequacy. The generally accepted velocities of 15 g/kg/day or 10 to 30 g/day for weight gain and 1 cm/wk for OFC and length have been shown to fit growth references only for a limited age range and may under- or overestimate appropriate growth outside that range. Rather, utilizing grams per day and centimeters per week to determine growth needed to maintain weight and length z-scores and comparing that to the actual growth velocity has utility in diagnosing malnutrition. Postnatal weight loss and delayed return to birth weight may reflect malnutrition, so the neonatal practice guideline examines days to regain birth weight as an indicator for malnutrition. However, this should be utilized in conjunction with an assessment of nutrient intake. Given that head growth is believed to be spared in periods of undernutrition, faltering head growth (in conjunction with faltering in weight and length growth) may support the diagnosis of moderate to severe malnutrition. Discussing, evaluating, and identifying malnutrition and faltering growth should be a priority during hospitalization, as this period of critical growth can have long-term consequences for an infant’s health and neurodevelopment.
Many studies are beginning to shed light on the role of the intestinal microbiota and their maturation in normal growth and the absence of their maturation in undernutrition. Therapies targeting the microbiota, such as probiotics, are attractive to those attempting to prevent or treat undernutrition. There have been many studies examining how perinatal exposure to probiotics affects fetal and neonatal growth. Probiotics taken during pregnancy and breastfeeding alter the cytokine profile of breast milk and increase secretory immunoglobulin A in infant stool samples. This may help to moderate excessive weight gain in infancy/early childhood. One study examining perinatal probiotic supplementation in addition to dietary counseling examined fetal and infant growth up to 24 months of age. There was no statistically significant difference in weight or length in the groups getting probiotics compared with those receiving placebo plus dietary counseling and the control groups. Studies examining postnatal administration of probiotics have shown mixed results regarding the effects of probiotics on growth. Hartel and colleagues demonstrated that probiotics were associated with improved growth among very low-birth-weight (VLBW) infants who had also received postnatal antibiotics. In another study, extremely low-birth-weight (ELBW) infants given Lactobacillus reuteri had improved rate of head growth in the first 28 postnatal days compared with infants who received placebo. Infants given Bifidobacterium breve and demonstrating colonization with B. breve had better growth between 4 and 8 weeks of life. A meta-analysis examining 25 randomized controlled trials demonstrated better weight gain and growth velocity among infants receiving probiotic supplementation. However, there have been several studies showing no clear association between probiotics and growth. The PREMAPRO study, a randomized controlled trial of Bifidobacterium probiotics supplements, showed no differences in growth—including measurements of weight, length, and head circumference—compared with infants receiving placebo. A separate randomized controlled trial of Lactobacillus sporogenes probiotic in VLBW infants showed no effect on growth. A review of four randomized controlled trials examining the effects of probiotics on growth in formula-fed preterm infants showed no difference in weight gain when compared with controls. Ultimately, the data have been mixed with regard to probiotics and growth and more research is needed; however, probiotics remain a promising target to prevent and treat undernutrition and improve growth among infants.
As reviewed by Saenger and colleagues in 2007, the definitions of IUGR and SGA are often confused; even the medical literature is not always clear. IUGR indicates a documented decrease in intrauterine growth noted by two fetal ultrasound examinations; a baby with IUGR may have a temporary problem that will still allow a normal or near-normal birth weight and length. Thus, the accuracy of fetal ultrasonography becomes an issue in this diagnosis. Because many infants are born with low birth weights, the description of SGA is in fact a proxy for IUGR in many but not all situations.
SGA refers to an infant whose weight and (presumably) length are below some limit. Usually weight or crown-heel length at birth for an SGA infant is at least 2 SD less than the mean for the infant’s gestational age, equivalent to the 2.5 percentile, based on data derived from an appropriate reference population. In some of the literature, however, SGA refers to babies with birth weights below the 3rd, 5th, or 10th percentile for gestational age, confusing interpretation of clinical studies during various eras. Furthermore, the difference in percentiles used for diagnosis is of practical importance, because medical insurance companies may adhere to one diagnosis and not cover treatment of children fulfilling other criteria. The WHO defines low birth weight as a weight at birth less than 2500 g in an infant of any gestational age; this inclusion of all gestational ages becomes problematic, because long-term outcomes are likely to differ between a baby who was premature but appropriate for gestational age (AGA) and a term infant who is SGA but of the same weight.
A consensus conference on treating SGA babies with growth hormone (GH) suggested a diagnostic criterion of a weight or length more than 2 SD below the mean for gestational age as a definition of SGA ; that convention is used in this chapter. SGA may be further classified as SGA with a low birth weight, SGA with a below-normal birth length, or SGA with a low birth weight and below-normal length, and the difference may change the outcomes of therapy. Two other descriptors of IUGR are used: symmetric IUGR denotes normal body proportions (a small head and a small body) and may be considered a more severe and long-standing form of IUGR, dating from the second trimester; asymmetric IUGR denotes small abdominal circumferences (due mostly to a small liver), decreased subcutaneous and abdominal fat, reduced skeletal muscle mass, and a head circumference in the normal range, probably resulting from stress effects in the third trimester. These definitions do not consider ethnic background, parity, or maternal size, so further classification is possible but not objectively defined, and the clinical utility of such costly and complex growth standards is unknown.
A low ponderal index (birth weight/length ) is also a marker for asymmetric growth restriction and is associated with an increased risk of cerebral palsy.
Evidence from longitudinal (as well as less powerful cross-sectional) studies shows that up to 90% of term SGA babies catch up in height for age, although often incompletely. In fact, approximately one-fifth of extremely short children have a history of IUGR, which presents at birth as SGA. Although premature infants are expected to have catch-up growth by 2 years, similar to term SGA infants, it is those born after 29 weeks’ gestation who exhibit catch-up growth; those born before 29 weeks more likely have a decreased rate of length and weight gain, which may be noted in the first week after birth and lasts up to 2 years. In addition, preterm SGA infants had slower growth velocity for height and weight up to 4 years of age, suggesting that preterm SGA infants did not catch up in growth compared with preterm AGA infants and term SGA infants. One study found that for extremely preterm young adults, height at 2 years of age was a stronger predictor of height at 18 years of age than midparental height, suggesting that medical conditions early on in life influence adult height more than genetic predisposition. A longitudinal study looking at the growth of VLBW infants (birth weight <1500 g) from birth until 20 years of age found that adult males with a history of being VLBW and SGA were significantly more likely to have weights and heights that were more than 2 SD below the mean than those who were VLBW and AGA. This difference was not seen in SGA versus AGA VLBW females. Male and female VLBW infants had similar rates of intrauterine and neonatal growth failure; however, females demonstrated greater catch-up growth than male counterparts, such that females born VLBW did not differ significantly in weight, height, or BMI by 20 years when compared with normal-birth-weight control subjects, suggesting that negative effects of neonatal illness on 20-year height attainment was greater among males. For ELBW infants (birth weight <1000 g), only 60.2% of SGA ELBW infants caught up in height by 2 years corrected age and 72.2% by 5.5 years of age. There were no differences in perinatal characteristics that could explain why some SGA ELBW infants caught up whereas others did not. In summary, it appears that those who are at greatest risk of growth failure are preterm SGA VLBW infant boys and ELBW infants.
It has been demonstrated for decades that catch-up growth can be shown soon after birth for term SGA infants if careful measurements are used. This seems to be a crucial time frame because much of the catch-up growth occurs in this period. Indeed, an acceleration of growth in length is noted in the first 3 months in SGA babies who will catch up. Minimal catch-up growth occurs after 2 years of age; thus those who do not catch up and remain short at 2 years of age have a greater risk for shorter adult stature. In general, children born SGA will achieve an adult height SDS equivalent to their SDS in childhood because skeletal development is normal. Children with a history of SGA at birth without catch-up by 2 years of age may have other conditions that limit growth, such as GH deficiency, thus an endocrine evaluation is warranted.
Longitudinal evaluation of growth in neonates in NICUs demonstrates an almost universal growth impairment. Gestational age is the most important predictor of growth impairment, followed by birth weight and length SDS. The babies born at younger gestational age with higher weights were at greatest risk of both types of growth impairment. However, one recent study reported that optimization of nutritional practices can results in no early postnatal growth failure, demonstrating that such children can grow along their newborn growth percentile without faltering.
A history of very preterm birth and/or VLBW is associated with more neurodevelopmental impairment and lower academic achievement scores. , Children born preterm with IUGR/SGA appear to have lower cognitive scores than preterm AGA infants, especially preterm IUGR/SGA boys. There is more controversy about long-term effects of SGA on intellectual function in term infants. However, most of the literature has found that even term infants who are SGA have lower neuropsychologic test scores, which appears to be due to IUGR, those who were constitutionally small without a history of IUGR had test scores similar to those of control subjects. These differences in neurocognitive functioning are likely related to the finding of an almost 6% reduction in total brain volume in young adults born term but SGA versus AGA control subjects.
Adults born with lower birth weights are at higher risk of impaired glucose tolerance, type 2 diabetes, and cardiovascular death. This was initially noted in several studies showing that death rates from cardiovascular disease were inversely related to adult height and paralleled previous geographical history of infant mortality. This original observation grew into the theory of fetal and infant origins of adult disease, also coined the Barker hypothesis. It states that undernutrition in utero and during infancy exerts long-term changes in the cardiovascular, endocrine, and metabolic regulatory systems, leading to poor cardiovascular health in adult life, and that these changes may be transgenerational. , Thus, the poorly nourished fetus is given a forecast of the nutritional environment following birth, and processes are adapted to survive under conditions of poor nutrition. These adaptations become detrimental when the postnatal environment differs from the forecasted environment and nutrients are normal or excessive, which leads to abnormal growth and obesity, thus increasing the individual’s susceptibility to fetal and infant origins of adult disease. , More recently, this hypothesis evolved into the concept of developmental plasticity—that is, that changes in gene expression during infancy and childhood influenced by environmental forces occur through epigenetic processes such as DNA methylation and histone modification, which can have lifelong consequences.
In addition to SGA status, rapid growth in such children also leads to adult risk of cardiovascular disease and glucose intolerance, as reviewed by Gluckman and colleagues. This tendency is already apparent at 2 to 4 years of age. SGA infants who experience catch-up growth demonstrate a higher degree of insulin resistance than AGA infants. In particular—according to longitudinal studies in Avon, Stockholm, and other sites—weight gain and fat mass accrual during the first months after birth may be more of a risk factor for obesity, insulin resistance, and metabolic syndrome than later weight gain during childhood. , Infants with the greatest increase in weight and linear growth during the first 2 weeks after birth have endothelial dysfunction up to 16 years later to a degree as significant as that seen with insulin-dependent diabetes mellitus or smoking in adults. Likewise, measures of insulin resistance are higher in those with the greatest weight gain in the postnatal period. Therefore it has been suggested that a degree of slow growth in the postnatal period may not be undesirable. Alternatively, minimizing EUGR of preterm infants, thus removing the need for catch-up growth, may lead to improved neurocognitive outcomes without the potential complications of insulin resistance and metabolic syndrome related to rapid weight gain. It is also postulated that early improvement in postnatal nutrition, especially with protein/amino acids, may have a larger impact on β-cell growth and function leading to less type 2 diabetes mellitus.
Most preterm infants grow poorly during their initial hospital stay, with up to 90% leaving the hospital with weight and length below the 10th percentile for age. This EUGR is associated with poorer neurodevelopment and increased rates of neurologic impairment. Therefore many recommend early aggressive nutrition to minimize caloric and protein deficits and to prevent EUGR and associated negative cognitive and neurodevelopmental outcomes. However, it is difficult to mimic the intrauterine environment for preterm infants, as the extrauterine environment has added stressors that affect growth, including temperature regulation, feeding intolerance, insensible water losses, infectious agents, and medical interventions that increase energy expenditure and nutrient losses.
Preterm birth itself was also found in meta-analyses to be associated with increased risk of decreased insulin sensitivity and type 2 diabetes. Premature babies who are born AGA and demonstrate slow catch-up weight gain nonetheless have postnatal insulin resistance to the same degree as SGA babies. Rapid, excessive weight gain in term neonates also has been correlated with the development of hypertension, obesity, and comorbid illnesses by the age of 30 years. ,
It appears that cardiovascular risk factors are affected differently in preterm girls versus preterm boys. Prematurity is associated with higher blood pressure in adolescent girls and a more atherogenic lipid profile and reduced insulin sensitivity in adolescent boys. The differences in these outcomes strengthened after excluding infants born SGA, suggesting that these differences are not solely due to restricted intrauterine growth. There was also evidence to suggest a dose-response relationship between shorter length of gestation and cardiovascular risk factors.
The effect of prematurity on all features of the metabolic syndrome may not be lifelong. Two meta-analyses found no differences in BMI, waist-to-hip ratio, brachial artery flow-mediated dilation, carotid intimal-medial thickness, high-density lipoprotein levels, and triglycerides in adults born preterm compared with those born at term. , One meta-analysis demonstrated no difference in percent fat mass in adults born term versus preterm, whereas another found no difference in levels of low-density lipoprotein (LDL). The two meta-analyses did find that blood pressure—including 24-hour ambulatory monitoring—was significantly higher in adults born preterm than in those born at term. , Parkinson and colleagues found that adults born preterm had significantly higher fasting LDL and total cholesterol. Markopoulou and colleagues found that preterm birth was associated with higher body fat mass, higher fasting glucose and insulin levels, estimated insulin resistance, and higher total cholesterol levels compared with term-born adults. Some have demonstrated that differences in adult blood pressure may be related to gender; 24-hour ambulatory blood pressure measurements were significantly higher in women but not in men born preterm. A separate meta-analysis found that preterm and VLBW status is associated with higher resting systolic blood pressure as well as essential hypertension (blood pressure ≥140/90 mm Hg) later in life versus those born at term.
Low birth weight can affect pubertal development in girls. Girls born with low birth weights extending to the classification of SGA defined previously tend to have early onset of puberty, precocious puberty, or exaggerated precocious adrenarche. In addition, girls with low birth weights have an increased prevalence of insulin resistance by 1 year of age and a tendency toward developing polycystic ovarian syndrome, which is characterized by hyperinsulinemic hyperandrogenism. Girls with average birth weight who display early breast development may have an extended duration of time between breast development and menarche; therefore the age of menarche is relatively stable even if the age of onset of breast development is more variable. However, girls with low birth weight do not demonstrate such an extension of the time between breast development and menarche ; treatment with metformin in childhood attenuated this rapid progression. Thus it has been postulated that the hyperinsulinemia characteristic of these girls leads to early menarche. The effect of low birth weight on the reproductive axis in males is not well known, but increased aromatase activity leading to elevated estradiol levels, increased 5-α-reductase activity reflected by elevated dihydrotestosterone, and elevated inhibin B levels reflecting activity of the seminiferous tubules are inversely proportional to birth weight. At present, the effects of these changes are not known.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here