Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Massive hemoptysis, defined as expectoration of 300 mL of blood or more within a 24-hour period, is a life-threatening condition. The most common mechanism of death from massive hemoptysis is asphyxiation, not exsanguination. Asphyxiation is caused by a reduction of respiratory surface area for oxygen exchange. Over the years, emergency management of massive hemoptysis has changed. Medical treatment consisting of blood replacement, emergency bronchoscopy, and insertion of a protective cuffed endotracheal tube in the noninvolved main-stem bronchus is still appropriate. However, in cases of massive hemoptysis, medical therapy alone has an approximate mortality rate of 50%. Emergency surgery with lung resection and various types of endobronchial laser coagulation has been used successfully with a mortality rate of approximately 17%. However, a high percentage of patients with massive hemoptysis have advanced bilateral pulmonary disease and/or severely reduced pulmonary function that render them poor candidates for emergency surgery.
The first reports of the use of bronchial artery embolization for the treatment of hemoptysis appeared in the mid-1970s. It is important to remember that in approximately 95% of cases the hemoptysis originates from systemic arteries, with the bronchial artery almost always involved ( Figs. 53.1 and 53.2 ). However, in addition to the bronchial artery, other systemic arteries such as branches of the subclavian and axillary arteries, internal mammary, intercostal, and the phrenic arteries can contribute blood supply to pathologic pulmonary tissue through the pleura (and diaphragm in the case of the phrenic artery) in up to 45% of patients with hemoptysis. ( Figs. 53.3 and 53.4 ) Pulmonary artery blood supply is responsible for hemoptysis in only 5% of cases ( Table 53.1 ).
| HEMOPTYSIS: Conditions with Systemic Artery Supply (95%) | HEMOPTYSIS: Conditions with Pulmonary Artery Supply (5%) |
| Cystic fibrosis Bronchial carcinoma Tuberculosis Postirradiation Aspergillosis Congenital heart disease Bronchiectasis Pulmonary embolus |
Swan-Ganz catheters |
The pathophysiology of massive hemoptysis is as follows:
Chronic inflammation or tumor → bronchial artery hypertrophy → small bronchial artery to pulmonary artery communications → high systemic (bronchial) artery perfusion pressure on small pulmonary arteries/arterioles → rupture into adjacent bronchi → massive hemoptysis.
Indications for embolotherapy are both massive hemoptysis and recurrent hemoptysis. Before initiation of embolotherapy for hemoptysis, most patients have undergone chest radiography and possibly bronchoscopy. Often a patient with hemoptysis can discern from which side the bleeding originates based on symptoms. A contrast-enhanced chest computed tomography (CT) scan can reveal enlarged bronchial and/or other systemic arteries that supply abnormal pulmonary parenchyma or pulmonary artery abnormalities ( Fig. 53.5 ). The preprocedure CT findings often significantly help to tailor the angiogram and embolization.
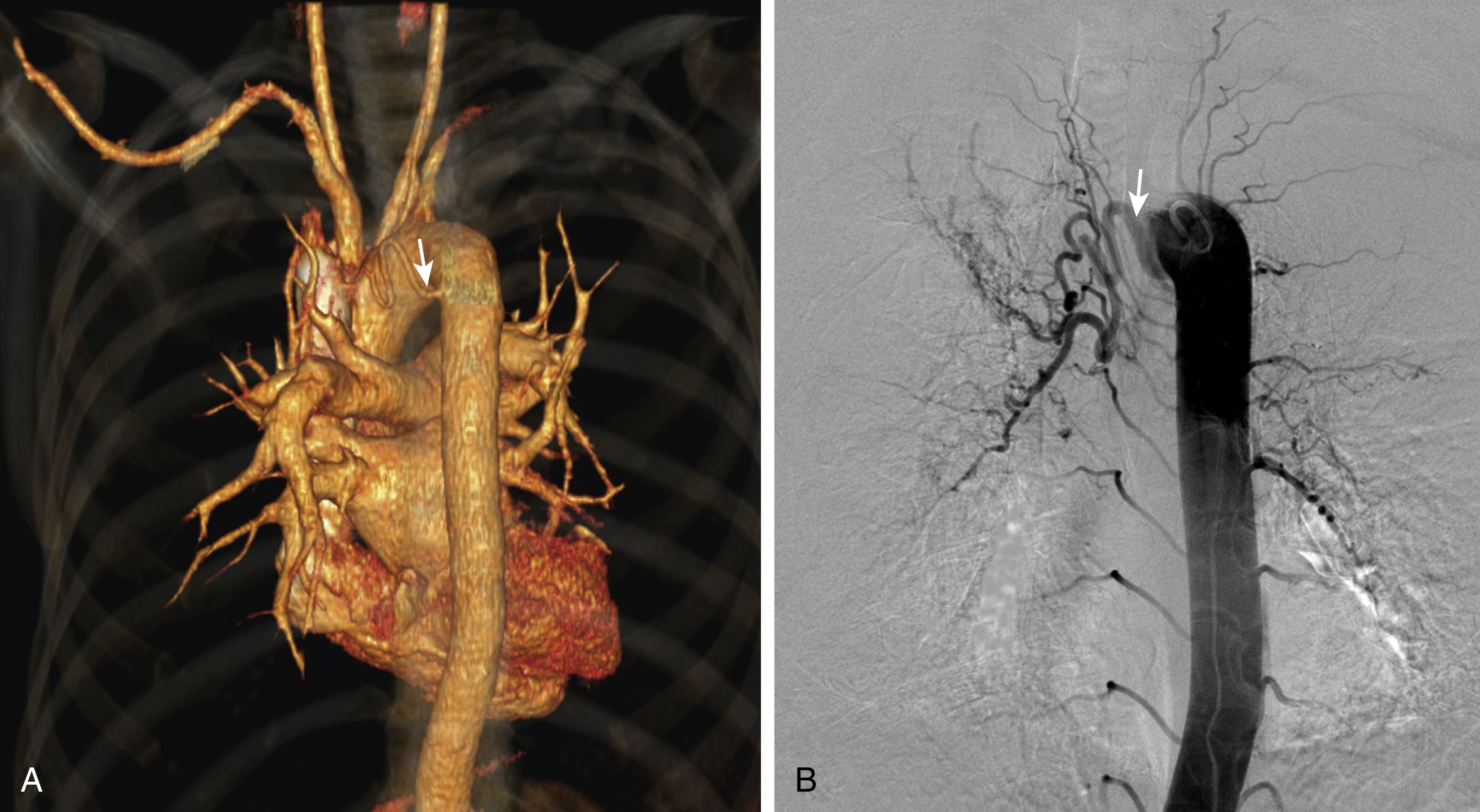
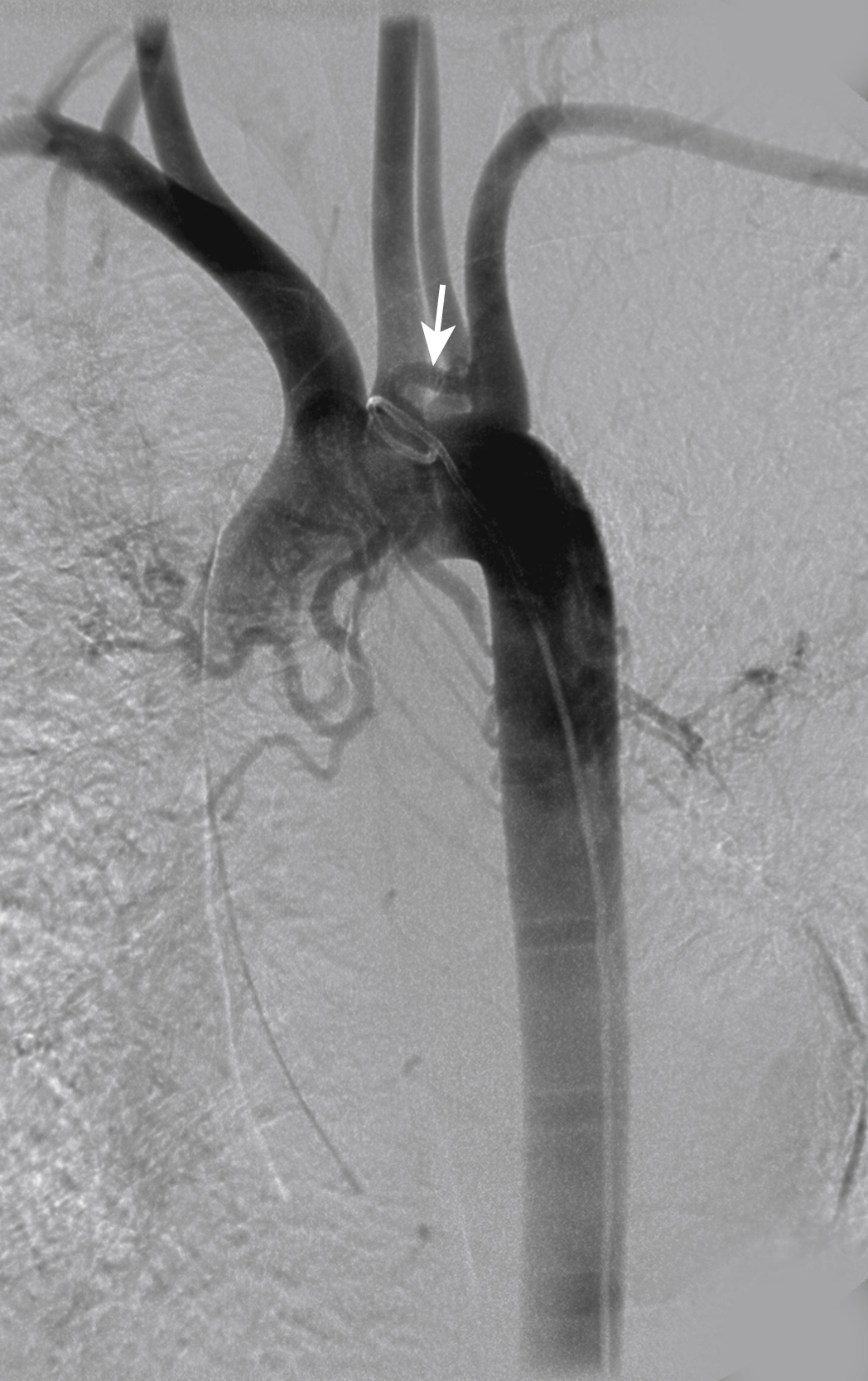
Table 53.2 describes the most commonly found variations of the bronchial arteries. The bronchial arteries usually originate from the anterolateral surface of the descending aorta at the T5, T6 level, often in the area of the left main-stem bronchus. This is a good place to start searching for their origin. However, one must be aware that bronchial arteries can originate from multiple, diverse sites, including the internal mammary, thyrocervical, innominate, and subclavian arteries ( Fig. 53.6 ) and also directly from the aortic arch. If after several minutes searching the usual site of origin of the bronchial arteries is not successful, an arch aortogram may be helpful in localizing it ( Fig. 53.7 )
| Right Single 60% Common trunk with intercostal 90% Supply to spinal cord 5-10% Left Double 60% Single 30% Common Trunk: 25% |
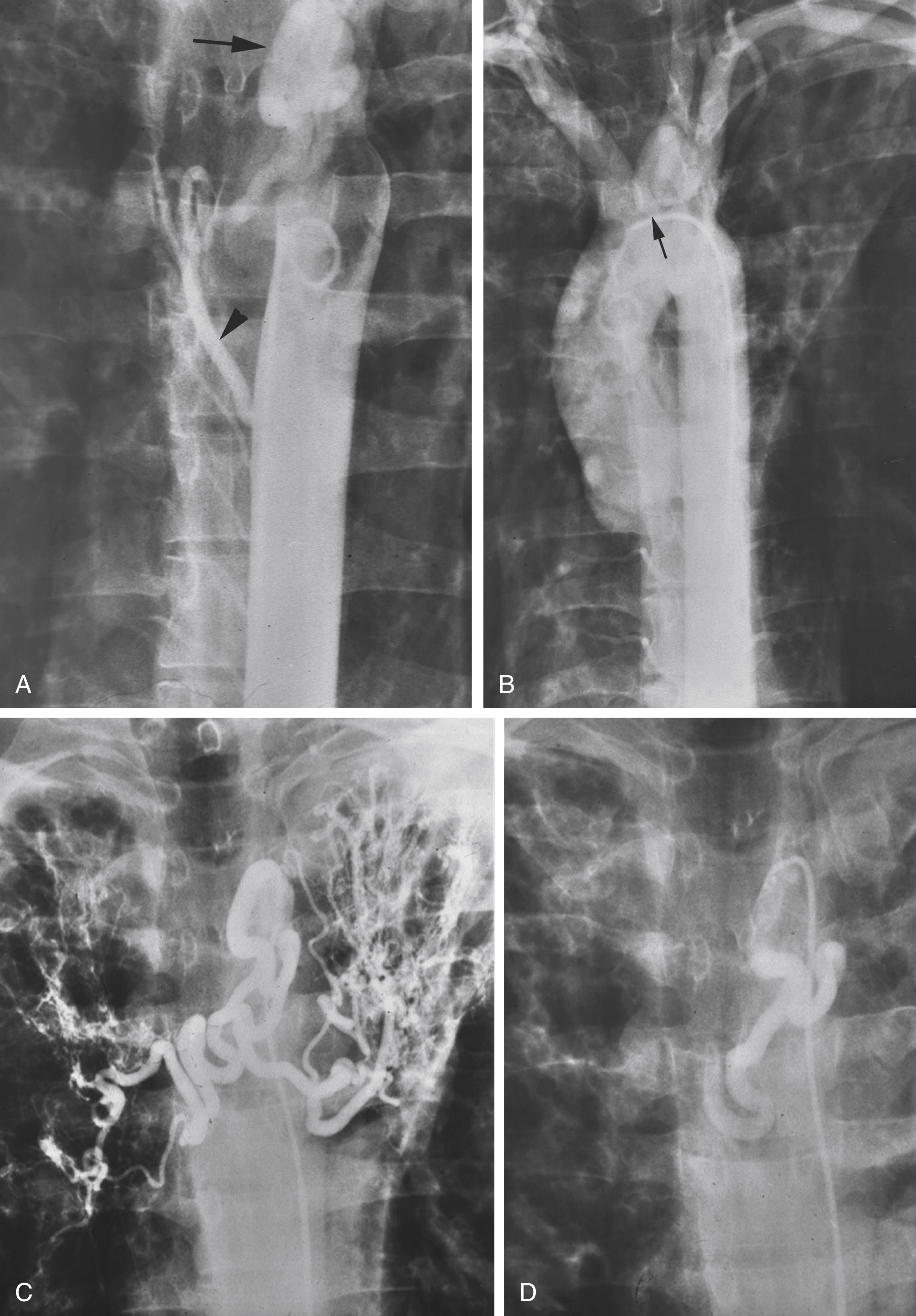
In our practice we usually start the angiographic procedure with a descending aortogram using a pigtail catheter that is placed in the distal aortic arch. This allows visualization of any enlarged bronchial, intercostal, or phrenic arteries. If no abnormalities are seen on the descending aortogram, the next step is a selective subclavian arteriogram on the side suspected to be responsible for the bleeding, especially if the abnormal pulmonary parenchymal findings are present in the lung apex on preprocedural chest radiograph or CT. The subclavian arteriogram demonstrates the subclavian and axillary branches and also the internal mammary and thyrocervical trunk branches that can be the source of systemic contribution to hemoptysis. Pulmonary parenchymal abnormalities at the lung bases are often supplied by the phrenic artery, and posterior parenchymal abnormalities may have intercostal artery supply. Finally, if after a careful search, no enlarged abnormal bronchial artery or other systemic arterial supply is found, a pulmonary arteriogram is indicated.
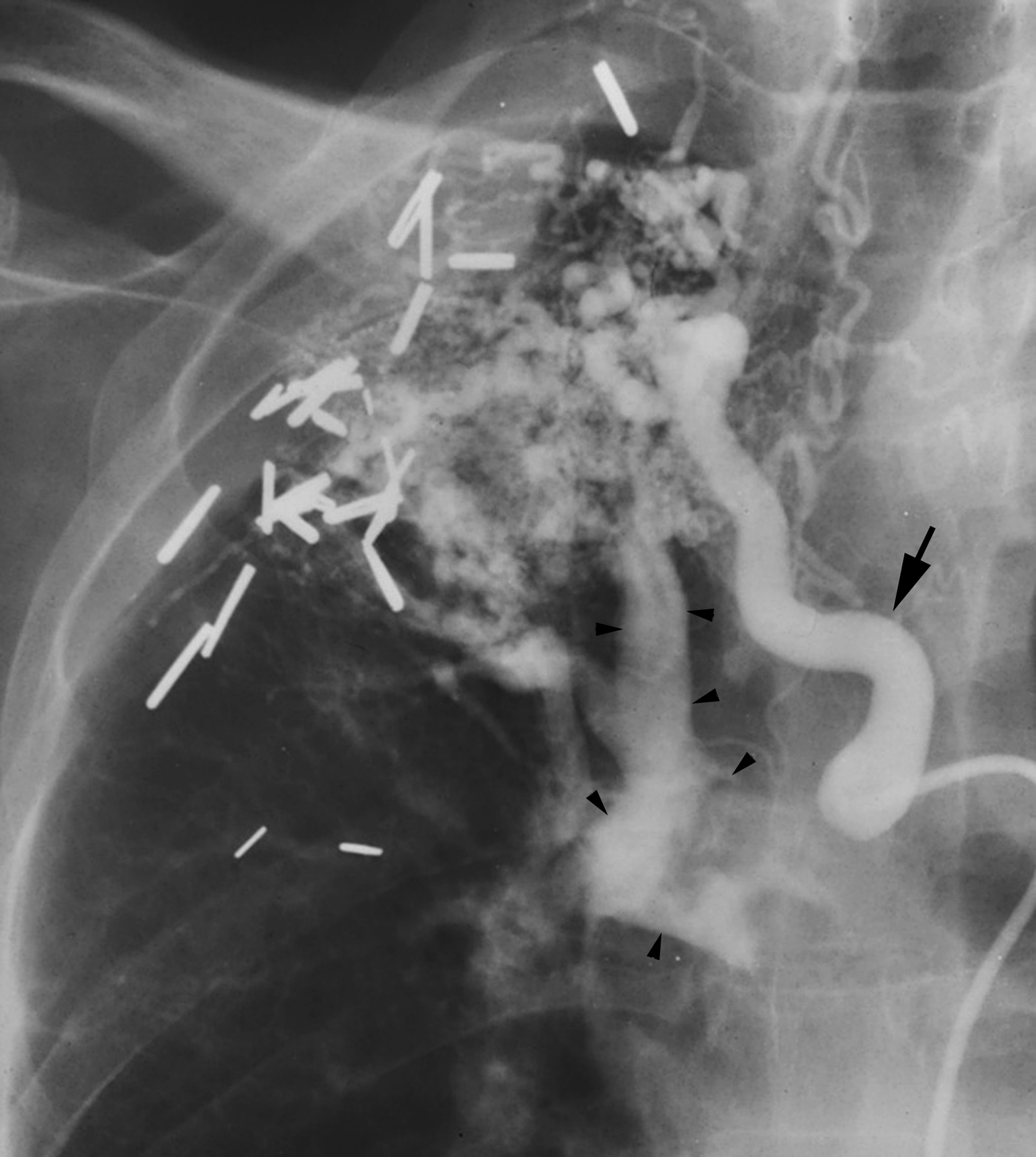
When the bronchial artery is catheterized ( Table 53.3 ), careful bronchial arteriography is performed. One must be sure to extend the imaging field far enough medially to include the entire width of the spine so that any contribution to the anterior spinal artery, if present, is not coned off the image. In addition, it is important to search for any other potentially “dangerous” anastomoses ( Fig. 53.8 ) arising from the bronchial artery where, if unnoticed, embolization could result in catastrophic complications. In most cases, a microcatheter will be coaxially inserted into the base selective catheter and advanced distally to a suitable location for the embolization.
| Headhunter1 (Merit Medical OEM, Salt Lake City, UT) Simmons 1 (Merit OEM) Rösch celiac 1or 2 (William Cook, Bloomington, IN) Mikaelsson (Merit OEM) Rösch left gastric Renal double curve (Merit OEM) |
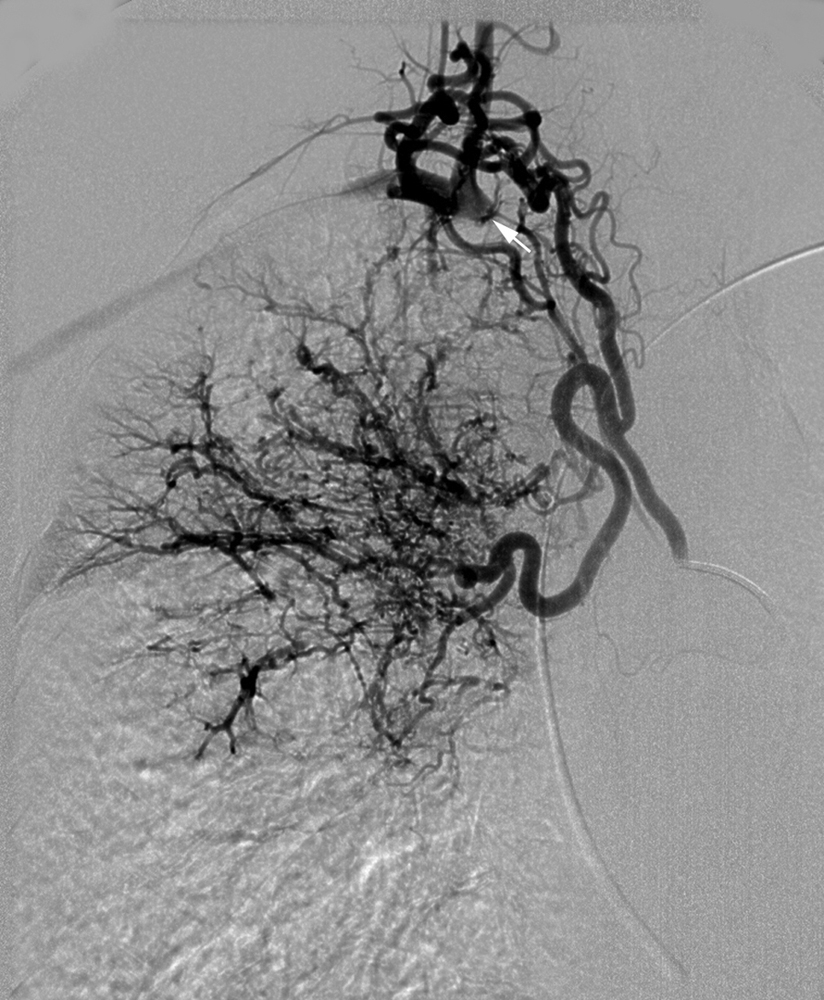
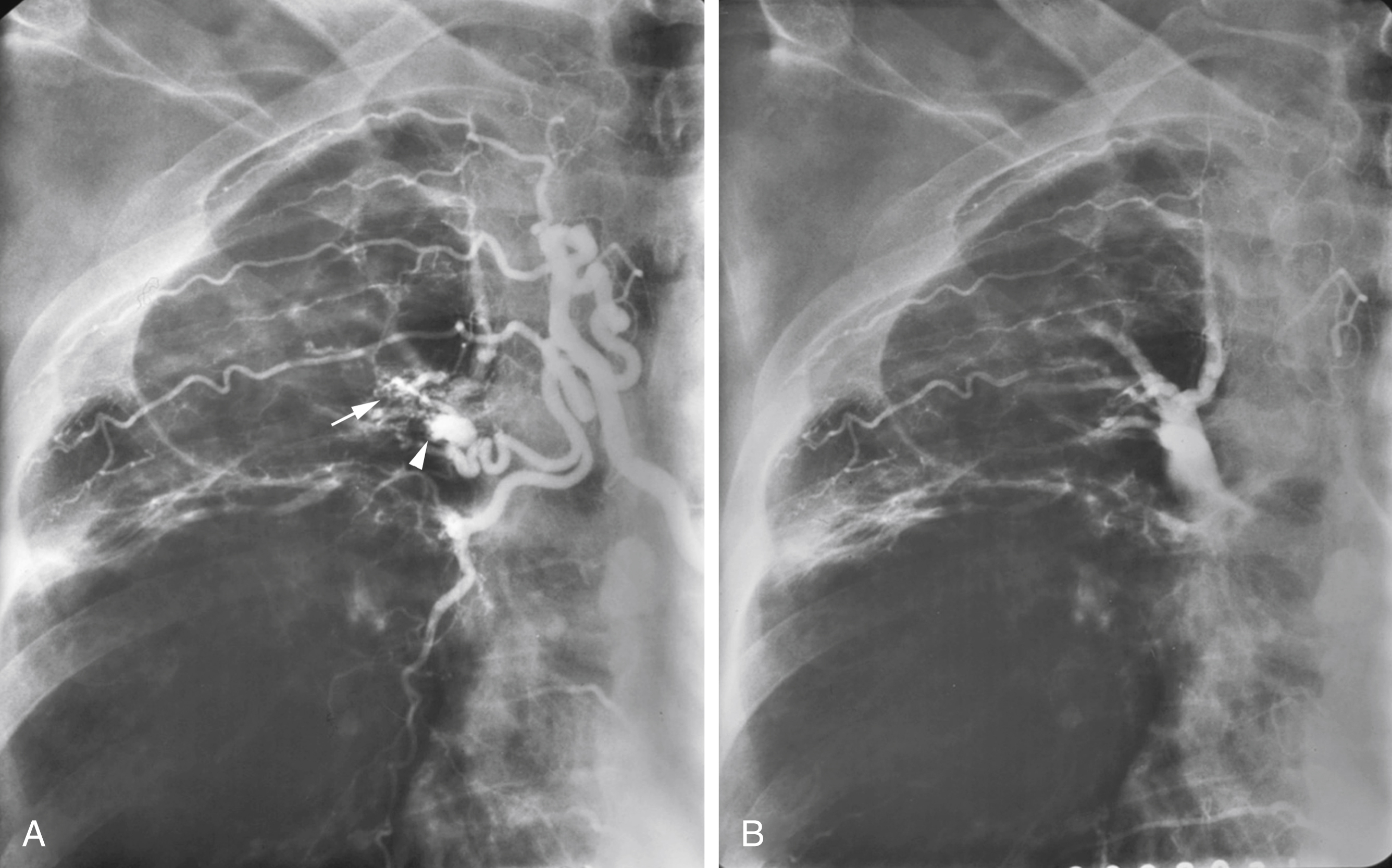
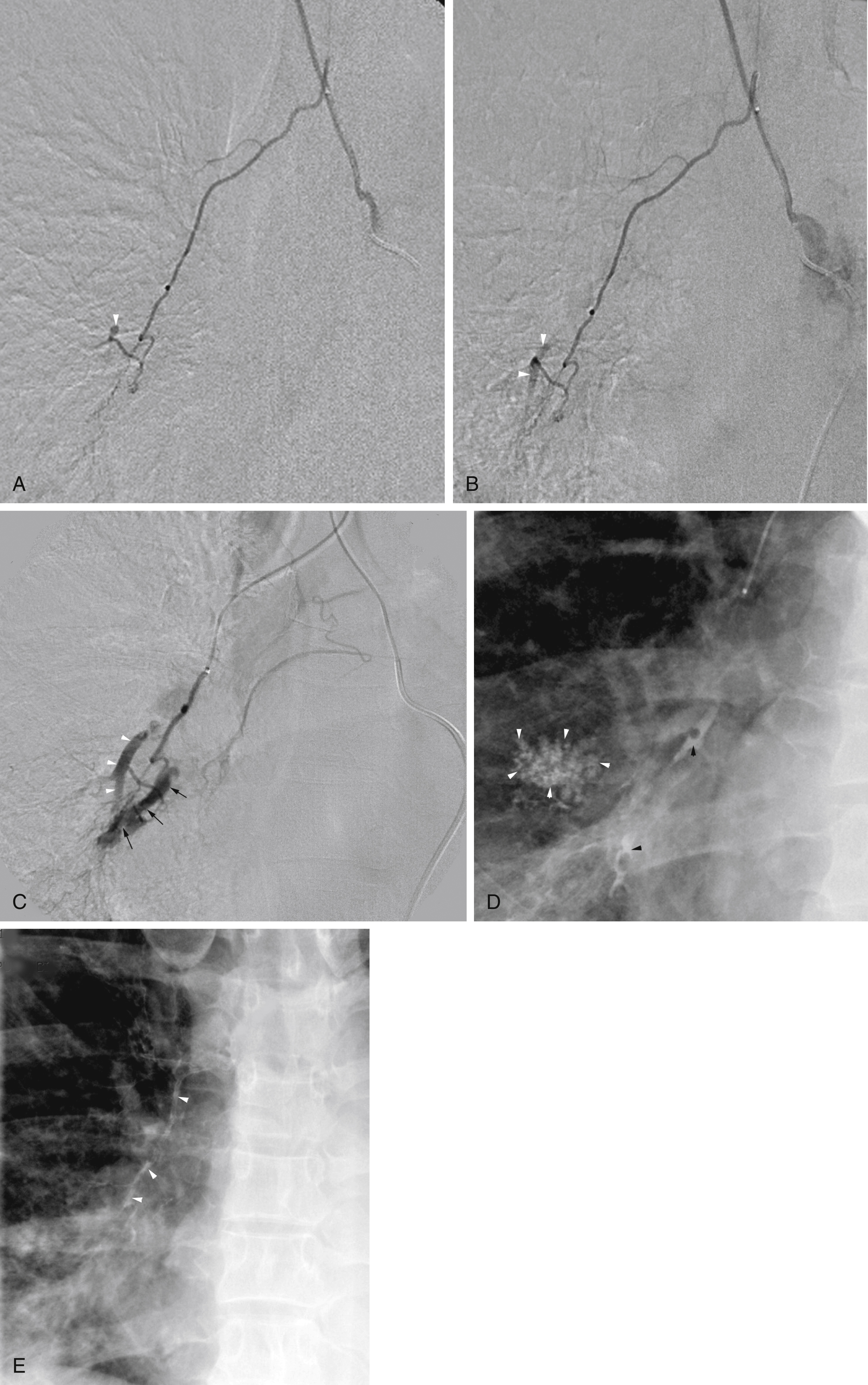
Unlike gastrointestinal bleeding, in which extravasation of injected contrast material into the lumen of the gastrointestinal tract is the hallmark finding, extravasation of contrast into the bronchus in patients with hemoptysis is very rare. Therefore, one must rely on secondary findings to indicate which systemic arteries are responsible for hemoptysis. These secondary angiographic findings for hemoptysis include (1) enlarged bronchial arteries (or other systemic arteries) that supply the abnormal pulmonary parenchyma ( Fig. 53.9A ); (2) hyperemic, hypervascular pulmonary parenchyma supplied from systemic arteries ( Fig. 53.10 ); (3) pulmonary artery opacification from systemic arterial contrast injection, i.e., systemic artery to pulmonary artery shunting ( Figs. 53.3B, 53.9B, and 53.10 ); and (4) bronchial artery aneurysm or pseudoaneurysm ( Figs. 53.9A and Fig. 53.11 ). In addition to these secondary angiographic findings of hemoptysis, there is the direct, rarely seen, primary finding of extravasation of contrast material into the bronchi or alveoli ( Fig. 53.11A–E ) .
Many embolic materials have been used for embolization in the treatment of hemoptysis, most commonly particles. In early cases of embolization for hemoptysis, absorbable gelatin sponge (Gelfoam) was used. However, Gelfoam is not a permanent occlusive agent. Therefore, once polyvinyl alcohol foam (Ivalon), a permanent embolic material, became commercially available, it replaced Gelfoam and significantly lowered the rate of recanalization. Today, various brands of acrylic microspheres 350–500 μm in diameter are commonly used. Particles smaller than 300 μm can pass through the systemic artery to pulmonary artery shunts, with resultant nontarget embolization of normal lung tissue. These should be avoided when systemic artery to pulmonary artery shunting is present on the angiogram.
Cyanoacrylate glues that have controlled polymerization times are favored by some interventionalists. They are particularly useful in situations where the embolization catheter cannot be advanced as far distally as desired. The glue can be mixed with ethiodized oil to control its polymerization time. Depending upon the volume of ethiodized oil mixed with the cyanoacrylate glue, the polymerization time can be adjusted to allow the glue to penetrate to the desired level of occlusion before it polymerizes. Some authors have described decreased rates of recurrent hemoptysis after embolization with the use of glue compared with particulate agents.
Absolute ethanol has also been used in the past as an embolic agent to treat hemoptysis. However, after the catastrophic complication of infarction with necrosis of the left main-stem bronchus was reported after ethanol for embolization, this agent was largely abandoned. Coils are to be avoided because they cannot be positioned sufficiently close enough to the actual site of the parenchymal disease and thus result in a proximal occlusion of the bronchial artery. This favors enlargement of other systemic collaterals that resupply the diseased parenchyma. Furthermore, coils often prevent future access to the previously embolized artery in the event that reembolization is necessary.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here