Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There has been a dramatic change in surgical care over the past 30 years with the introduction of digitization, miniaturization, improved optics, advanced diagnostic and therapeutic tools, and computerized information systems in the operating room (OR). Whereas surgery has traditionally required large incisions sufficient to allow the surgeon to introduce his/her hands into the body and to allow sufficient light to see the structures being operated upon, innovations have stimulated a radical change in the way surgical procedures are performed. For many surgical procedures, image-guided techniques have now become the standard of care, and the technology continues to evolve, rendering it safer and more seamless for optimizing patient outcomes. These can be done by manipulating instruments from outside the patient, while directing them by looking at displays of direct images of the target tissues (e.g., endoscopic or laparoscopic surgery) or at indirect images of the region of interest (e.g., endovascular catheter-based treatments, image-guided energy ablation of specific targets). Image-guided surgery has enabled the use of significantly smaller incisions to introduce surgical instruments into specific compartments and perform a procedure that would otherwise not be possible without a traditional incision ( Fig. 15.1 ). In other cases, the surgical instruments can access the target tissues through anatomic conduits (e.g., arteries or veins) or natural orifices (e.g., mouth, anus, vagina, or urethra) without the need for any visible incision. These digital platforms have also opened the door to an entire world of opportunities to provide surgeons with real-time data and guidance to improve performance and patient safety, including the use of augmented reality, telementoring by an expert outside the operating theater, and the emerging field of artificial intelligence (AI) to provide computer-augmentation of intraoperative performance. Furthermore, newer technologies have created an explosion of available diagnostic and therapeutic tools that can potentially be used to improve patient outcomes after surgery.
Although patients may benefit substantially from new technologies that minimize the invasiveness of surgical therapies and improve the safety of surgery altogether, employment of novel techniques often requires an entirely new set of skills for the surgeon and his/her team. While the concept of the procedure itself may be familiar to the operating team, the aptitudes required to perform the procedure using a new approach and using new tools are different (e.g., developing a mental model of the anatomic landmarks from a different vantage point or developing the psychomotor skills necessary to effectively maneuver a new tool). Learning and practice have become fundamental aspects of introducing new technologies in the OR in order to avoid the risk of complications during this transition phase. In addition, “new” does not always equate to “better,” and critical assessment of the utility, safety, and cost-effectiveness of new technology remains a cornerstone of the process of adopting innovations in surgery.
This chapter describes recent groundbreaking surgical innovations, highlights emerging technologies that are poised to change the OR significantly in the near future, discusses important elements and hurdles for introducing technologies in the OR, and addresses approaches to training and establishing proficiency as new technologies emerge.
Accessing internal body cavities, such as the chest, abdomen, and pelvis, requires making an incision, the size of which is determined by the surgeon’s need to visualize, feel, and manipulate the target tissues, and knowing the dimensions of any tissues that need to be extracted. Minimal-access surgery (such as laparoscopic surgery) provides the means to diminish the trauma of access using image-guided systems to perform the operation without compromising its overall goal. The “cost” to the patient of the access incision is multifactorial. Generally, larger incisions are associated with more postoperative pain, longer recovery periods, a period of physical disability, greater morbidity in cases of wound infection, more risk of incisional hernias, a higher rate of symptomatic adhesive bowel obstruction in the future, and poorer cosmetic results. While minimally invasive surgery has already been widely adopted as the standard of care for many surgical procedures for over two decades, it nevertheless continues to evolve, with more advanced optical systems, integrated digital platforms, and incorporation of more sophisticated therapeutic and diagnostic tools. As surgeons have become more skilled in laparoscopy, these systems are increasingly being adopted for procedures that have traditionally been either too complex or difficult to accomplish without an open approach, bolstered by evidence of effectiveness and safety. Relative contraindications have continued to diminish, and, currently, most elective and many emergency abdominal surgical procedures are frequently done laparoscopically. Despite their advantages, the smaller incisions present some specific challenges to the operating surgeon, and conversion to an open operation should always be considered a possibility.
One of the advantages of minimally invasive image-guided surgery is the illumination of target tissues, which conveys a bright, magnified, high-definition image to the surgeon through an attached or incorporated camera system (i.e., endoscope). This view, particularly when using newer platforms with high-definition (4K or 8K) cameras, is startling in its clarity ( Fig. 15.2 ). It eliminates shadows and affords all members of the operating team an identical view of the surgery. An important limitation of endoscopic imaging is that it is generally monocular (compared to the binocular view of open surgery), since traditional scopes have a single lens system. With a monocular scope, the surgeon obtains a two-dimensional view of the body displayed on a video monitor. Other sensory cues must be used as part of the surgeon’s mental model to appreciate the relative positions of the instruments and tissues in a three-dimensional (3D) space. This depth perception is a learned skill, and most surgeons are able to adjust to laparoscopic imaging with adequate training. Newer platforms have recently emerged to provide minimally invasive surgeons with binocular perception to provide a better sense of stereopsis. Evidence suggests 3D camera technology shortens the learning curve during the acquisition of laparoscopic skills for novices and may enhance proficiency in complex tasks such as fine dissection and suturing for even experienced surgeons. , Limitations involve increased cost, the need for the surgeon to wear 3D glasses in order to properly see the image, and reduced visualization when using energy devices due to the snow-like appearance of surgical smoke that can obstruct visualization of the field.
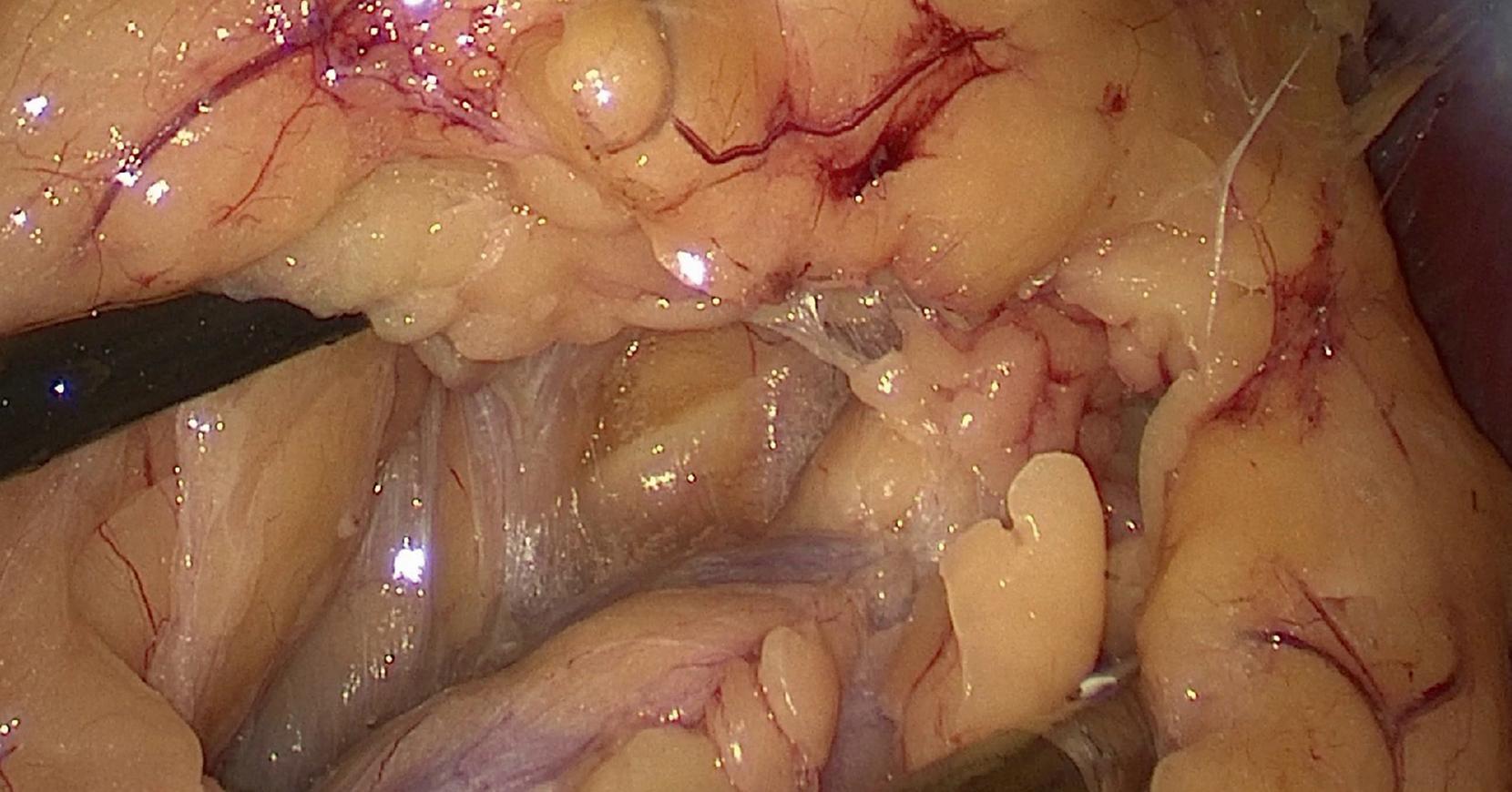
Another drawback of endoscopic surgery is the limited field of view, requiring the scope to be moved dynamically to maintain an ideal image. The closer the scope is to the target, the better the illumination, magnification, and image detail but the more limited the field of view. Constant communication between the surgeon performing the operation and the assistant managing the telescope is essential for safe surgery. New automated navigation systems for both endoscopic and robotic surgery have begun to be developed and could potentially be integrated into the operating theater of the future.
Whereas in open surgery the surgeon can palpate and compress tissues to evaluate tissue and assess pathology, including that which lies beneath the surface, laparoscopic images give the surgeon a view of the surface of tissues without the ability for direct manual assessment. Interposing the laparoscopic instrument between the surgeon’s hands and the target tissue significantly dampens tactile feedback. This significantly limits the acquisition of tactile feedback from the environment that helps surgeons fine-tune their understanding of relevant anatomy and pathology (i.e., their mental model). In order to augment one’s mental model intraoperatively, various methods have been introduced, including the use of augmented reality platforms that can superimpose anatomic reconstructions onto the display monitor (see section below), intraoperative imaging modalities (e.g., laparoscopic ultrasound), or other innovations such as near-infrared fluorescent imaging (e.g., real-time indocyanine green cholangiography). Novel instruments for minimally invasive surgery that incorporate tactile feedback have also recently been developed and have shown to improve kinesthetic perception.
Perhaps some of the greatest advances in minimal-access surgery are in cardiovascular surgery using catheter-based therapies. Vascular surgery has traditionally involved replacing or bypassing occluded or aneurysmal vessels. For several decades, endovascular procedures have revolutionized vascular surgery in much the same way that laparoscopy has impacted abdominal and thoracic surgery. Imaging is provided by fluoroscopy, and contrast solution is injected to outline the vascular anatomy. By accessing the vascular system through puncture or cut-down, instruments can be threaded inside the vessel, stenotic vessels can be dilated with balloons, and intraluminal stents can be threaded into position, guided by real-time fluoroscopic imaging. Large incisions required for access in patients with serious comorbidity can be avoided entirely. Short-term results of endovascular procedures are excellent, recovery is hastened, and the requirements for prolonged hospitalization and intensive care unit care are reduced. , Newer and more sophisticated tools, such as drug-eluting stents, drug-coated balloons, and fenestrated and branched endografts for thoracoabdominal aortic aneurysms, are being used with increasing technical success and long-term patency to treat complex pathologies. In cardiac surgery, similar transcatheter endovascular approaches have been used to treat coronary artery disease, close septal defects, dilate stenotic valves, and even replace cardiac valves. The idea of avoiding the stress and morbidity of a major incision is particularly appealing in these patients with serious underlying disease. Despite this, the effectiveness and durability of these less invasive therapies must be compared to traditional surgical approaches, and selection of appropriate candidates for these operations is paramount.
One of the limitations to image-guided surgery is the constraint in range of motion afforded to the operator to perform very delicate and complex tasks in a confined and restricted space (e.g., hand-sewn anastomosis). Unlike in open surgery, whereby the surgeon has the flexibility to utilize many degrees of freedom via the shoulder, elbow, wrist, and joints of the hand, laparoscopic instruments have a long shaft whose translational motions within an internal cavity are largely limited to a single axis via a trocar through the abdominal wall and to rotational motions along three axes of the instrument about a fulcrum. This has stimulated the development of novel laparoscopic instruments that offer additional degrees of freedom with articulating components within the instrument in order to overcome this limitation. However, similar to other technologies, the psychomotor skills required to operate these instruments effectively are not inherent to a laparoscopic surgeon and require training prior to their utilization in the OR.
Having realized the tremendous benefit of laparoscopy, there has been a consistent desire to further reduce the overall stress of surgery and improve recovery after surgery by diminishing the trauma of access to internal body cavities. This has promoted a trend to using flexible endoscopy and other transluminal approaches to accomplish increasingly complex procedures. Natural orifice transluminal endoscopic surgery (NOTES) is a method whereby access to a body cavity is achieved without any incision in the body wall. This is truly scarless surgery, conducted by accessing the target organ through a natural orifice (such as the mouth, rectum, or vagina). After placing a flexible or rigid endoscope through a natural orifice, an organ (esophagus, stomach, colon, or vagina) is intentionally perforated and the scope is advanced directly to the target tissue. One way this is accomplished is by passing a flexible endoscope through the mouth into the stomach, then through the stomach wall into the abdominal cavity. Other surgical instruments are then advanced through or around the gastroscope, out this opening, and into the abdominal cavity. After the procedure is completed, the resected tissue is retrieved through the mouth and the organ perforation is closed with clips or sutures.
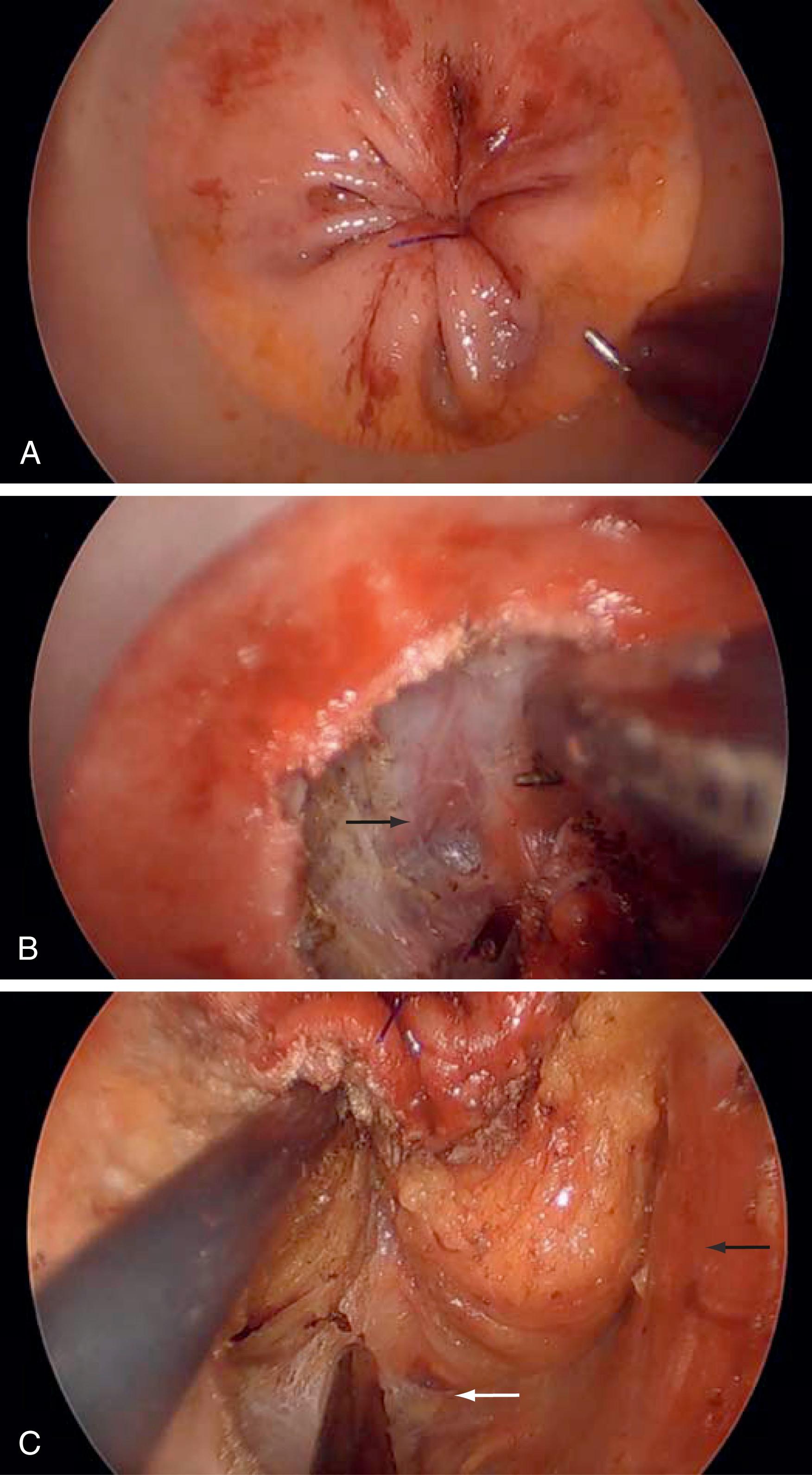
This concept has similarly been adopted for nongastrointestinal operations, such as in transoral endoscopic thyroidectomy vestibular approach, whereby the thyroid gland is excised endoscopically by obtaining access through the mouth. In this technique, standard laparoscopic equipment is used to develop a working space in the neck and perform the dissection. Preliminary case series have demonstrated feasibility and safety with this approach, but, while its cosmetic advantages seem promising compared to a traditional cervical incision, its role currently remains unknown. In colorectal surgery, transanal total mesorectal excision has gained significant popularity to address tumors in the lower third of the rectum, which tend to pose significant technical challenges due to the anatomic constraints of the bony pelvis and thin mesorectum at that level. In this approach, the total mesorectal excision (one of the core surgical principles to optimizing long-term oncologic outcomes for rectal cancer) proceeds transanally (“bottom-up”), enhancing the surgeon’s view of the total mesorectal excision planes compared to conventional transabdominal total mesorectal excision ( Fig. 15.3 ). Despite this, the procedure is technically challenging and is fraught with potential complications (e.g., urethral injury) during the initial learning curve if performed with inadequate training.
As might be imagined, natural orifice surgery is challenging, including the need for very long instruments, which are difficult to maneuver without also moving the field of view from the visualization platform (endoscope). In addition, NOTES requires an iatrogenic perforation to obtain access. Any failure of healing can result in serious consequences, such as peritonitis or dyspareunia.
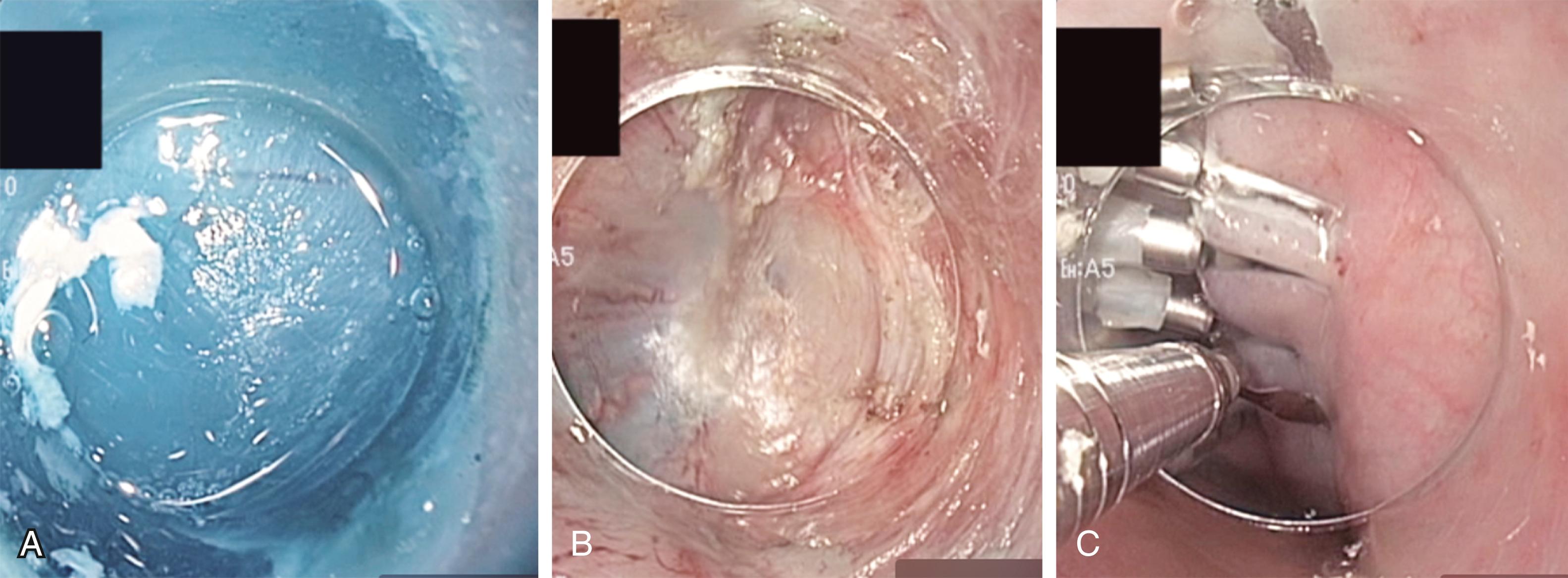
Novel platforms are increasingly pushing the boundaries of what can be treated endoscopically through the upper and lower gastrointestinal (GI) tract. This can be an attractive option, especially if the procedure can be performed with only topical anaesthesia or intravenous sedation. Endoscopes can now be equipped with more advanced functions to perform more complex tasks, such as endoscopic suturing, balloon dilatation, and placement of more intricate stents. , These devices have paved the way for surgeons to perform procedures endoscopically that were traditionally performed using an open or laparoscopic approach. For instance, peroral endoscopic myotomy (POEM procedure; Fig. 15.4 ) has gained popularity over the last decade for the treatment of achalasia. This is a natural orifice technique that involves creation of a long esophageal myotomy using a flexible GI endoscope. After incising the esophageal mucosa, a tunnel is created in the esophageal wall, the circular muscle is divided to a point distal to the lower esophageal sphincter, and the esophageal mucosal opening is closed with clips. While long-term data are still lacking, preliminary data in skilled hands have shown short-term effectiveness with POEM, equivalent to the gold-standard laparoscopic Heller myotomy to control dysphagia, with low morbidity and rapid recovery. However, the procedure does appear to be associated with a higher rate of gastroesophageal reflux compared to a Heller myotomy combined with an antireflux procedure. ,
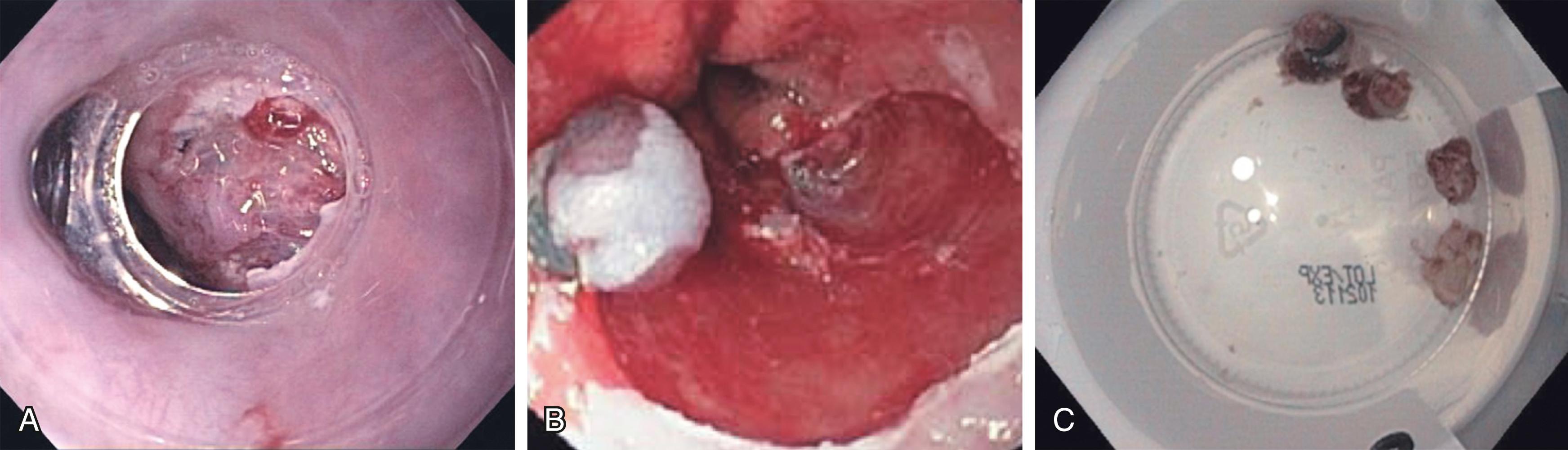
As the scope and breadth of advanced endoscopic procedures continue to gain momentum, this technology has also been used to resect tumors in the esophagus, stomach, colon, and rectum, thereby sparing the patient an anatomic organ resection for early stage cancers in which lymphadenectomy is not required or would not be tolerated due to poor performance status. Techniques currently employed include endoscopic mucosal resection ( Fig. 15.5 ) and endoscopic submucosal dissection ( Fig. 15.6 ). While these techniques are not necessarily new, there is a constant evolution of highly specialized equipment required to perform them, including endoscopes with enhanced range of motion at the tip and additional working and water irrigation channels, as well as specialized endoscopic dissection and hemostasis tools.
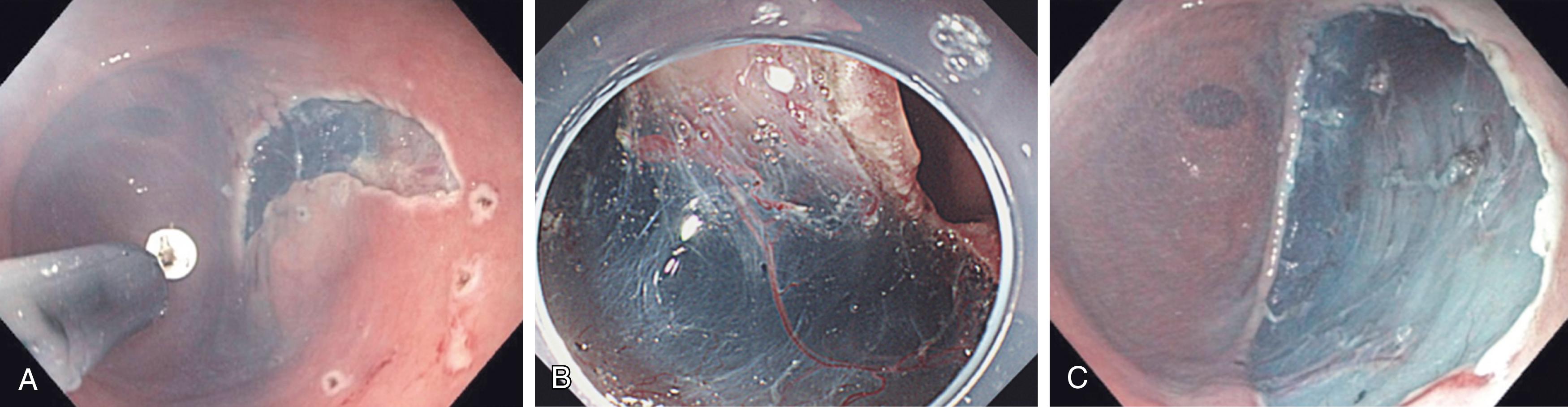
Recent advances have also combined the outstanding imaging capability of the flexible endoscope with an ultrasound transducer at the distal end. Applications in the GI tract (endoscopic ultrasound [EUS]) and bronchial tree (endobronchial ultrasound) extend the capability of the endoscope to visualize the complete thickness of the wall of the organ (for staging of tumors), adjacent lymph nodes that can be biopsied, and adjacent structures (e.g., evaluation of the common bile duct or pancreas through the duodenum or stomach during EUS). Surgical procedures can now be performed using EUS guidance, such as drainage of pancreatic pseudocysts, debridement of walled-off pancreatic necrosis into the stomach, and gastrojejunal stenting to palliate obstructing pancreatic tumors. While technically challenging, the endoscopic approach in these cases is associated with significantly lower morbidity and mortality compared to an open approach.
Robotic surgical systems were designed to overcome some of the limitations of endoscopic surgery by using enabling characteristics of robots to improve the capabilities of the surgeon compared to working freehand. , Unlike the use of robotics in industry, surgical robots rarely work autonomously but rather act as an interface between the operating surgeon and the patient. In this master-slave relationship, the master (surgeon) sits at a console in an ergonomically sound position and uses movements of both hands and feet to control movements of the laparoscope and wristed instruments inside the patient attached to the robot arms (slave) ( Fig. 15.7 ). The most widely used robotic system in North America uses a proprietary laparoscope with two optical systems providing binocular (3D) vision and additional depth perception. The surgical instruments have additional articulating components near their distal tips (i.e., “wristed”) so that the movements of the surgeon’s hands can be reproduced by the instruments without the usual limitations of the fulcrum effect seen with traditional laparoscopic instruments. This enables surgeons to perform finer motor movements within a small, confined, and deep space. For a demonstration of robotic-assisted dissection, see Video 1.
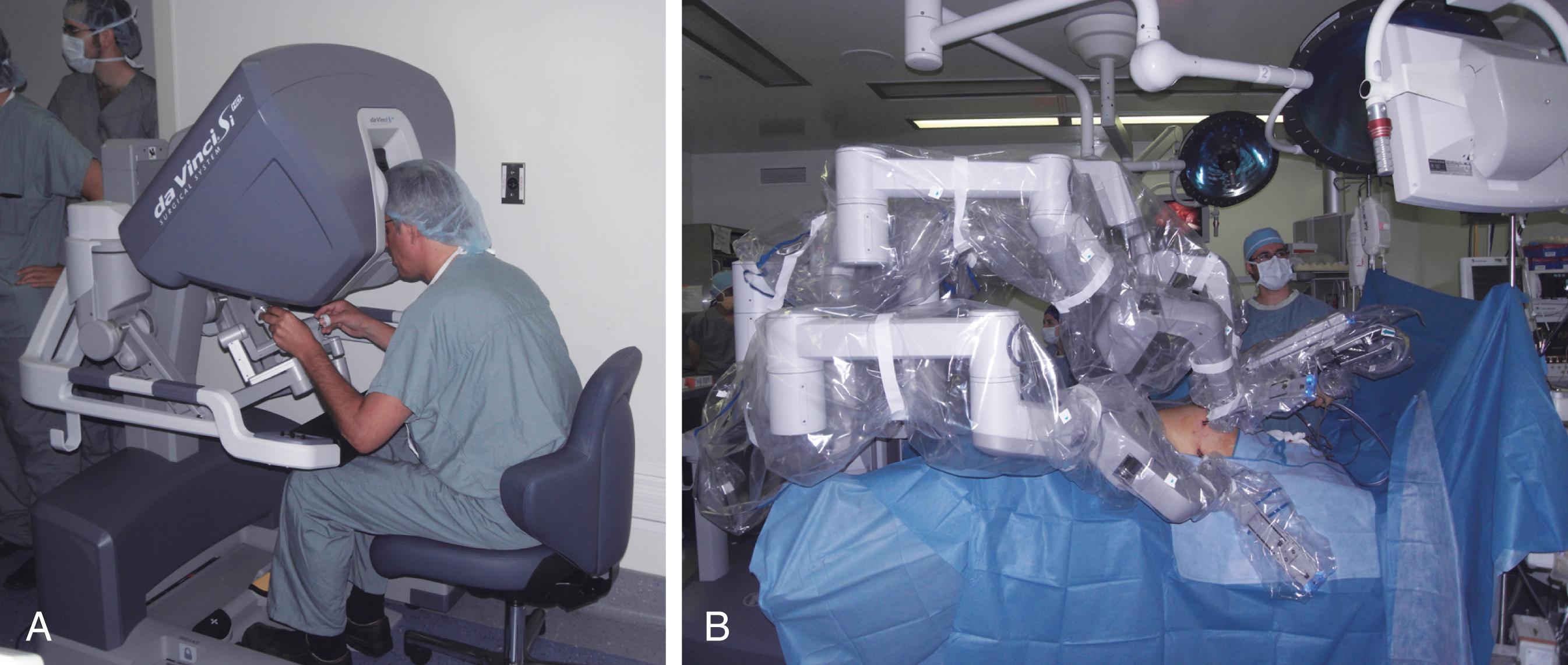
Since there is no direct contact between the surgeon at the console and the instruments, the surgeon can also work from a remote location or even long distances, paving the way for telesurgery. Robotic surgical platforms are especially appealing for providing care to patients in hostile environments, such as those in the military, on outer space missions, on deep-sea explorations, and on polar expeditions. Despite the theoretical advantages of providing surgical care to remote regions, the costs and resources required to deploy such a system have limited its practicality and implementation. For instance, trained personnel would still be required on site to prepare the patient, insert the ports, dock the robot, change instruments, and intervene to treat complications or unexpected findings that cannot be controlled robotically. Also, issues relating to licensing and liability have yet to be addressed, and latent delays between motions performed by the surgeon and the movement of the instrument on site must be resolved. The longer the distance that the data needs to be transmitted from the console to the patient, the greater the latent delay, as even 250 millisecond can have significant impact on the quality of the operation. It is important to highlight that a negative feature of this interface is that the surgeon has no tactile sense of the tissues and, as a result, must adapt by using only visual data. However, integrated tactile feedback into the next generation of minimally invasive robotic systems has thus far shown to improve tissue handling and will undoubtedly help address some of the platform’s limitations in the future.
Robotic surgery provides other exciting opportunities to enhance surgical performance. Since there is an interface between the surgeon and the effector instruments, it is possible to modulate the relationship between the surgeon’s movement and that of the instrument using complex computer algorithms. The platform can adjust the gain or the scale of movement. In this way, the surgeon may make larger movements to affect very fine movements of the instrument tip. This can be very helpful for procedures that require precise movements, such as microvascular anastomosis. Algorithms can also be incorporated to dampen tremor using embedded filters. Over the last few years, robotic surgery has been carried out in conjunction with robotic-assisted anesthesia. This is an automated platform in which anesthesia agents are controlled using computer-assisted devices that calculate moment-to-moment anesthesia doses in a closed-loop system to provide optimal dosing—as either a completely automated or a semiautomated system.
Currently, minimally invasive robotic systems are widely used in urologic surgery and gynecology and to a lesser extent in cardiac surgery, general surgery, and endocrine surgery. , , The main drawbacks are costs, bulkiness, and set-up time for the equipment and absence of compelling data to show superiority of robotic operations over those done by well-trained laparoscopic surgeons. Nevertheless, several new robotic surgery platforms have recently entered the market and will undoubtedly stimulate competition and innovation to overcome these limitations and expand the role of robotics in surgical care. Indeed, one of the greatest influences of robotics may be in flexible endoscopic procedures. The single-operator nature of contemporary endoscopes strongly limits the ability to expose tissues and perform advanced motor movements (e.g., traction/countertraction, dissection, suturing). As a result, robotic endoscopes have been equipped with advanced navigation systems and instruments that can be maneuvered and articulated with more flexibility, making it possible to execute complex tasks. Various platforms have recently become commercially available, and preliminary data demonstrating their effectiveness, safety, and feasibility will begin to surface in the near future.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here