Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver’s essential structure is one of layers of metabolically active hepatocytes arrayed along and around a vascular network carrying nutrient-rich blood derived from the products of intestinal absorption, otherwise semi-isolated from the systemic circulation. The biliary ducts are interleaved and intimate within this system and provide an excretory apparatus linking back with the intestinal tract.
The pancreas, by contrast, has two completely independent functional units. One is a relatively simple exocrine repository of initially inactive digestive enzymes; the other is a complex array of endocrine cells devoted to various homeostatic processes and feedback loops.
The first period of development is considered to extend from fertilization to form a single-celled zygote, or ovum, in the Fallopian tube to the implantation of the multicellular blastocyst into the wall of the uterus and lasts about seven days. Gastrulation in week three generates the three embryonic tissue layers: the ectoderm, mesoderm, and endoderm, which will contribute to various cell types within different organs. The first eight weeks post-fertilization are conventionally described as the embryonic period . They involve the formation of the major organs of the body and are followed by the fetal period , which involves further growth and maturation, extending to the time of birth and delivery.
Both the liver and pancreas start to develop during the embryonic period. Despite their very different functions and structures, the liver and pancreas initially arise from the same population of bipotent endodermal cells (Sox17, FoxA, Hhex, Gata-expressing), which can form either the liver or pancreas, depending on the growth factor signals from surrounding tissues. This region of endoderm also gives rise to the gallbladder and extrahepatic biliary system. Specification into the two different organs is dependent on the differential expression of the transcription factors PDX1 and PTF1A for the pancreas and HHEX, FOXA1/2, GATA4, HNF1β, and HNF4α for the liver. GATA4 and FOXA1/2 are known as “pioneer” transcription factors that are pre-assembled at liver-specific genes such as albumin, allowing a rapid induction of liver fate.
Gestational age is different from post-fertilization because it is measured from the last menstrual period, which effectively adds two weeks to the timeline described. Actually, this is a term seldom used by embryologists, but becomes more valid clinically by the end of the whole process.
Much of the work detailing the anatomy and timing of embryologic events was performed by Franklin Mall and later George Streeter in the Carnegie Institution in Baltimore, MD during the first part of the 20th century. From this ensued the widely-used classification of Carnegie Staging (stages 1–23).
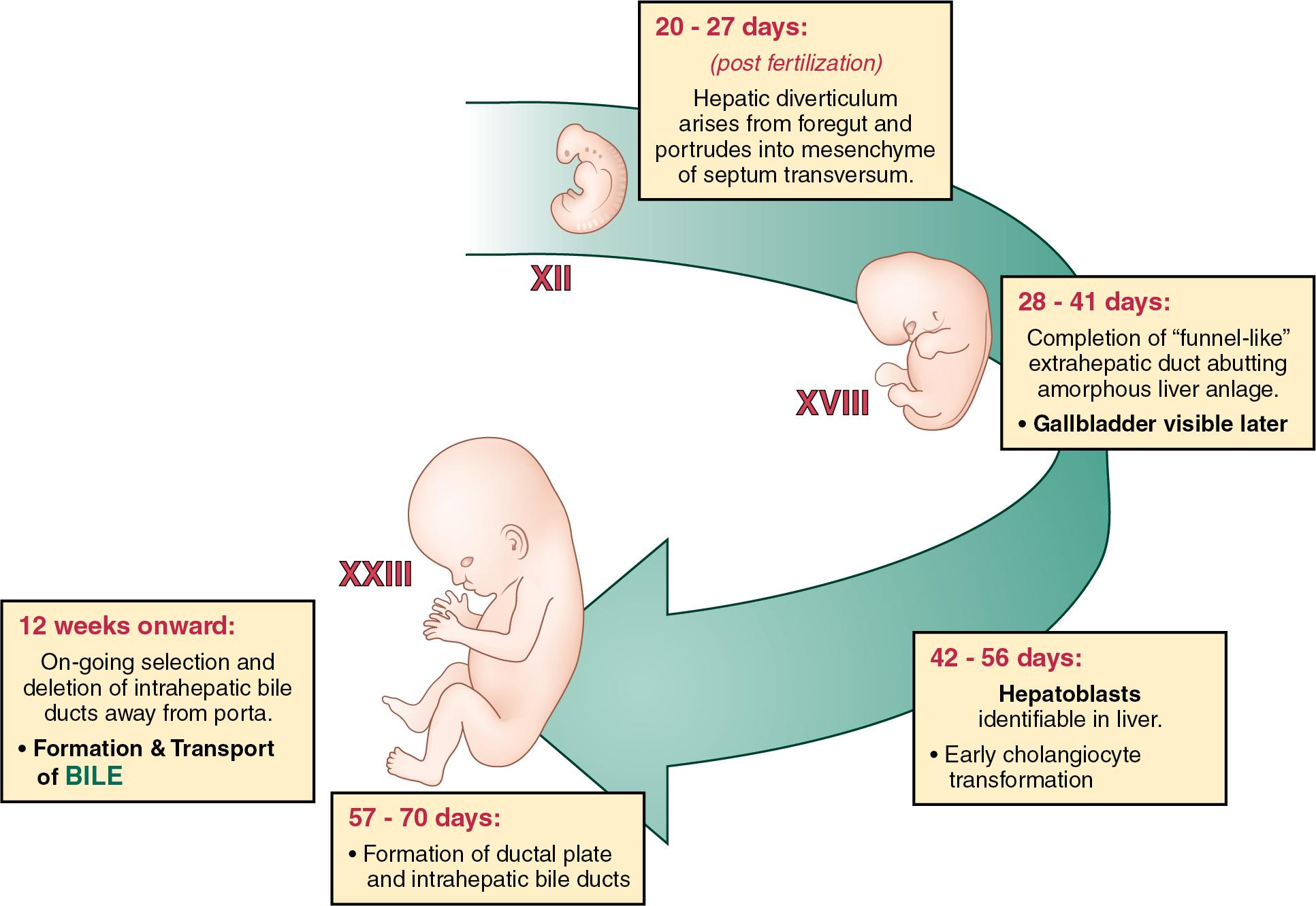
Development of the liver can be divided into several phases ( Fig. 1.1 ). During the early embryonic phase, there is induction of the liver diverticulum from the endoderm and growth with the formation of hepatoblasts to form the liver bud. The late embryonic period is defined by the onset of generation of hepatocytes, which will form the bulk of the liver, and of cholangiocytes, which will form the bile ducts. These two cell types arise from the bipotential hepatoblast that expresses markers of both hepatocytes and cholangiocytes. The fetal period is characterized by further differentiation of hepatocytes and cholangiocytes, which become organized into liver lobules and bile ducts, respectively. During this phase, there is maturation of the hepatocytes, together with further expansion of the liver and bile ducts at the periphery of the liver.
This chapter provides an overview of key events and the growth factor signals and transcriptional factors required for liver development. More detailed information can be found in Gordillo et al., Ober and Lemaigre, and Peruggoria et al.
At the start of this period, the embryo measures about two to three mm, has implanted into the endometrial layer of the uterine wall, and externally is characterized by the formation of somites. The primitive endodermal intestinal tube has formed but is blind, occluded at either end by the buccopharyngeal and cloacal membranes and conventionally divided according to its three supplying arteries as foregut, midgut, and hindgut.
The liver and biliary tract arises initially as an endodermal bud from the distal foregut within the ventral mesogastrium, projecting into the mesenchyme of the septum transversum . This endodermal bud is formed from two endodermal origins: two lateral domains and the ventral midline of endoderm lip (VMEL), where the endoderm folds into the anterior intestinal portal. The lateral and VMEL endodermal domains will generate the posterior and anterior parts of the liver, respectively. The liver primordia develops into a funnel-shaped structure with a lumen evident throughout, and from about 45 days the thicker-walled gallbladder also becomes evident.
Induction of the liver diverticulum is controlled by growth factors: bone morphogenetic proteins (BMPs) from the septum transversum and lateral plate mesoderm, together with fibroblast growth factors (FGFs) from precardiac mesoderm and signals from the surrounding endothelial cells ( Table 1.1 ). Specifically, BMPs specify liver fate in the VMEL precursors, whereas FGFs induce liver formation in the lateral endodermal hepatic precursors. These growth factors induce or maintain the expression of the transcription factors FOXA1&2, HHEX, HNF1β, HNF4α, and GATA4&6, which specify the liver primordium and are needed for hepatoblast differentiation. Repression of the β-catenin (canonical Wnt) and Notch signaling pathways are also required for liver induction.
| DERIVATION | POSSIBLE FUNCTION | CHROMOSOME | |
|---|---|---|---|
| BMP | Bone morphogenic protein | Growth factor family; multifunctional role. |
|
| FGF | Fibroblast growth factor | Growth factor family; multifunctional role. |
|
| VEGF | Vascular endothelial growth factor | The protein acts on endothelial cells, increasing permeability and inducing angiogenesis. | Ch6 |
| PDX1 | Pancreas/duodenum homeobox protein 1 | Transcription factor; pancreas development | Ch13 |
| HES-1 | Hairy and enhancer of split 1 ( Drosophila ) | Transcription factor family; target of Notch signaling | Ch3 |
| PROX-1 | Prospero homeobox 1 | Homeobox transcription factor; needed for hepatocyte formation. | Ch1 |
|
Hepatocyte nuclear factor | Transcription factor; needed for cholangiocyte formation. | Ch15 |
| NOT CH 1-4 | Mutation produced irregular (“notches”) in wing tips of Drosophila . | Receptors for signaling network, which regulates interactions between physically adjacent cells. | Ch9 |
| NEUROG3 | Neurogenin 3 | Basic Helix-loop-helix transcription factor; essential for endocrine lineage | Ch10 |
| SOX-9 | SRY (sex determining region Y)-box 9 | Forms DNA-binding proteins; needed for cholangiocyte differentiation. | Ch17 |
| WNT | Wg & Int standing for wingless-related integration ( Drosophila ) |
|
(Depends on member) |
| SHH | Sonic hedgehog | Early embryonic patterning, cell proliferation, and survival | Ch7 |
| LGR-4 | Leucine-rich repeat containing G protein-coupled receptor. | Gallbladder maturation | Ch11 |
| PAX-6 | Paired box - 6 | Transcription factor; for endocrine cell lineage | Ch11 |
| (C/EBP) | CCAAT/enhancer binding protein | Transcription factor; needed for hepatocyte formation. | N/A |
| PTF1A | Pancreas-specific transcription factor 1A | Transcription factor; pancreas development | Ch10 |
From the cranial aspect of the endodermal diverticulum emerge the primordial liver cells, known as the hepatoblasts . Hepatoblasts are the precursors of both hepatocytes and cholangiocytes and express markers of both lineages: hepatocytes (e.g., α-fetoprotein, albumin, HNF4α, HHEX) and cholangiocytes (HNF1β and CK19, a late differentiation marker). Initially hepatoblasts are cuboidal cells lining the invaginating diverticulum. Proliferation results in a “multilayered” pseudostratified endodermal structure and requires HHEX. Subsequently, the hepatoblasts reduce their cell–cell contacts, delaminate, and migrate into the septum transversum to become arranged in plates, initially three to four cells thick, which line the vascular sinusoids. This delamination phase requires the transcription factors PROX1, HNF6 (ONECUT1), and ONECUT2. The ingrowing columns of cells, known here as “cords” (sometimes “chords”), also have an intimate relationship with the mesenchymal-derived endothelial cells lining primitive vascular sinusoids. Proliferation and cell survival are controlled by paracrine Wnt, FGF, and hepatocyte growth factor (HGF) signaling from adjacent mesodermal cells, including from the closely opposed liver sinusoidal endothelial cells.
The embryonic liver is populated not only by the hepatoblasts but also by hematopoietic cells, originally derived from the yolk sac and then from the aorta-gonad-mesenchyme region and placenta. Hematopoietic cells will form the bulk of the liver, and hematopoietic maturation becomes the dominant feature during the second trimester. Hematopoietic cells produce Oncostatin M (OSM), which also increases hepatoblast proliferation, contributing to liver growth until late fetal development.
The liver is by now a large, rounded mass of tissue called the liver anlage (German for “rudimentary organ or part”) and the dominant organ by mass within the abdomen. Later, it will push the embryonic gut into the base of the umbilicus before it returns, and complete closure of the anterior abdominal wall occurs at around 12 weeks gestation. Failure of this return phase leads to an omphalocele (or exomphalos), of which the liver can form a major part.
Hepatoblasts are the parental cells for two key cellular progeny, hepatocytes and cholangiocytes , and differentiation occurs later at or around the seventh week (see Fig. 1.1 ). This common cellular origin has implications for malignant disease in adults. Notch signaling from the portal vein mesenchyme promotes cholangiocyte formation, and hence ductal development, whilst inhibiting hepatocyte development. Other growth factors needed for cholangiocyte cell fate include transforming growth factor (TGF)-β, BMPs, and FGFs. Cholangiocyte differentiation requires the HNF1β, HNF6, and SOX9 transcription factors. In contrast, hepatocyte induction requires FGF, HGF, and OSM signaling from the surrounding mesodermal cells, including the hematopoietic precursors and mesothelial cells that surround and line the liver lobes. Hepatocyte fate requires the transcription factors HNF4α and C/EBPα. The transcription factor PROX1 (and TBX3) determines which cell fate develops from the bipotential hepatoblasts; in the absence of PROX1, fewer hepatocytes are formed, which is associated with an increase in cholangiocyte number.
Intrahepatic bile ducts only appear distinctly from about seven weeks gestation, and formation is organized by the portal vein. , At seven weeks, the portal venous network is infiltrating the liver anlage and becomes surrounded by a layer of mesenchyme. From this a cylindrical double cell layer of darkly staining cells adjacent to the portal vein emerges, which is termed the ductal (or limiting) plate . The bile ducts are generated from the dual-layer by a unique process of so-called transient asymmetry , whereby ductal plate cells resembling cholangiocytes (expressing SOX9 and CK19) on the side facing the portal tract are matched by ductal plate cells resembling hepatoblasts (expressing HNF4α) on the side facing the parenchyma. After the formation of a lumen, the nascent bile duct becomes symmetrical, as hepatoblasts are replaced by cholangiocytes to form a double cell layer composed entirely of cholangiocytes, which will remodel to form a single-cell layer bile duct. Bile duct development is discontinuous along the portal vein, and selective remodeling of this layer generates an interconnected, single cell–lined network of small bile ducts within the mesenchymal architecture. Ductal progression and elongation proceeds from the hilum to the periphery and appears to be controlled by the noncanonical Wnt pathway. Cholangiocytes that are not incorporated into the bile ducts dedifferentiate into hepatocytes. At some point, extra- and intrahepatic systems coalesce at the interface of the porta hepatis, although the process is again imperfectly understood. , Hepatic arteries develop in association with the developing bile ducts and are thought to be induced by VEGF signals from the ductal plate.
The onset of hepatocyte polarity and generation of the polygonal shape characterizes the latter stages of this phase with structurally distinct apical, lateral, and basolateral domains. The basolateral surface abuts the blood-containing sinusoids, but is separated from them by the cell-free space of Disse. The apical aspect abuts onto adjacent hepatocytes, within which is the biliary canaliculus, the smallest component of the biliary network. These are delineated by tight junctions (desmosomes), with the remainder of this apical surface given over to gap junctions, which can be conduits between cells. The surface area of both the canaliculus and basolateral surfaces are multiplied by the presence of microvilli. This implies that the canaliculus is of hepatocyte origin, with later connections to the intrahepatic bile ducts emanating from the center. Nevertheless, the hepatocyte differentiation phase is most pronounced during the fetal stages and is linked to the reducing hematopoietic cell number as hematopoiesis shifts to the bone marrow.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here