Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The placenta produces a greater diversity of hormones in greater quantity than any other single endocrine tissue. Near term, steroid hormones (primarily estrogens and progestins) are being made at the rate of 0.5 g/day, and protein hormones (lactogens, growth factors, and other hormones similar to those of the hypothalamic-pituitary-adrenal axis) are being made at more than twice this rate. Although the secretory nature of the placenta was recognized by the early 1900s, , it was not until the 1950s that the placenta was recognized as part of a highly regulated endocrine system incorporating the fetus and mother. Placental hormones, made either directly by the placenta or dependent on placental synthesis of precursors, are critical for fetal growth and for metabolic adjustments in response to environmental factors of pregnancy in both mother and fetus. These hormones may act as endocrine, paracrine, and autocrine signals that link maternal physiology to fetal development through placental alterations in structure, secretion, or growth.
The human hemochorial placenta allows maternal blood to have direct contact with fetal tissue through controlled invasion of the maternal vascular system by fetal trophoblasts. This invasion peaks around 12 weeks gestation although placental maturation continues well into the third trimester. During the initial invasion of the maternal spiral arteries and formation of chorionic villi, trophoblasts differentiate along two major pathways: into invasive extravillous cytotrophoblasts and into a fused layer of syncytiotrophoblasts ( Fig. 11.1 ). Syncytiotrophoblasts are the primary hormone-producing cells in the placenta, making both peptide and steroid hormones, whereas cytotrophoblasts appear to make a limited set of peptide hormones. Additional hormones are made in adjacent fetal and uterine tissues, including amnion, chorion, and decidua. The placenta is not only a producer of hormones but also acts as a barrier to hormone transfer (i.e., thyrotropin-stimulating hormone and insulin) from the mother to the fetus or as a modifier of endocrine signaling through specific metabolism of maternal or fetal hormones.
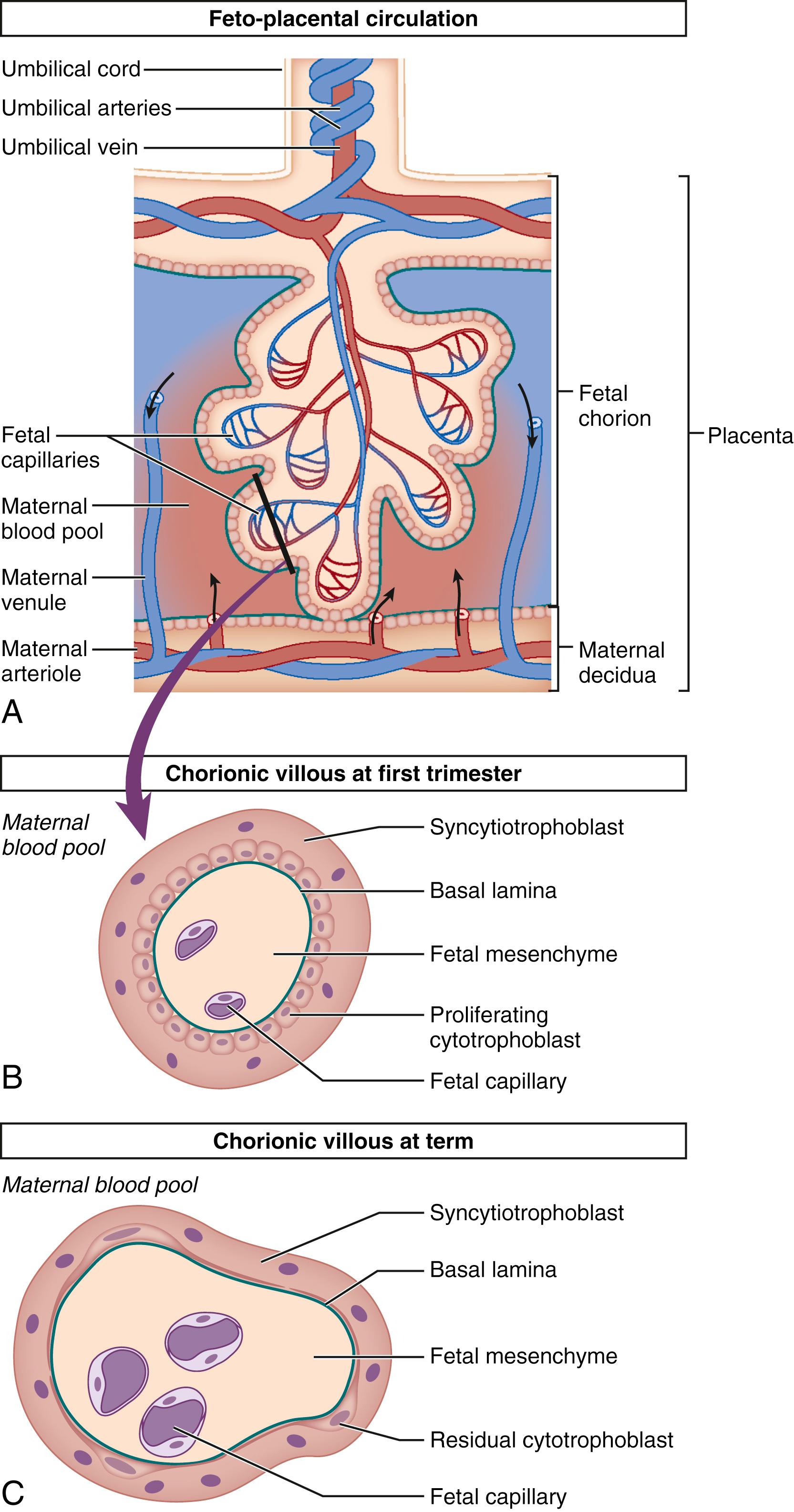
The human placenta has multiple intrinsic physiologic functions and produces many factors that regulate them. However, the placenta does not act in isolation. Its functions are integrated with those of other intrauterine tissues, such as the maternal uterus, chorion, amnion, decidua, amniotic fluid, and the fetus. These other intrauterine tissues produce or use some of the same hormones and carrier proteins that regulate placental hormone activity. Metabolic signals and precursors, as well as hormones, are carried by maternal and fetal blood or transported from cell to cell from the uterus, decidua, fetal membranes, and amniotic fluid to and from the placenta. Integration of activities across multiple tissues provides the normal physiology of pregnancy, allowing its maintenance and timely parturition.
This chapter focuses on the major endocrine, paracrine, and autocrine signals made by the placenta; given that the human placenta is capable of synthesizing most of the hormones identified to date, there are many factors that will not be discussed and likely many more will be discovered in the future. Table 11.1 highlights major peptide and steroid factors described in placental endocrine function. Understanding is still limited regarding the roles that many of these hormones play in the local endocrinology of placental development or in the broader regulation of the materno-feto-placental system required for successful pregnancy outcome. This chapter highlights what is currently known about the expression, function, and regulation of the major categories of peptide and steroid placental hormones.
| Steroid Hormones | Pituitary-Like Hormones, Including Growth Factors | Hypothalamic-Like Hormones | Neuropeptides | Placental Cytokines | Eicosanoids |
|---|---|---|---|---|---|
| Estriol Estradiol Estrone Estetrol Progesterone Allopregnanolone Pregnenolone5α-DHP Cortisone Testosterone Androstenedione DHT DHEA |
hCG hCS hGH-V IGF-I IGF-II Activin Inhibin Follistatin β-Endorphin Oxytocin ACTH MSH Relaxin |
CRH Urocortins GnRH-I, GnRH-II GHRH Somatostatin TRH PRH |
Serotonin Dynorphin Met-enkephalin ANP Leptin Ghrelin Adiponectin Neurotensin Substance P Melatonin Cholecystokinin Galanin Neuropeptide Y Endothelin VIP |
TNF-α LIF Interferon-α Interferon-β Interferon-γ IL-1 IL-2 IL-6 IL-8 IL-10 |
Prostaglandins Leukotrienes Prostacyclin Thromboxane |
Human chorionic gonadotropin (hCG) is one of the first hormones of pregnancy, produced by trophoblasts even before placenta formation. After placentation, hCG is synthesized primarily by the syncytiotrophoblasts and passes into the maternal circulation via secretion into the intervillous space. hCG can be detected in human serum or urine within a week of conception and is the most frequently used biochemical marker for pregnancy.
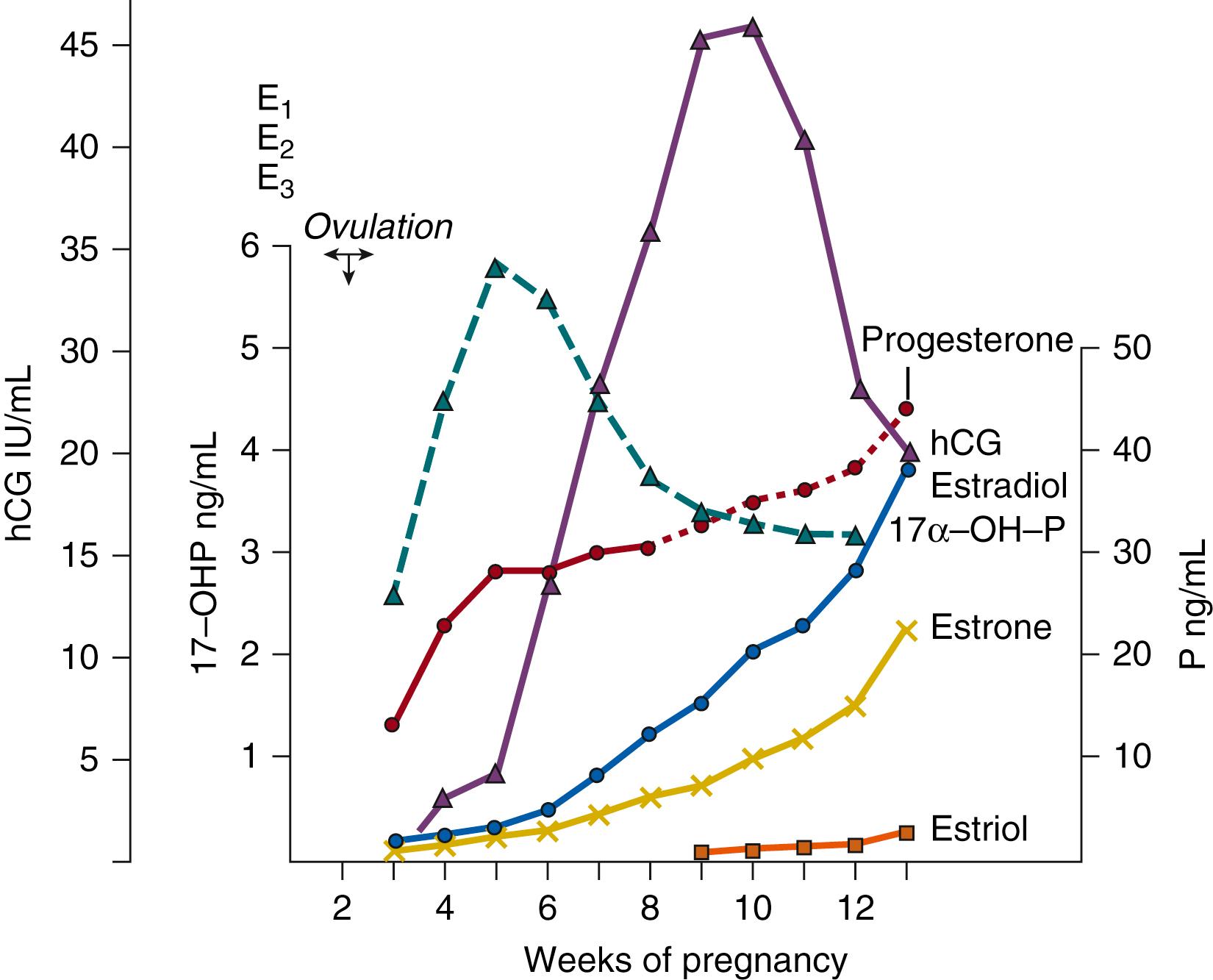
The primary biologic role of hCG is to maintain progesterone production by the corpus luteum until this function shifts to the maturing placenta. This transition from ovarian to placental steroid production, required to sustain human pregnancy, is referred to as the ovarian-placental shift. This progesterone shift starts at the end of the sixth gestational week and is complete by the ninth week, at least 2 weeks before the level of placental hCG peaks ( Fig. 11.2 ), minimizing the chance of loss of the progestational environment.
hCG is unique to human pregnancy; primate placentas express similar gonadotropins, but the vast majority of placentas from other species do not. hCG is a glycoprotein heterodimer (36 to 40 kDa) composed of an α-subunit and β-subunit encoded by genes on chromosome 6 and chromosome 19, respectively. , The α-subunit is homologous to pituitary thyroid-stimulating hormone (TSH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), whereas the β-subunit is homologous to LH. hCG can be immunologically distinguished from LH with use of antisera directed against the C-terminal amino acids of its β-subunit, which are not present in LH. β-subunit hCG–specific antisera is the basis for most current pregnancy tests.
Intact hCG (i.e., having both an α-subunit and a β-subunit) is required for hCG endocrine activities. Because it shares a receptor with LH, the LH chorionic gonadotrophin receptor (LHCGR), hCG mimics the function of LH. Both hormones activate second-messenger pathways, primarily cyclic adenosine monophosphate (cAMP) pathways, via LHCGR. However, the functions of LH and hCG are quantitatively different owing to the longer half-life of hCG and its relatively stable presence in the bloodstream compared with the pulsatile release of pituitary LH.
Variations in both glycosylation and subunit availability appear to regulate hCG activity. A hyperglycosylated form (hCG-H) has been detected in early pregnancy, as well as in choriocarcinoma cells. hCG-H appears to enhance invasive cytotrophoblast activity independently of LHCGR, possibly through a transforming growth factor receptor pathway. hCG-H may be a very early biomarker of placental invasion of the endometrium. A decreased level of hCG glycosylation in very early pregnancy has been correlated with early pregnancy loss, although this measure is not currently used clinically. , , Isoform production may also regulate activity. Initially, β-subunit production exceeds α-subunit production, but this ratio rapidly shifts to an excess of the α-subunit, and the ratio increases as gestation progresses. As a result, little free β-hCG is secreted, and circulating hCG is mostly intact hCG or free α-hCG. It has been proposed that the ratios of hCG isoforms (intact hCG, independent subunits, and nicked breakdown products) present in maternal blood and urine might be useful for detection of pregnancy-related disorders because only intact hCG is fully active and abundance of other isoforms may modulate this activity.
Clinically, hCG doubling time may be used in early gestation to predict general pregnancy outcome. After hCG can first be detected, its level increases with a doubling time averaging 2.11 days. It reaches peak levels of approximately 50 international units per milliliter at 9 to 10 weeks from the date of the last menstrual period, declining to 1 IU/mL by mid-gestation (see Fig. 11.2 ). An abnormally slow doubling time of hCG concentration is considered to be a sign of a poor prognosis for pregnancy outcome, whereas a rising hCG level without detection of an intrauterine embryo suggests an ectopic pregnancy.
Both local and systemic factors can influence hCG production. Locally, hCG expression is regulated by a releasing factor, gonadotropin-releasing hormone (GnRH; isoforms I and II), produced largely in the cytotrophoblasts. , Additional factors, including neurotransmitters, cAMP, epidermal growth factor (EGF), activin, cytokines, prostaglandins, and hCG itself regulate hCG production. Each of these factors is produced by the placenta, as well as by other trophoblastic tissues. hCG is known to affect placental steroidogenesis by stimulating both progesterone and estrogen formation. Estrogens can inhibit GnRH stimulation of hCG, thereby completing a feedback axis in the paracrine placenta. Other hormones such as inhibin have also been shown to modulate this axis.
Although most of our understanding of hCG expression, function, and regulation comes from studies of its role as an endocrine signal for the maternal corpus luteum, hCG has multiple activities that regulate placental structure and function and modify the intrauterine environment to support implantation and fetal survival. In addition to its well-known endocrine function for the corpus luteum, hCG acts as an autocrine signal in trophoblasts expressing LHCGR. In trophoblasts, hCG regulates the differentiation of cytotrophoblasts into syncytiotrophoblasts, thus amplifying hCG production because it is made primarily by the syncytiotrophoblasts. Phosphorylation of the receptors via this pathway also decreases LHCGR expression in differentiating syncytiotrophoblasts, thus completing a feedback loop.
More recently identified activities of hCG also include roles in endometrial angiogenesis, maintaining uterine quiescence, and enhancing immunotolerance to the fetus. , In addition, hCG appears to have maternal thyrotropic activity because of its partial homology to TSH. However, recent studies demonstrate that although elevated hCG levels suppress maternal TSH levels, leading to elevation of the levels of free thyroxine, this action is rarely associated with maternal hyperthyroidism. Continued research is required to fully define the wide-ranging effects of this classic human pregnancy hormone given its potential impact on multiple aspects of pregnancy.
A hormone similar to pituitary growth hormone (GH) and prolactin was first extracted from human placenta in the early 1960s. , This hormone was initially named human placental lactogen, but was later renamed human chorionic somatomammotropin (hCS), to reflect its GH-like activity and its lactogenic activity; both names remain in use.
Expression of hCS can first be detected in trophoblast tissue within 10 days of conception and in maternal serum by the third to fourth week of gestation. The hormone is a single 191 amino acid nonglycosylated peptide chain with considerable homology to GH (96%) and prolactin (67%); it is transcribed from a gene cluster on chromosome 17 containing two genes for hCS, one hCS pseudogene, and two GH genes. , It is synthesized by the syncytiotrophoblasts at a constant rate during gestation, so as placental mass expands, the hCS levels reflect total placental mass and thus gross placental function. By term, hCS is the most abundant placental hormone, produced at more than 1 g/day, representing 10% of total placental protein synthesis.
Despite hCS being so abundant and having been identified more than 50 years ago, understanding of its function in pregnancy remains limited. It is almost exclusively found in maternal rather than fetal circulation. This has led to the hypothesis that the primary role of hCS is to ensure adequate fetal nutrition because, in maternal circulation, it induces metabolic changes such as mobilization of fatty acids, insulin resistance, decreased utilization of glucose, and increased availability of amino acids through decreased maternal use of protein. The levels of circulating maternal glucose and free fatty acids are thus increased. Although glucose readily crosses the placenta, fatty acids cross slowly, thus biasing glucose delivery toward the fetus and use of fatty acids for maternal energy, especially during maternal fasting. hCS is considered one of the major diabetogenic factors of pregnancy, along with placental steroids, placental human GH variant (hGH-V), and maternal cortisol. Within the placenta, hCS may regulate insulin-like growth factor (IGF) I and alter fetal growth through direct action on placental nutrient transport systems. Loss of hCS and hGH-V may result in severe fetal growth restriction, although healthy pregnancies have also been reported in the absence of hCS.
In addition to its metabolic activity, the lactogenic activity of hCS suggests a synergistic role with prolactin and steroids in preparation of the breast for lactation. Most recently, a role for hCS as a placental angiogenic factor has been suggested.
Regulation of hCS release also remains poorly defined. The hypothalamic-like releasing and inhibiting factors found in the placenta do not appear to effect hCS release, in contrast to hypothalamic actions on pituitary GH release. In vitro studies show that hCS can be stimulated by high-density lipoproteins, apolipoproteins, , angiotensin, cAMP, arachidonic acid, insulin, and IGFs, and is inhibited by the prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α), catecholamines, phorbol esters, and diacylglycerols. Dopaminergic agents may also inhibit hCS release.
Placental human GH variant (hGH-V) is encoded by the same gene cluster as hCS and pituitary GH on chromosome 17. In syncytiotrophoblasts, two transcripts are generated from the hGH-V gene, one major form and one alternatively spliced version. Secreted hGH-V is translated from the major version and is produced in a highly bioactive 22-kDa non-glycosylated form and to a lesser degree in a 25-kDa glycosylated form. Early in pregnancy, maternal pituitary GH is produced, but from 15 to 20 weeks gestation to term, hGH-V secretion increases, suppressing maternal GH to undetectable serum levels by 24 weeks gestation. The level of hGH-V peaks about 1 month before term delivery and it disappears from the maternal circulation immediately after delivery. hGH-V is not detected in the fetal circulation, but acts as an endocrine factor in the maternal circulation that indirectly affects fetal growth and possibly as a paracrine factor in the placenta.
Much like hCS, hGH-V plays a significant role in modifying maternal metabolism to meet fetal needs. hGH-V primarily appears to control maternal IGF-1 production. In mice overexpressing hGH-V (not normally found in rodents), body weight was increased, IGF-1 levels were elevated, and insulin resistance developed, suggesting that hGH-V strongly contributes to the normal hyperinsulinemia and lack of responsiveness to insulin that characterizes the second half of human gestation. hGH-V expression thus increases the risk for gestational diabetes and other pregnancy-related disorders. This risk is counterbalanced by placental lactogens, hCS, and prolactin, which induce increased insulin secretion by pancreatic β cell expansion. hGH-V itself is not a lactogen. Thus, a combination of pituitary-like GHs is required to support fetal growth while maintaining maternal metabolic homeostasis.
hGH-V secretion is tonic, in contrast to pulsatile pituitary GH secretion, and is not regulated by hypothalamic releasing factors. Secretion is inhibited by elevated glucose levels and mildly increased by hypoglycemia, creating a feedback loop that may ensure constant delivery of nutrients to the developing fetus.
IGF-1 and IGF-2 are highly homologous single-chain polypeptides with similarities to proinsulin. Both are made in human placental tissues. Most of the components of the insulin-IGF system are found in the placenta (IGF-1, IGF-2, and IGF-binding proteins [IGFBPs] 1-6). The exception is insulin, which is not made by the placenta and does not cross the placenta, although insulin has profound indirect effects on fetal growth and well-being. IGFs are the primary somatotrophs in gestation, as GH receptors are expressed at only low levels in fetal tissues. Within the placenta, IGF-1 is expressed predominantly in syncytiotrophoblasts throughout gestation, with some expression in cytotrophoblasts as well. In contrast, IGF-2 is not found in syncytiotrophoblasts but is expressed in cytotrophoblasts with a declining expression level across gestation. , These hormones mediate a variety of metabolic and mitogenic effects by binding to specific receptor tyrosine kinases. At physiologic concentrations, both IGF-1 and IGF-2 bind to IGF-1 receptor (IGF1R). The localization of IGF1R shifts during gestation; initially it is predominantly expressed on the syncytiotrophoblasts (closer to the maternal circulation) and by term it is mainly expressed on the fetal cytotrophoblast side, presumably reflecting the shifting activity from maternal to fetal growth control. The IGF-2 receptor (IGF2R; also known as the cation-independent mannose 6-phosphate receptor ) controls extracellular IGF-2 concentrations by mediating the endocytosis and degradation of IGF-2 rather than transducing a signal. Mouse experiments suggest that an additional receptor, possibly a variant of the insulin receptor, may mediate some of the fetal growth effects of IGF-2. IGFs are thought to primarily act locally but can circulate, primarily in bound forms. IGF-2 has its highest serum levels in the fetal circulation, although whether the placenta contributes to this circulating IGF-2 is unclear.
Although IGF-2 is often considered the primary fetal GH, IGF-1 plays a significant role in fetal growth as well. Information on the role of IGFs in fetal growth comes from genetic manipulation in mouse models, as well as examination of human tissues, especially in pregnancies having compromised fetal growth. In mice, disruption of the IGF-1, IGF-2, or IGF1R gene retards fetal growth, whereas disruption of the IGF2R gene or overexpression of IGF-2 enhances fetal growth. In humans, IGF-1 or IGF1R mutations are extremely rare, and no IGF-2 deletions have been reported. However, IGF-2 is an imprinted gene normally expressed exclusively from the paternal allele in placenta and fetal tissues. Changes in IGF-2 expression due to abnormal imprinting has been linked to both overgrowth (Beckwith-Wiedemann syndrome) and growth restriction (Russell-Silver syndrome). Whether placentally derived IGFs, as opposed to fetal IGFs, directly contribute to these fetal growth changes is uncertain, as these factors also have paracrine effects in the placenta that determine nutrient transport and placental growth.
Additional roles of IGFs in fetal growth are mediated by their potentiation of EGF activity, stimulation of decidual prolactin production and enhanced progesterone production. In addition, production of placental thromboxane, a potent vasoconstrictor, is specifically inhibited by IGF-1. Thus the production of placental IGFs is thought to be of major importance for normal intrauterine fetal growth.
IGF-1 and IGF-2 play significant, but seemingly distinct, roles in paracrine and/or autocrine signaling in the placenta. The expression of IGF1R in human trophoblasts as noted above also supports paracrine or autocrine effects in the placenta. IGF-1 can regulate the differentiation of cytotrophoblasts into syncytiotrophoblasts, whereas IGF-2 appears not to have this function despite its very early placental expression. Placental mass may also be regulated directly by both placentally produced IGFs. Cytotrophoblast proliferation and survival is mediated by IGFs produced in isolated placental explant cultures. However, in vivo a decreased level of IGF-2 reduces the placental surface area available for gas and nutrient exchange more than loss of IGF-1. Both IGF-1 and IGF-2 alter nutrient transport especially of amino acids. , The increased amino acid transporter expression caused by increased levels of IGF-1 and IGF-2 in vitro is paralleled by elevated fetal amino acid transport associated with gestational diabetes.
hGH-V concentrations correlate with IGF-1 levels throughout pregnancy, and hGH-V appears to be the critical regulator of placental IGF levels. In addition, IGF-1 and IGF-2 effects are modulated by high-affinity IGFBPs (IGFBP-1 to IGFBP-6) that bind these IGFs with different affinities. IGFBPs are carrier proteins protecting IGF from degradation while blocking its bioactivity. IGFBPs are expressed in human placenta. , , In addition to placental IGFBPs, IGFBP-1 is produced by the decidua in large amounts. IGFBPs are themselves regulated by protease activity and posttranslational modifications, adding a further layer of regulatory complexity.
Additional growth factors have been identified in the placenta and chorionic membranes. The early blastocyst expresses platelet-derived growth factor A, transforming growth factor α, and transforming growth factor β. These factors are thought to be involved in signaling implantation. Other growth factors such as EGF, basic fibroblast growth factor, nerve growth factor β, and granulocyte colony-stimulating factor are not detected in early gestation, but are expressed by the placenta at later gestation stages. Receptors for EGFs, as well as for many other growth factors, have been identified in the placenta and membranes. Most of the EGF receptors in the placenta are localized to the syncytiotrophoblast and correlate with induction of trophoblast differentiation rather than proliferation. The finding that EGF stimulates hCG and hCS secretion supports this hypothesis. EGF also stimulates prostaglandin synthesis. Another growth factor, hepatocyte growth factor, also appears to be essential for placental development. ,
Inhibin and activin, so called because they are, respectively, an antagonist and an agonist of pituitary FSH, have also been found in placental cytotrophoblasts, as well as in fetal membranes. , Activin receptors are expressed in syncytiotrophoblasts but not cytotrophoblasts. Follistatin and follistatin-related gene (activin-binding proteins), which functionally inhibit FSH secretion in the maternal circulation, are also made in the placenta. Inhibin has been shown to inhibit GnRH stimulation of hCG and chorionic GnRH production and reduce progesterone production. Activin potentiates the GnRH-stimulated hCG release and progesterone production. Follistatin can reverse activin potentiation of hCG. Elevated levels of inhibin can be seen in fetal trisomy 21 cases, whereas elevated activin levels have been reported in the setting of preeclampsia and diabetes. During pregnancy these hormones are actively involved in the GnRH-hCG-steroid-prostaglandin axis of the placenta and may serve as potential biomarkers of placental disorders.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here