Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter, the student should be able to answer the following questions:
How does the action potential contribute to excitability and contraction in heart muscle?
What is automaticity, and how does it differ from excitability? How do derangements of these properties contribute to arrhythmias?
What is the structural basis of the electrocardiogram?
What are preload and afterload and how do they regulate contraction of the heart?
What is the local control theory of cardiac excitation-contraction coupling?
What changes occur in atrial and ventricular pressures and volumes during a cardiac cycle, and what is their temporal relation to the electrocardiogram?
How does the relation between end-diastolic volume and left ventricular developed pressure define the Frank-Starling law of the heart and regulate the force of cardiac contraction?
What is the pressure-volume loop of the left ventricle, and how does it define changes in left ventricular function?
How is cardiac metabolism linked to O 2 consumption, and how are these processes affected by changes in cardiac work?
The human heart typically beats over 3 billion times during an average lifetime, accomplishing a truly extraordinary amount of physical work. Essential to this task is the ability to adjust the rate of beating, the strength of contraction, and the rate of relaxation. Efficient ejection of blood into arteries requires precise timing of electrical activation and contraction of the ventricles, as provided by specialized pacemaker tissue and a conduction system. The cellular processes that comprise excitation-contraction (E-C) coupling produce a change in cytoplasmic [Ca ++ ] of the cardiac myocytes that activates the contractile proteins (actin, myosin). E-C coupling processes are regulated by hormones and the autonomic nervous system. The output of the heart is also affected by conditions in the systemic vasculature, such as total peripheral vascular resistance and venous capacitance. Finally, the need for constant and considerable metabolic energy is associated with a high reliance on oxidative phosphorylation of available metabolic substrates.
Cardiac myocytes, like neurons, are excitable cells capable of generating action potentials. However, the primary function of the heart is to pump blood throughout the circulatory system. For this to occur, an orderly sequence of events must take place at a given interval. This is accomplished through the initiation of an action potential and the propagation of this impulse throughout the heart. The cardiac action potential is important not only because it is the trigger that initiates contraction of individual myocytes through the process of E-C coupling, but also because it synchronizes the contraction of the entire heart as it is conducted from cell to cell. Furthermore, while the heart possesses the property of intrinsic automaticity, it is continually under the influence of both intrinsic and extrinsic mechanisms that modulate its activity to meet the demands of the body. Therefore, altering the electrical properties of the heart is an important means by which cardiac function can be controlled. In this section, the electrical properties of cardiac cells and tissues are described. In addition, how these electrical properties account for the electrocardiogram (ECG) is discussed. The coupling of electrical and mechanical activity of cardiac cells is considered in a later section.
The human heart contains billions of cardiac myocytes, and although they are all capable of generating an action potential, not all action potentials are alike. The actual configuration of the action potential is related to the functional role of the myocyte in which it occurs. However, in general, cardiac myocytes can be categorized as exhibiting either fast-response or slow-response action potentials. Examples of each are illustrated in Fig. 16.1 .

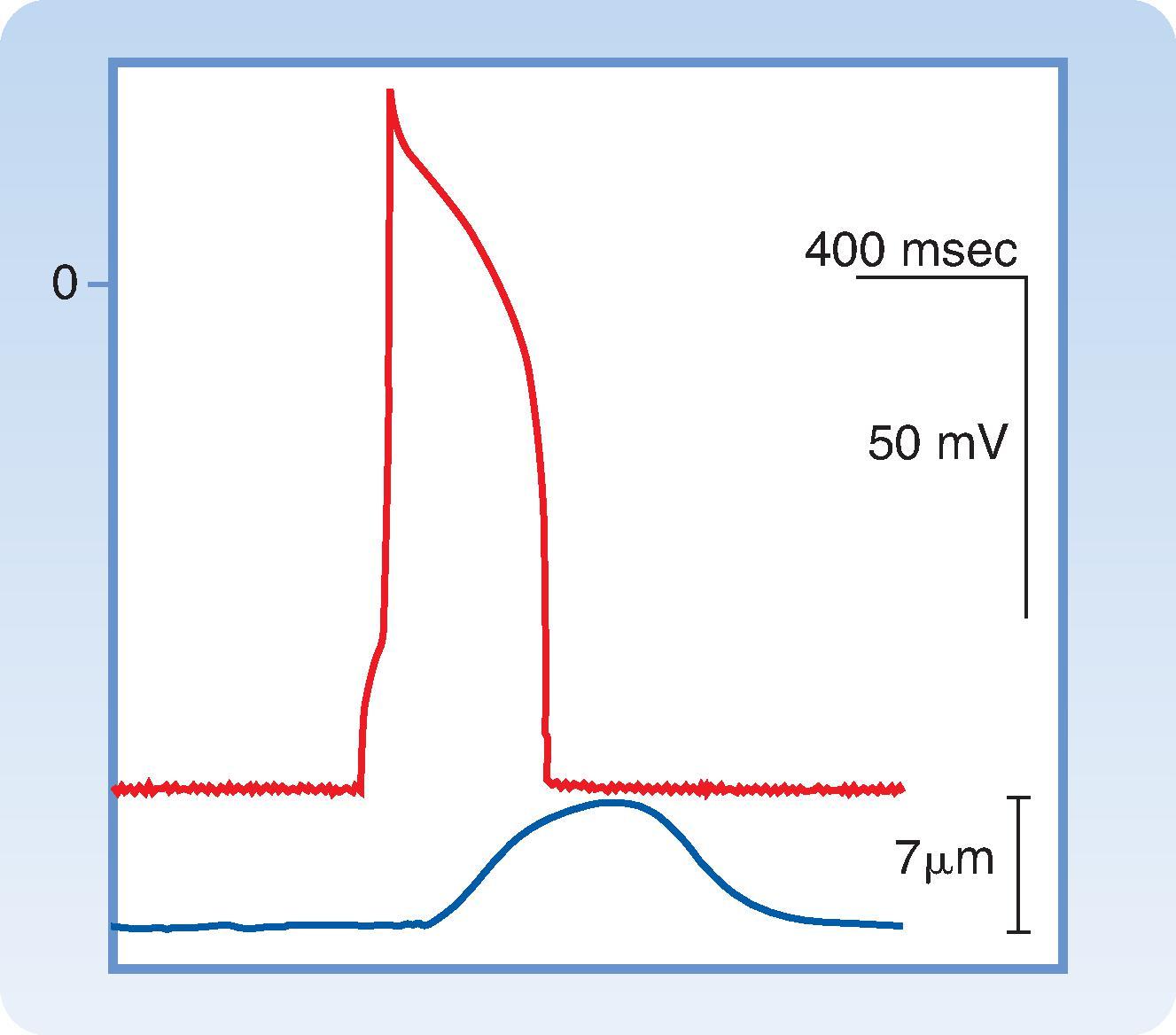
Fast-response action potentials are found in atrial and ventricular myocytes , which make up the bulk of the contractile cells found in the heart. Fast-response action potentials are also found in myocytes like those that make up Purkinje fibers , which are part of the specialized conducting system responsible for transmitting electrical impulses rapidly to all areas of the ventricular myocardium. A fast-response action potential can be divided into five phases: upstroke (phase 0), initial repolarization (phase 1), plateau (phase 2), final repolarization (phase 3), and resting membrane potential (phase 4). These action potentials have several other key characteristics. First, as the name implies, they exhibit a rapid change in membrane potential during the upstroke (phase 0). This facilitates rapid propagation of impulses from cell to cell. These action potentials also exhibit a pronounced plateau (phase 2), which gives them the characteristic long duration (200–400 msec). Another important property is that during diastole (phase 4), which is when the heart is relaxed or at rest, the membrane potential is quite negative (approximately −90 mV). The relationship between the fast-response action potential and contraction of a ventricular myocyte is shown in Fig. 16.2 . Rapid depolarization (phase 0) precedes cell shortening, and completion of repolarization occurs just before peak shortening. Relaxation of the muscle takes place mainly during phase 4 of the action potential. The duration of contraction usually parallels the duration of the action potential.
Slow-response action potentials are found in myocytes that make up the sinoatrial (SA) node, which is the normal pacemaker region of the heart, and the atrioventricular (AV) node, which is the specialized tissue that conducts electrical impulses from the atria to the ventricles. These action potentials have just three phases: upstroke (phase 0), final repolarization (phase 3), and diastole (phase 4). A key feature of these action potentials is the slow upstroke velocity (phase 0). This contributes to slow conduction of impulses, which is particularly important in the AV node, which contributes to the delay between contraction of the atria and ventricles. After reaching a peak, slow-response action potentials begin to gradually repolarize (phase 3). There are no initial repolarization or plateau phases. Furthermore, the membrane potential during diastole (phase 4) is more depolarized (less negative) than that found in fast-response cells, and it never stabilizes at a fixed level. Once the membrane reaches its most negative value, called the maximum diastolic potential , it begins to spontaneously depolarize.
The actual membrane potential of a cardiac myocyte, like any excitable cell, is a function of the unequal distribution of ions across the plasma membrane. The ions that are most important in determining the membrane potential of a cardiac myocyte are K + , Na + , and Ca ++ , and the typical intracellular and extracellular concentrations of these ions are listed in Table 16.1 . Even though the concentration gradient for each of these ions is large, that alone does not determine whether they will diffuse across the membrane and contribute to an action potential. Because of their charged nature, the movement of ions is also affected by the membrane potential (V M ) of the cell. The potential difference that offsets or balances the concentration gradient of an ion is referred to as the equilibrium potential (E ion ) for that ion, and it can be calculated using the Nernst equation, as described in Chapter 1 . The equilibrium potentials for K + , Na + , and Ca ++ in a typical cardiac myocyte are included in Table 16.1 . Thus, the potential for any ion to move across the plasma membrane is determined by the difference between the membrane potential and the equilibrium potential for that ion (V M − E ion ). This is referred to as the electrochemical gradient or driving force .
| Ion | Extracellular Concentrations (mmol/L) | Intracellular Concentrations (mmol/L) * | Equilibrium Potential (mV) |
|---|---|---|---|
| Na + | 145 | 10 | 71 |
| K + | 4 | 135 | −93 |
| Ca ++ | 2 | 10 −4 | 129 |
* The intracellular concentrations are estimates of the free concentrations in cytoplasm. Data from Ten Eick RE, et al. Prog Cardiovasc Dis. 1981;24:157.
Even in the presence of a significant driving force, an ion cannot affect the membrane potential of a cell unless there is a means for it to cross the membrane. Ions are unable to freely diffuse across the lipid membrane of a cell on their own. This process relies on the presence of ion channels, which are membrane proteins that form aqueous pores providing a pathway for passive transport. Ultimately, the actual contribution any ion makes to the membrane potential depends on the relative permeability or conductance of the membrane to that ion, as described by the chord conductance equation (discussed in Chapter 2 ):
This relationship says that the size of the contribution that any ion makes to the membrane potential is determined by the relative magnitude of the conductance of the membrane to that ion (g ion ), expressed as a fraction of the total membrane conductance to all ions (g M ), multiplied by the equilibrium potential for that ion. Put another way, V M is simply a weighted average of the equilibrium potentials of the ions that the membrane is permeable to, where the weighting factor for each ion is the fraction of the total membrane conductance attributable to that ion. The bottom line is that V M will move toward the equilibrium potential of the ion to which the membrane has the greatest conductance.
In a simple example that illustrates how this relationship works, if the membrane is permeable to only one type of ion (g ion /g M = 1), then the membrane potential will approach the equilibrium potential for that ion. This is close to the situation that exists in a ventricular myocyte at rest (phase 4), where the conductance of the membrane is primarily due to K + (g K /g M ≈ 1). The membrane has only a very low permeability to either Na + or Ca ++ at that time. As a result, the resting membrane potential of a ventricular myocyte is very negative because it closely follows the equilibrium potential for K + (E K ). However, during the upstroke of the ventricular action potential (phase 0), this situation reverses. The conductance of the membrane to Na + increases, and the conductance to K + decreases. As a result, the membrane potential depolarizes as it moves away from E K and toward the equilibrium potential for Na + (E Na ). Using this basic principle, the ionic basis of an action potential can then be explained if one knows what types of ion channels are present and the factors that influence the opening and closing, or gating , of those channels.
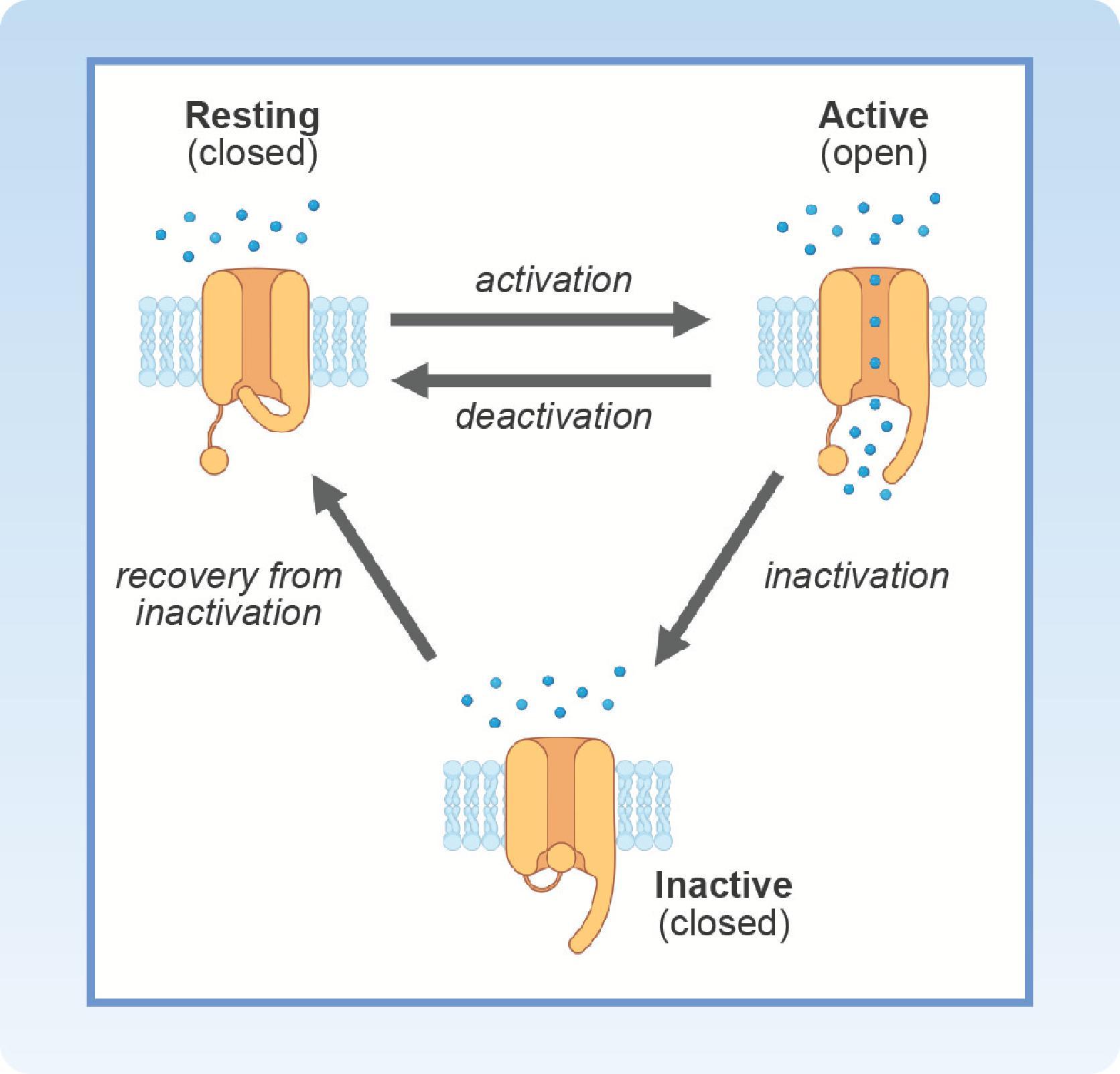
Most cardiac ion channels are voltage-dependent, which means their gating is affected by changes in membrane potential ( Fig. 16.3 ). Activation is the gating process a channel goes through when transitioning from a resting (closed) state to an active (open) state. When open, the conductance of the membrane to ions that are permeable through the channel can then affect the membrane potential. Channels can return to their resting closed state by one of two general mechanisms. The first involves a reversal of activation, through a process called deactivation . The second involves a process called inactivation . A channel that undergoes inactivation is closed, and therefore cannot contribute to the membrane potential. However, an inactivated channel cannot be reactivated, or reopened, unless it first returns to its resting state through a process called recovery from inactivation . All these steps are voltage-dependent.A key to understanding the ionic basis of the cardiac action potential is remembering which channels open and close through a process that involves activation and deactivation (i.e., inward rectifier and delayed rectifier K + channels), and which channels open and close through a process that involves activation, inactivation, and recovery from inactivation (i.e., Na + , Ca ++ , and transient outward K + channels).
The upstroke of a ventricular action potential is normally initiated by depolarization of the membrane by an electrical impulse propagating from an adjacent cell. This causes activation of voltage-dependent Na + channels. If enough of these channels open, the resulting increase in g Na ( Fig. 16.4 ) will cause the membrane potential to move toward E Na , resulting in further depolarization. This, in turn, activates even more Na + channels, facilitating an even greater increase in g Na and a more rapid movement of the membrane potential toward E Na . This type of positive feedback response, together with the fact that these Na + channels respond to changes in membrane potential extremely rapidly, explains the fast depolarization of the membrane during the upstroke of a ventricular action potential. However, after opening, these Na + channels quickly close by inactivating, and they cannot reopen until they recover from inactivation, which only occurs after the membrane repolarizes. It is important to understand that both activation and inactivation of these Na + channels are caused by membrane depolarization, and both processes are very rapid. Activation occurs over a time course of a few microseconds; inactivation occurs over a time course of a few milliseconds.
The ionic current conducted through individual membrane channels can be measured with the patch clamp technique. Channels open and close repeatedly in what often appears to be a random manner. This process is illustrated in Fig. 16.5 , which shows the current flowing through Na + channels in a myocardial cell. In this experiment the membrane potential was initially held constant or “clamped” at −85 mV, where these channels exist in a resting state. At the time point indicated by the arrow, the potential was suddenly changed to −45 mV to activate these channels, and the membrane potential was held there for the remainder of the recording. Immediately after the membrane was depolarized, one channel opened (1.5 pA in amplitude) and then a second(3 pA total current from both channels). These channels then proceeded to open and close multiple times before finally entering an inactivated state, where they remained closed. When viewed individually, the opening and closing of ion channels appears to be random. However, when the activity of an entire population of channels in a cell is added together, the behavior is quite predictable.
![Fig. 16.5, Current (in picoamperes [pA]) through two individual Na + channels recorded in a cardiac myocyte using the patch clamp technique. Membrane voltage was held at −85 mV and then abruptly changed to −45 mV (at the time indicated by the arrow ), where it was held for the remainder of the recording. Fig. 16.5, Current (in picoamperes [pA]) through two individual Na + channels recorded in a cardiac myocyte using the patch clamp technique. Membrane voltage was held at −85 mV and then abruptly changed to −45 mV (at the time indicated by the arrow ), where it was held for the remainder of the recording.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ElementsofCardiacFunction/4_3s20B9780323847902000163.jpg)
Depolarization of the membrane potential during the upstroke of the action potential also causes a decrease in the background K + conductance that is responsible for the resting membrane potential of ventricular myocytes. This large resting K + conductance is due to the activity of a specific type of K + channel, one that generates the inward rectifier K + current (I K1 ) ( Fig. 16.6 ). The term inward rectification simply refers to the fact that these are voltage-dependent ion channels that are activated as the membrane potential is made more negative ( Fig. 16.7 ). As a result, they can generate an inward current carried by K + when the membrane potential is more negative than E K , because of the inward driving force. At membrane potentials more positive than E K , when the driving force would be expected to cause an outward movement of K + ions, there is actually very little current because these channels close due to a deactivation-like process. This facilitates depolarization during the upstroke by decreasing g K , allowing the membrane potential to move away from E K . These channels are unusual in that the gating is due to the block and unblock of the channel pore by intracellular Mg ++ and positively charged molecules called polyamines. This is unlike other channels, where the voltage dependence is due to gating mechanisms that are intrinsic to the channel protein itself.
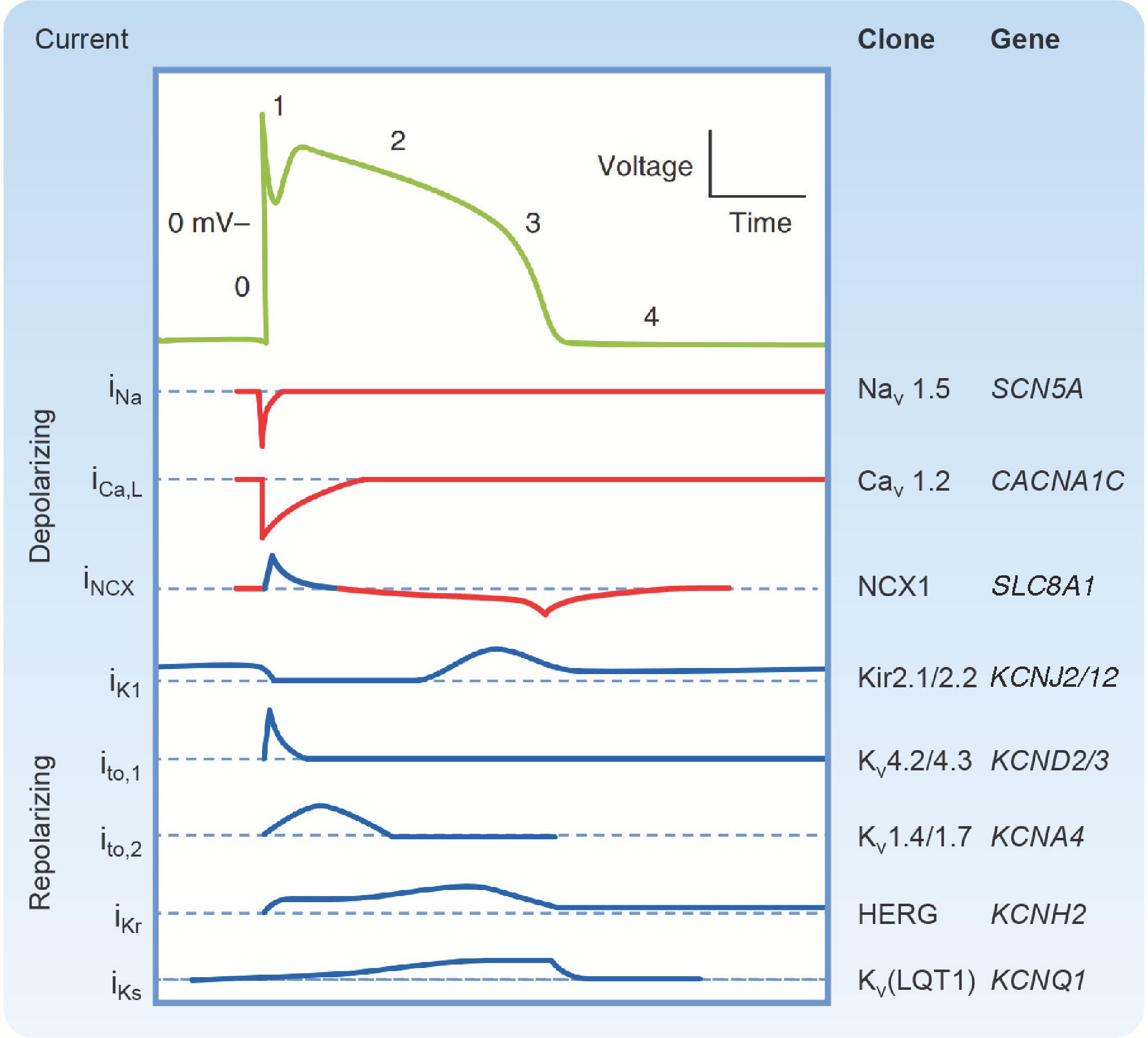
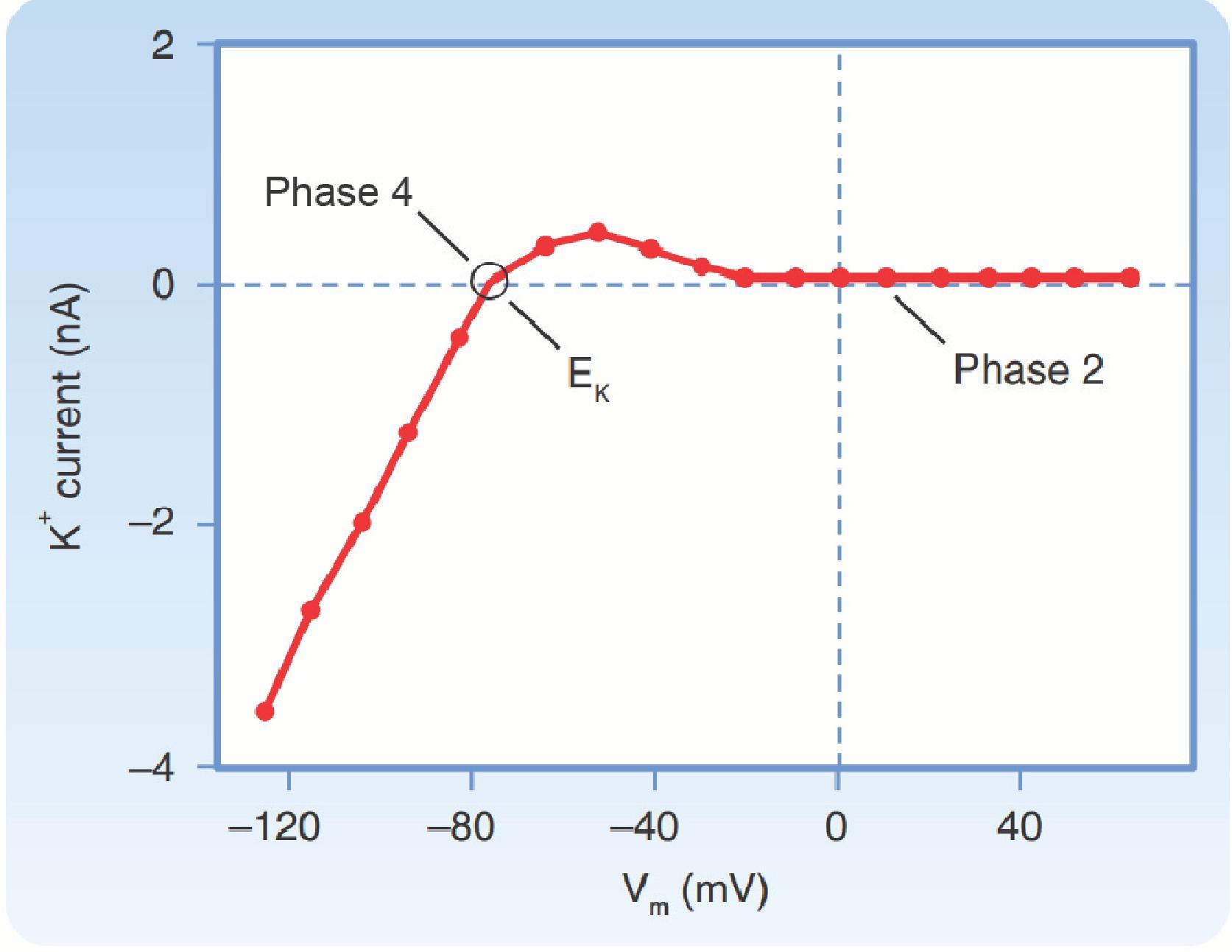
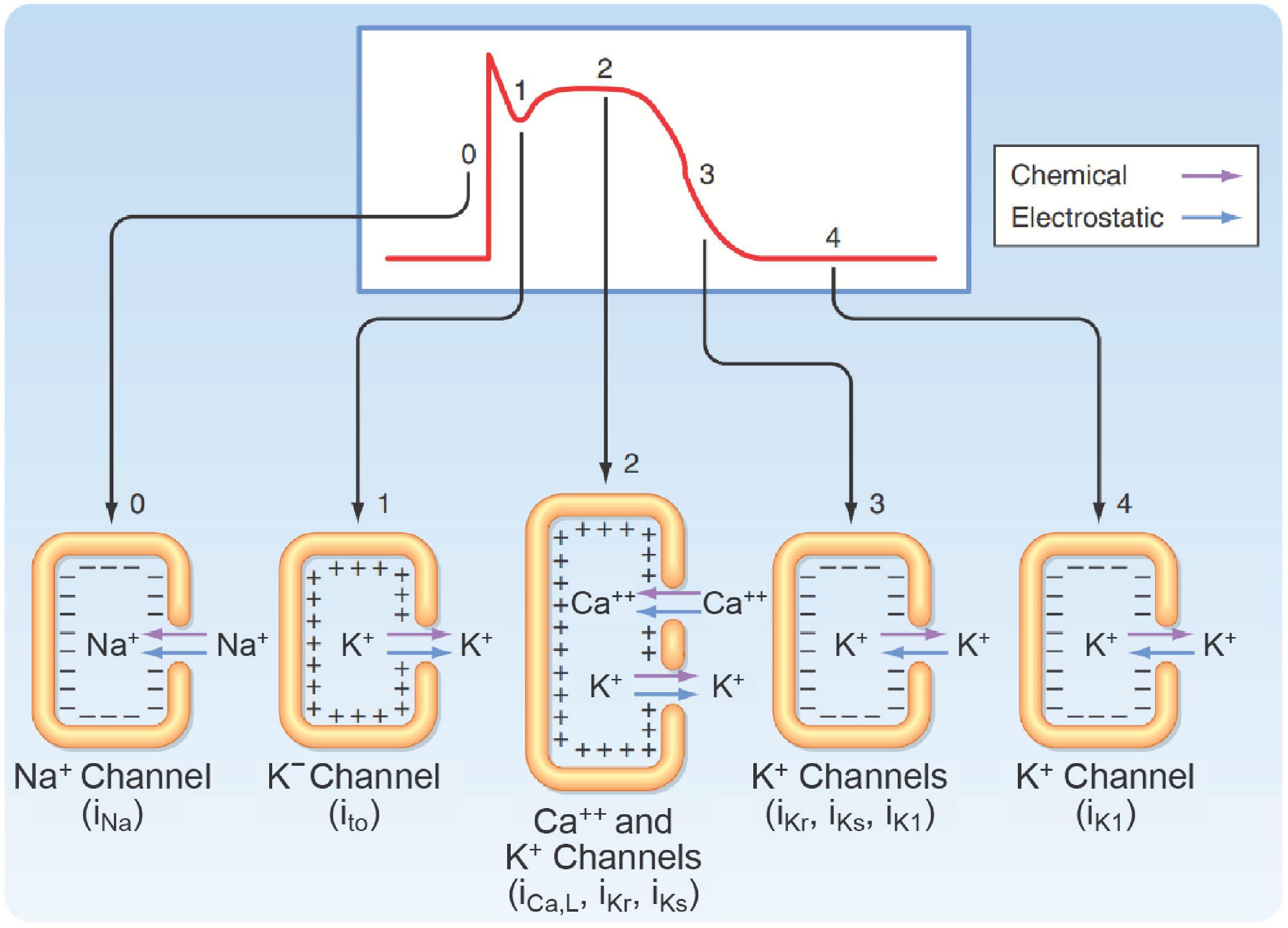
After the action potential reaches its peak, there is a brief period of limited repolarization that results in a notch between the upstroke and the beginning of the plateau (see Fig. 16.1 ). This is facilitated in part by the rapid decrease in g Na caused by voltage-dependent inactivation of the Na + channels responsible for the upstroke. The prominence of the initial repolarization phase can be quite variable. In cells where it is most noticeable, the notch is largely due to a transient increase in g K caused by voltage-dependent activation and subsequent inactivation of K + channels that generate a transient outward current (I to ) . There are at least two components to this current. They are conducted by K + channels that generate I to,1 and I to,2 ( Fig. 16.6 ).
The membrane of a fast-response cell does not return to its resting potential following initial repolarization because the depolarization that occurs during the upstroke also causes an increase in Ca ++ conductance (g Ca ) ( Fig. 6.4 ). In ventricular myocytes, this is due to the activation of channels that generate an L-type Ca ++ current (I Ca,L ) ( Fig. 16.6 ), which depolarizes the cell by attempting to move the membrane potential to E Ca . The voltage-dependent behavior of these channels is like that of the Na + channels responsible for the upstroke. They are activated by membrane depolarization, and after opening they undergo inactivation. However, there are two important differences. First, these Ca ++ channels require the membrane potential to be more depolarized to activate. Second, they respond more slowly to changes in membrane potential: They activate over a time course of a millisecond and inactivate over a time course of tens to hundreds of milliseconds. The influx of Ca ++ that occurs with the activation of I Ca,L channels also triggers myocyte contraction through a process that is discussed in the section “Excitation-Contraction Coupling” (see also Chapter 13 ). The prolonged increase in g Ca due to slow inactivation of these channels is the primary mechanism responsible for keeping the membrane depolarized during the plateau.
The autonomic nervous system regulates cardiac contractility in part through changes in L-type Ca ++ channel activity. The sympathetic neurotransmitter norepinephrine acts by stimulating β-adrenergic receptors found in the plasma membrane of cardiac myocytes. This interaction activates the membrane-bound enzyme adenylate cyclase, which then stimulates the production of cyclic adenosine monophosphate (cAMP; see Chapter 13 ). The rise in cAMP activates protein kinase A, which in turn causes a phosphorylation-dependent increase in channel activity, augmenting the influx of Ca ++ into the cell. Conversely, the parasympathetic neurotransmitter acetylcholine can antagonize this effect by acting through muscarinic receptors to inhibit the production of cAMP by adenylate cyclase.
Calcium channel antagonists such as verapamil, amlodipine, and diltiazem are drugs that block L-type Ca ++ channels. These compounds can inhibit the influx of Ca ++ into cardiac myocytes. This can result in a decrease in ventricular action potential duration as well as the strength of contraction (see Fig. 16.8 ). Blocking these channels can also decrease action potential firing frequency in the SA node as well as the speed of impulse propagation through the AV node. Calcium channel antagonists can also depress the contraction of vascular smooth muscle and thereby induce generalized vasodilation. This diminished vascular resistance reduces the counterforce (afterload) that opposes the propulsion of blood from the ventricles into the arterial system, as explained in Chapter 17 . Hence, vasodilator drugs such as the calcium channel antagonists are often referred to as afterload-reducing drugs.
![Fig. 16.8, Effects of diltiazem, a calcium channel antagonist, on the action potentials (in millivolts [mV]) and isometric contractile forces (in millinewtons [mN]) recorded from a papillary muscle in vitro. The tracings were recorded under control conditions (C) and in the presence of diltiazem at concentrations of 3, 10, and 30 µmol/L. Fig. 16.8, Effects of diltiazem, a calcium channel antagonist, on the action potentials (in millivolts [mV]) and isometric contractile forces (in millinewtons [mN]) recorded from a papillary muscle in vitro. The tracings were recorded under control conditions (C) and in the presence of diltiazem at concentrations of 3, 10, and 30 µmol/L.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ElementsofCardiacFunction/7_3s20B9780323847902000163.jpg)
Even though the driving force for K + is significant at depolarized membrane potentials, g K is quite low during much of the plateau. This is because I K1 channels open during phase 4 are deactivated during phase 0 and most I to channels activated during phase 1 have inactivated. This explains why the membrane potential during phase 2 of the ventricular action potential is maintained between E Ca and E K . The reduction in g K minimizes the amount of Ca ++ that needs to flow into the cell in order to maintain depolarization. It also minimizes the amount of K + that would otherwise flow out of the cell during the plateau. Both effects reduce the energy required to maintain the gradients for these ions through active transport mechanisms.
The end of the plateau and beginning of final repolarization of the fast-response action potential comes about when the balance between the Ca ++ and K + conductance of the membrane shifts back toward g K ( Fig. 16.4 ). There is a decrease in g Ca due to the time- and voltage-dependent inactivation of the L-type Ca ++ channels. There is also an increase in g K due to the time- and voltage-dependent activation of channels that generate a delayed rectifier K + current (I K ) . These K + channels are activated when the membrane depolarizes to potentials like those that activate the Ca ++ channels. However, an important distinction is that, as their name implies, delayed rectifier K + channels activate very slowly, even more slowly than the Ca ++ channels. Therefore, the increase in g K due to opening of these channels does not become significant until the end of phase 2. There are also multiple types of delayed rectifier K + channels. The two types most prominent in ventricular myocytes generate a rapidly activating delayed rectifier K + current (I Kr ) and a slowly activating delayed rectifier K + current (I Ks ) ( Fig. 16.6 ). Both contribute to an increase in g K that drives the membrane potential back toward E K . As the membrane potential becomes more negative, this causes these channels to close by deactivating late in phase 3. However, g K does not decrease. In fact, it continues to increase, because at the same time, I K1 channels begin to reactivate bringing the membrane back to its resting potential near E K .
Cardiac ion channels associate with various cellular proteins to form macromolecular complexes. These interactions are involved in many aspects of ion channel function, including trafficking, gating, and post-translational modification. For example, the L-type Ca ++ channel (Ca V 1.2) exists as a complex consisting of α 1 , β, α 2 δ, and γ subunits. While the α 1 subunit can function as an ion channel on its own, the β and γ subunits affect the voltage-dependent properties of the channel, and the β and α 2 δ subunits promote trafficking of the channel to the plasma membrane. The α 1 subunit also interacts with various signaling proteins, including calmodulin, which is involved in regulation of channel inactivation by intracellular Ca ++ , as well as A-kinase anchoring proteins, which act as scaffolds to bind various components of the protein kinase A signaling pathway. This multimeric complex also interacts with other membrane proteins including caveolin-3, which facilitates interactions with the stimulatory G protein G s , adenylate cyclase, and β-adrenergic receptors, all of which are involved in sympathetic regulation of this channel by the autonomic nervous system.
As described previously, the resting membrane conductance of a ventricular myocyte is dominated by its permeability to K + ( Fig. 16.4 ). As a result, the membrane potential during phase 4 closely follows E K . Because g K is so large, it very effectively stabilizes the membrane, preventing spurious depolarization, which might otherwise lead to the generation of arrhythmias. At the resting membrane potential there is actually very little movement of K + because the driving force is minimal. There is also very little movement of Na + and Ca ++ because the conductance of the membrane to these ions is very small, even though the inward driving force is quite large.
The upstroke of slow-response action potentials is due to the activity of L-type Ca ++ channels ( Fig. 16.9 ). The increase in g Ca caused by activation of these channels causes the membrane potential to depolarize, as it moves toward E Ca . Unlike fast-response action potentials, where Na + channels are involved, the rate of change in the membrane potential during the upstroke of the action potential in an SA node cell is much slower. This is because L-type Ca ++ channels activate much more slowly than Na + channels and the channel density is lower.
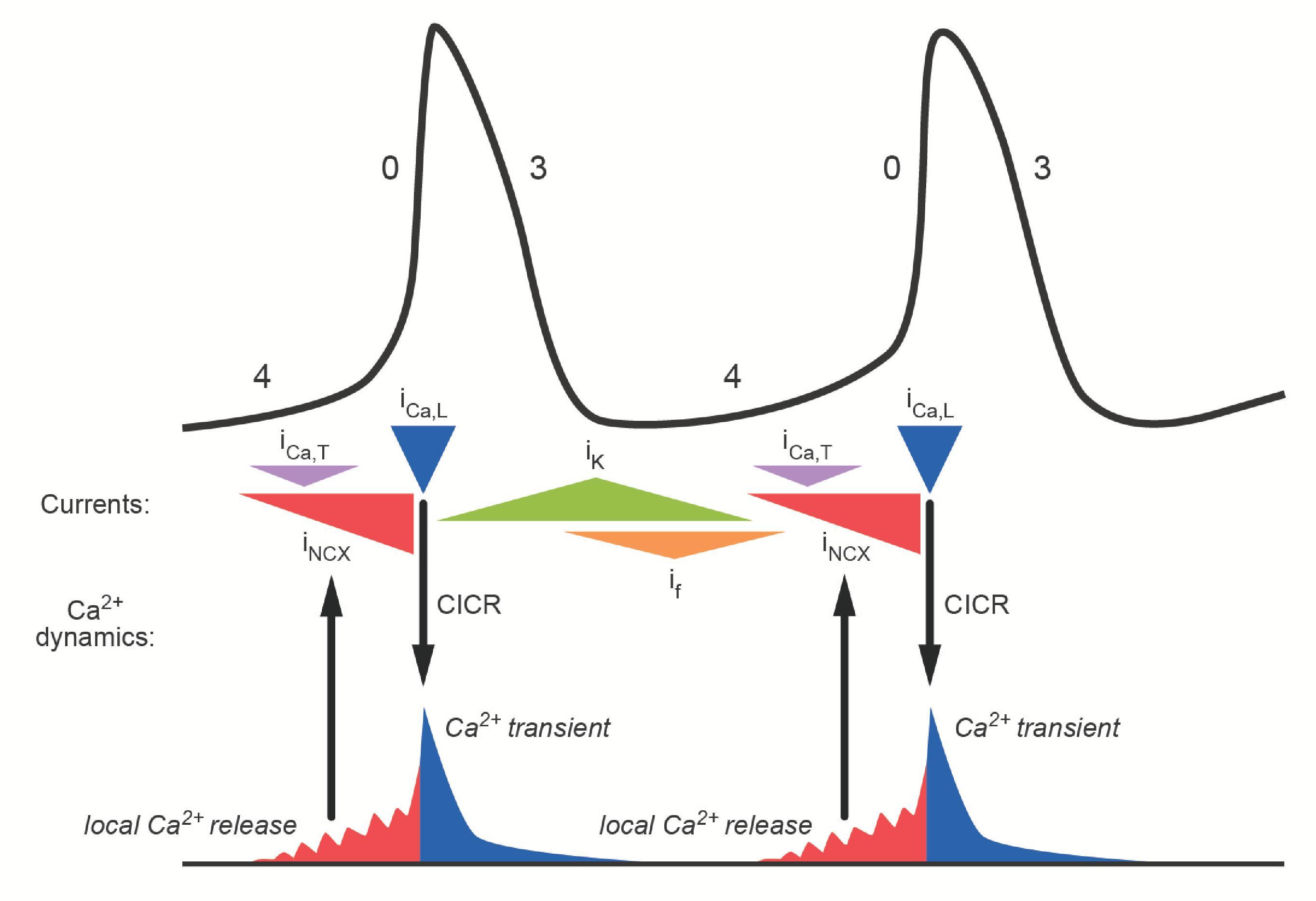
As mentioned earlier, there is no pronounced plateau phase in a slow-response action potential. However, action potential duration is determined by a similar process in both fast-and slow-response cells: a shift in the balance between g Ca and g K . There is a gradual decrease in g Ca as the L-type Ca ++ channels responsible for the upstroke slowly inactivate, and there is a gradual increase in g K due to activation of delayed rectifier K + channels ( Fig. 16.9 ). The increase in g K causes the cell to repolarize until it eventually reaches its maximum diastolic potential.
The maximum diastolic potential during phase 4 of the slow-response action potential in an SA node cell is less negative (−50 to −70 mV) than the resting membrane potential of a ventricular myocyte. This can be explained by the fact that SA node cells have a lower background g K , which is due to K + channels that generate an acetylcholine (ACh)-activated K + current (I K,ACh ) . The lower background K + conductance makes it easier for these cells to be depolarized by the activity of other ion channels and transporters.
One mechanism that contributes to spontaneous depolarization during phase 4 actually begins during phase 3. The delayed rectifier K + channels that contribute to final repolarization begin to close by deactivating as the membrane potential becomes more negative ( Fig. 16.9 ). However, this gating process is slow. The result is a time-dependent decrease in g K , during phase 4, which allows the membrane potential to slowly move away from E K , or depolarize.
Depolarization during phase 4 is also facilitated by the activation of channels that generate a pacemaker or funny current (I f ) . These are called “funny” channels because they activate upon hyperpolarization of the membrane, which was viewed as unusual at the time they were originally discovered. As such, these channels are closed when the membrane is depolarized during the action potential, but activate or open upon repolarization during phase 4 ( Fig. 16.9 ). Pacemaker channels are also unusual because they are permeable to both Na + and K + . So, when they are open, they cause the membrane to move toward their equilibrium potential, which is between E K and E Na (−15 mV). Because this is more positive than the maximum diastolic potential, the result is a net inward current carried by Na + moving down its electrochemical gradient.
The combination of factors described above eventually depolarizes the membrane enough to cause a brief increase in g Ca due to the activation of channels that generate a T-type Ca ++ current (I Ca,T ) ( Fig. 16.9 ). These channels activate upon membrane depolarization and then inactivate, similar to L-type Ca ++ channels. However, there are two main differences. T-type Ca ++ channels activate at more negative membrane potentials, and they activate and inactivate much more rapidly.
In addition to the effects of the time- and voltage-dependent ion channels described earlier in the chapter, SA node cells spontaneously release Ca ++ from the sarcoplasmic reticulum (SR) into the cytoplasm. The resulting rise in intracellular Ca ++ near the plasma membrane activates the Na/Ca exchanger (NCX), which couples the movement of extracellular Na + down its electrochemical gradient into the cell to the movement of intracellular Ca ++ out of the cell. Because this process involves three Na + ions moving in for each Ca ++ ion that moves out, the result is a net inward current ( I NCX ). This contributes to spontaneous depolarization late during phase 4 ( Fig. 16.9 ).
Changes in serum [K + ] can have significant effects on the cardiac action potential and the electrical properties of the heart. For example, an increase in serum [K + ] ( hyperkalemia ) will depolarize the resting membrane potential, and a decrease in serum [K + ] ( hypokalemia ) can hyperpolarize the resting membrane potential (see Fig. 16.10 ). These responses can be easily explained by the effect that changing the extracellular K + concentration has on E K . However, changes in serum [K + ] can have what appear to be paradoxical effects on action potential duration if one only considers what happens to E K . Remember, the chord conductance equation tells us that membrane potential is a function of the conductance of the membrane to an ion, as well as its equilibrium potential. It turns out that changes in extracellular [K + ] also affect the K + conductance of the membrane associated with delayed and inward rectifier K + channels: Increasing extracellular [K + ] increases g K and decreasing extracellular [K + ] decreases g K . The changes in g K have a greater effect on action potential duration than the changes in E K . As a result, hyperkalemia reduces action potential duration and hypokalemia prolongs action potential duration.
![Fig. 16.10, Effect of changes in extracellular [K + ] on action potentials recorded from a Purkinje fiber. The stimulus artifact (St in tracing D) appears as a biphasic spike to the left of the upstroke of the action potential. The horizontal dashed lines near the peaks of the action potentials denote 0 mV. When extracellular [K + ] is 3 mM (tracings A and F), the resting membrane potential (V M ) is −82 mV, and the slope of phase 0 is steep. At the end of phase 0, the overshoot attains a value of +30 mV. Hence, the action potential amplitude is 112 mV. The distance from the stimulus artifact to the beginning of phase 0 is inversely proportional to the conduction velocity. When extracellular [K + ] is increased gradually to 16 mM (tracings B to E), resting V M becomes progressively less negative. At the same time, the amplitude and duration of the action potential and the steepness of the upstroke all diminish. As a consequence, conduction velocity decreases progressively. At extracellular [K + ] levels of 14 and 16 mM (tracings D and E), the resting V M attains levels sufficient to inactivate all the fast sodium channels and leads to the characteristic slow-response action potentials. Fig. 16.10, Effect of changes in extracellular [K + ] on action potentials recorded from a Purkinje fiber. The stimulus artifact (St in tracing D) appears as a biphasic spike to the left of the upstroke of the action potential. The horizontal dashed lines near the peaks of the action potentials denote 0 mV. When extracellular [K + ] is 3 mM (tracings A and F), the resting membrane potential (V M ) is −82 mV, and the slope of phase 0 is steep. At the end of phase 0, the overshoot attains a value of +30 mV. Hence, the action potential amplitude is 112 mV. The distance from the stimulus artifact to the beginning of phase 0 is inversely proportional to the conduction velocity. When extracellular [K + ] is increased gradually to 16 mM (tracings B to E), resting V M becomes progressively less negative. At the same time, the amplitude and duration of the action potential and the steepness of the upstroke all diminish. As a consequence, conduction velocity decreases progressively. At extracellular [K + ] levels of 14 and 16 mM (tracings D and E), the resting V M attains levels sufficient to inactivate all the fast sodium channels and leads to the characteristic slow-response action potentials.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ElementsofCardiacFunction/9_3s20B9780323847902000163.jpg)
The experimentally induced changes in transmembrane potential shown in Fig. 16.10 mimic those that may take place in the cardiac tissue of patients with coronary artery disease. When regional myocardial blood flow is diminished, the supply of O 2 and metabolic substrates delivered to the ischemic tissues is insufficient. The Na + ,K + -ATPase in the membrane of cardiac myocytes requires considerable metabolic energy to maintain the normal transmembrane gradients of Na + and K + . When blood flow is inadequate, the activity of Na + ,K + -ATPase is impaired, and the affected myocytes gain excess Na + and lose excess K + to the surrounding interstitial space. This can critically disturb conduction of electrical impulses by causing fast-response cells to produce slow-response–like action potentials. The increase in [K + ] in the extracellular space causes a shift in E K , resulting in depolarization of the resting membrane potential of these cells. This leads to inactivation of voltage-dependent Na + channels that normally contribute to the upstroke. As a result, the upstroke may be slowed (as illustrated in Fig. 16.10 ) or even blocked. This often contributes to the generation of arrhythmias.
The ability to initiate an action potential in response to a stimulus is a characteristic of any excitable cell. Yet, for this to happen, the stimulus must depolarize the membrane to a threshold potential, the absolute value of which varies depending on the cell type and the timing. The threshold for initiating an action potential that can be propagated within a cell and from cell to cell in cardiac tissue depends on (1) whether fast- or slow-response action potentials are involved and (2) precisely when during the cardiac cycle the stimulus arrives.
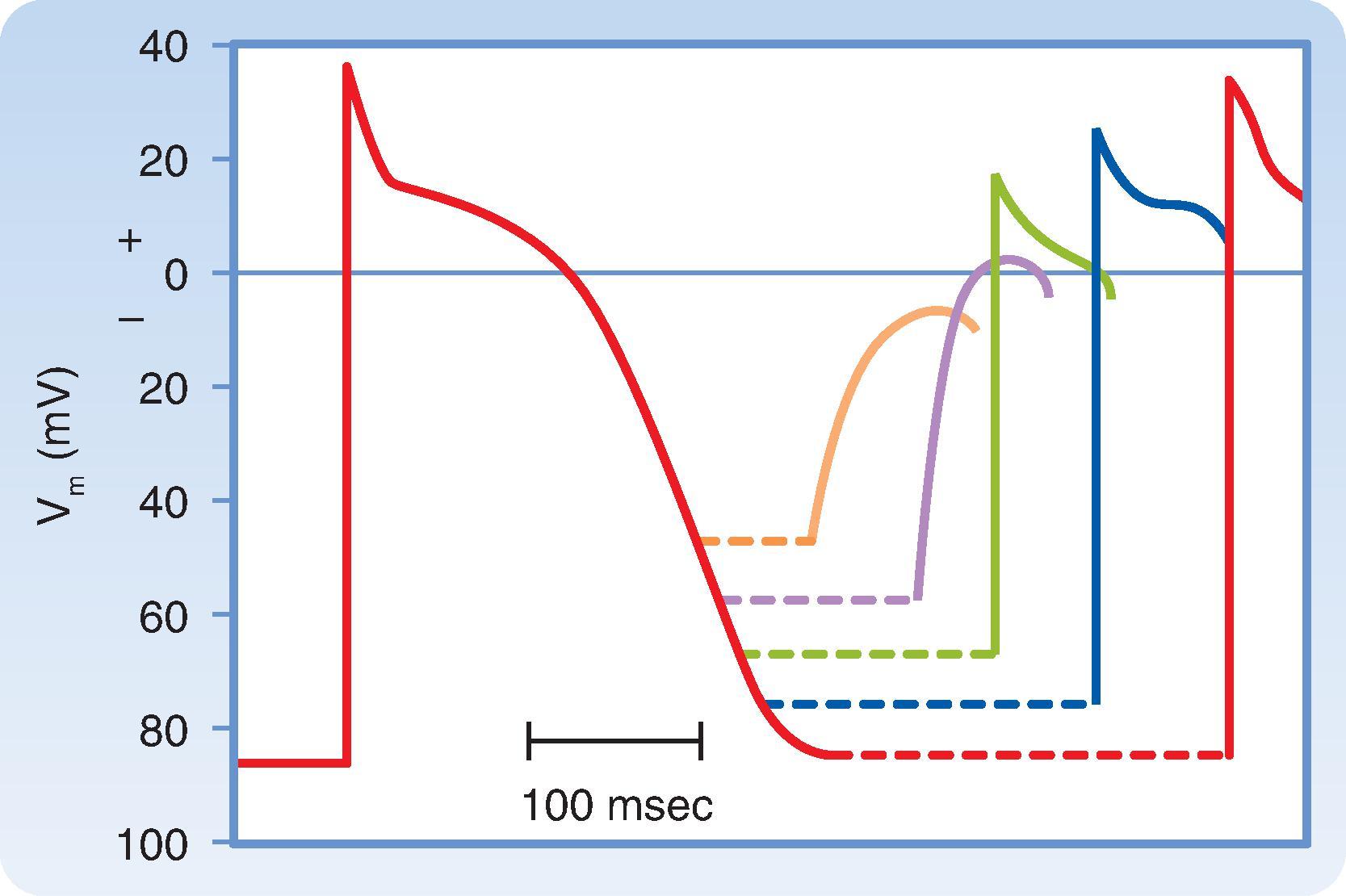
In fast-response cells, excitability depends on the availability of voltage-dependent Na + channels to generate the upstroke of the action potential. Once a fast-response action potential has been initiated, the depolarized cell cannot be re-excited until it has at least partially repolarized. This is because the Na + channels, which inactivated immediately following the upstroke, must recover from inactivation before they can reopen. This is a time- and voltage-dependent process that occurs at more negative membrane potentials. The interval from the beginning of the action potential until the cell can generate at least some type of action potential is called the effective refractory period . This extends approximately midway through repolarization, during phase 3. At that point, enough Na + channels have typically recovered from inactivation to restore some degree of excitability. However, fast-response cells are not fully excitable until they have completely repolarized. The time between the end of the effective refractory period and complete repolarization is referred to as the relative refractory period . When a response is evoked during the relative refractory period, its properties vary as a function of the membrane potential at the time the stimulus arrived ( Fig. 16.11 ). This reflects the difference in the number of Na + channels that have recovered from inactivation and are available to contribute to the upstroke. The earlier in the relative refractory period that the fast-response cell is stimulated, the slower the upstroke velocity and the smaller the amplitude of the resulting action potential. Consequently, conduction velocity also decreases. This effect can be mimicked by pharmacologically blocking Na + channels with the drug tetrodotoxin ( Fig. 16.12 ). The result is a slow-response–like action potential, due to activation of L-type Ca ++ channels.
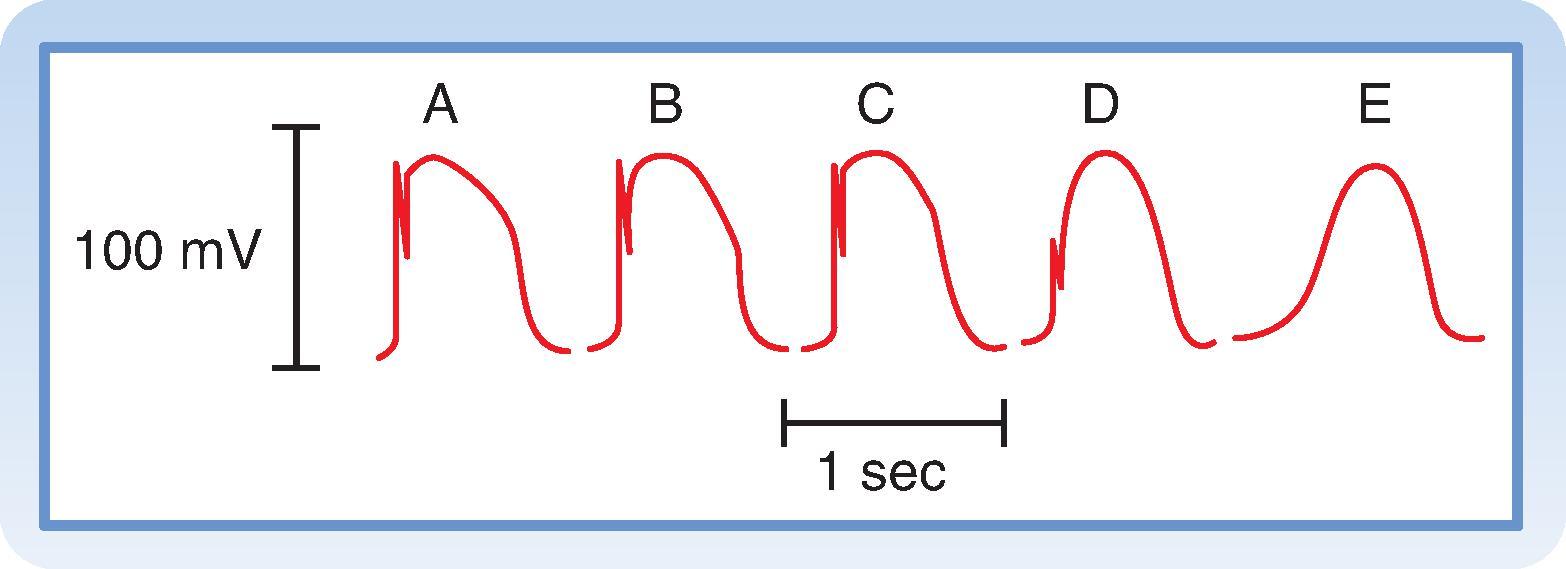
In slow-response cells, excitability depends on the availability of the L-type Ca ++ channels that generate the upstroke of the action potential. In these cells, the relative refractory period frequently extends well beyond phase 3. Even after the cell has completely repolarized, it may be difficult to evoke a propagated response for some time. This characteristic of slow-response cells is called postrepolarization refractoriness . Refractoriness of a slow-response cell is due to L-type Ca ++ channel inactivation, and normal excitability does not return until these channels have recovered. Just like Na + channels in fast-response cells, recovery from inactivation of Ca ++ channels in slow-response cells is a time- and voltage-dependent process. However, it occurs much more slowly, which explains the long refractory period duration. The amplitude and upstroke velocity of slow-response action potentials progressively improve the later the stimulus arrives in the relative refractory period ( Fig. 16.13 ). The fact that recovery of full excitability extends well beyond the point of complete repolarization is particularly important property of slow-response cells found in the AV node. The long refractory period makes it difficult for a premature stimulus arriving from the atria to be conducted to the ventricles. It can also lead to AV node conduction block. In some situations, the AV node may be able to conduct only a fraction of the impulses that arrive from the atria.
In a patient who has occasional premature atrial depolarizations (see Fig. 16.28 ), the timing of these early beats may determine their clinical consequence. If they occur late in the relative refractory period of the preceding depolarization or after full repolarization, the premature depolarization is inconsequential. However, if the premature depolarizations originate early in the relative refractory period of the ventricles, conduction of the premature impulse from the site of origin is slowed, and hence a cardiac impulse is more likely to re-excite some myocardial region through which it had passed previously (a phenomenon known as reentry ). If that reentry is irregular (i.e., if ventricular fibrillation ensues), the heart cannot pump effectively, and death may result.
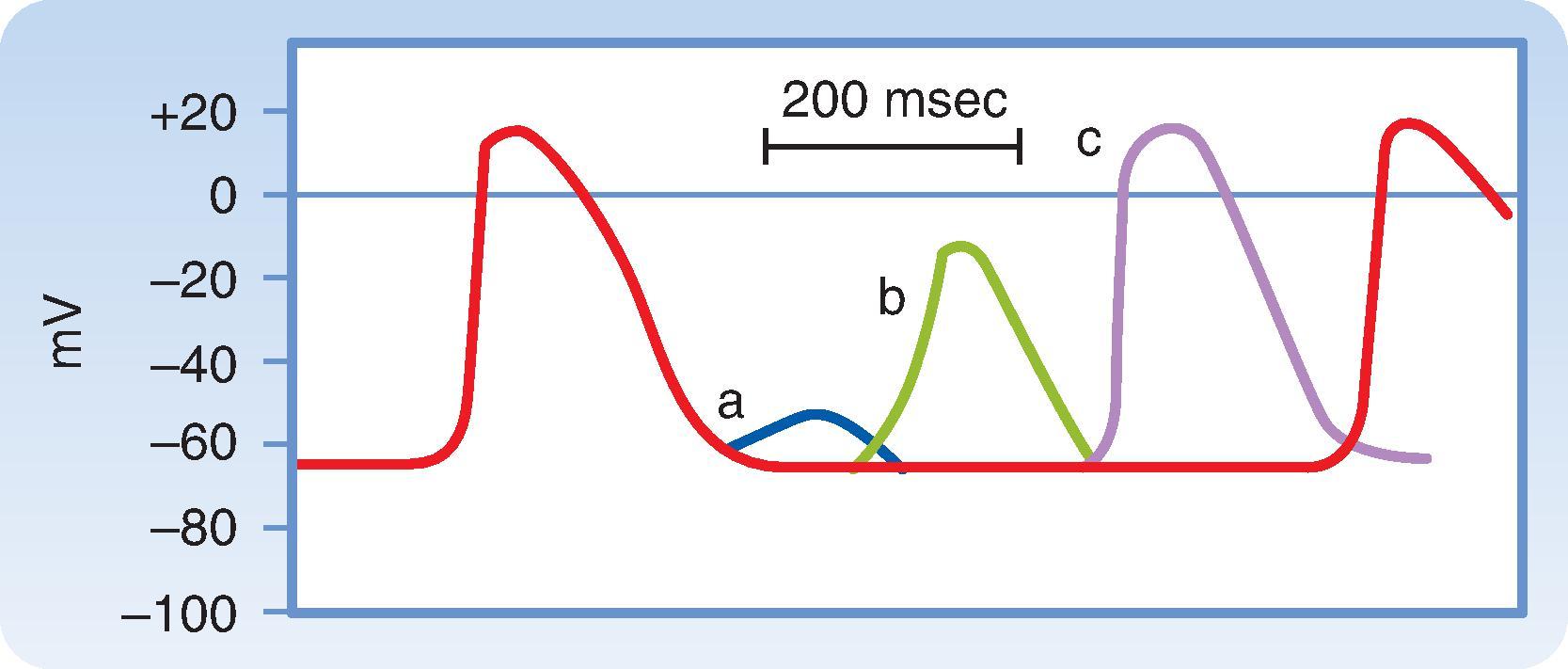
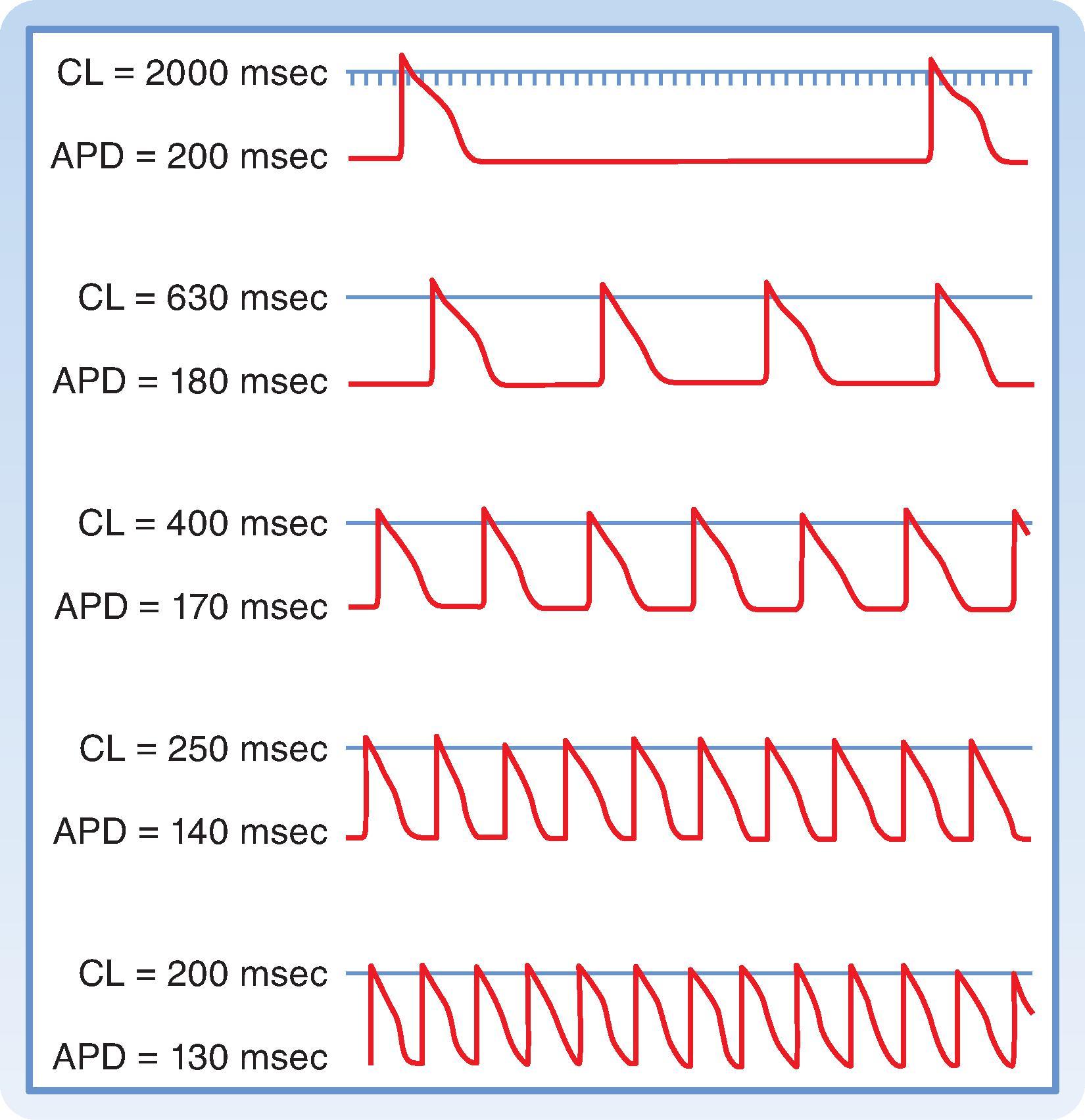
Excitability of a cardiac myocyte can also be affected by changes in cycle length, or the time between successive action potentials. This is because of the effect that cycle length has on action potential duration. As a result, changes in pacing frequency or heart rate are often important factors in the initiation or termination of certain arrhythmias (irregular heart rhythms). The changes in action potential duration produced by stepwise reductions in cycle length from 2000 to 200 msec in a Purkinje fiber are shown in Fig. 16.14 . Note that as cycle length diminishes, the duration of the action potential decreases. This direct correlation between action potential duration and cycle length can be explained by changes in g K that involve delayed rectifier K + (I K ) channels . These channels, which normally activate very slowly during the plateau of the action potential, also deactivate or close very slowly following repolarization. As the cycle length progressively decreases, so does the amount of time between action potentials, resulting in insufficient time for these channels to close. Consequently, there is an accumulation of channels that remain activated. The result is an increase in g K during the plateau, which results in earlier repolarization and shorter action potential duration.
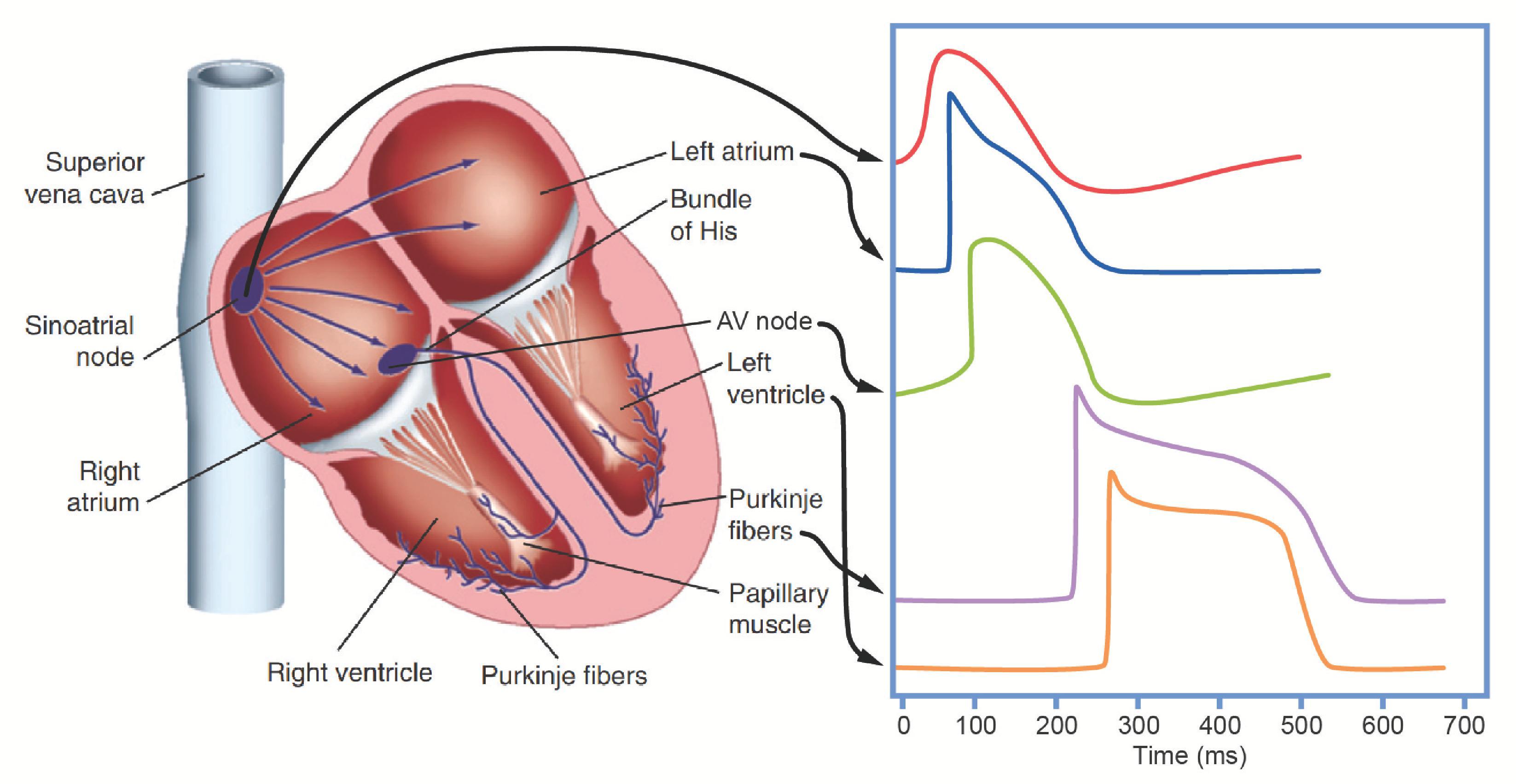
Each heartbeat starts with an electrical impulse that is generated in the SA node and then conducted throughout the atria. The impulse arriving from the atria is then slowed as it passes through the AV node to the His-Purkinje system, where it speeds up again, synchronizing the spread of electrical activity across the entire subendocardial surface of the ventricles. From there, the wave of excitation is conducted from cell to cell throughout the ventricular myocardium. Electrical activity initiated by the SA node and conducted throughout the heart in this manner ( Fig. 16.15 ) at regular intervals is referred to as normal sinus rhythm , and it typically occurs at a frequency between 60 and 100 beats/minute. Maintaining this rate, rhythm, and conduction pattern is essential to ensuring that the heart contracts in a coordinated manner, effectively pumping blood throughout the body.
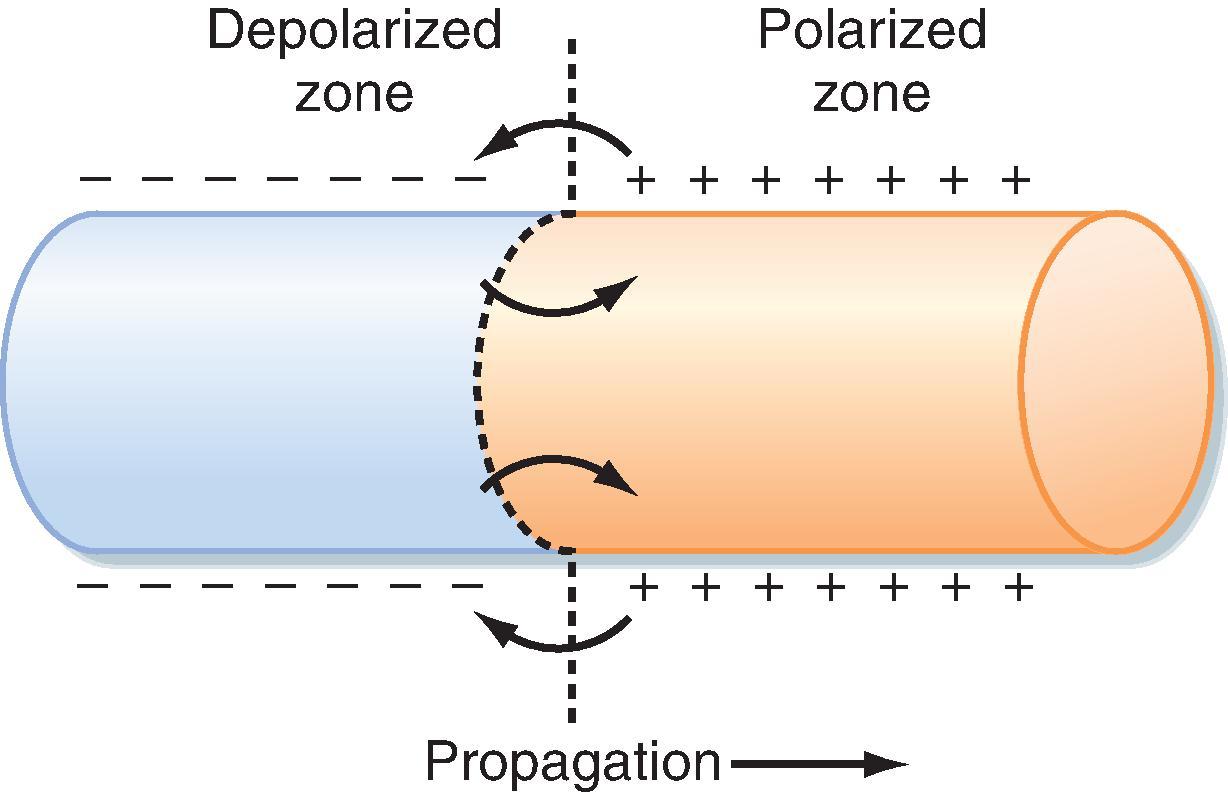
There are several factors that affect the speed and direction of impulse propagation throughout the heart. One set of these factors relates to the passive or cable properties of the cells and tissues. An action potential traveling from cell to cell along a cardiac muscle fiber is propagated by local circuit currents, much as it does in nerve and skeletal muscle fibers (see Chapter 5 ). These local currents, which are generated as the membrane is depolarized during the upstroke, flow along the inside of a cell, or from cell to cell, depolarizing adjacent areas of membrane or cells ( Fig. 16.16 ). As a result, the speed with which an action potential is propagated is affected by both intracellular and intercellular resistances to this local current flow.
The degree of (intracellular) resistance within a cell determines how far from the site of stimulation current will flow. The lower the intracellular resistance, the farther the current will reach and the faster the action potential will be propagated. Therefore, cells with a larger diameter and less complex morphology, such as those found in Purkinje fibers, can conduct impulses faster, and smaller cells, like those found in the AV node, will tend to conduct impulses more slowly.
The spread of an impulse from cell to cell is facilitated by the fact that cardiac myocytes are electrically coupled to one another via gap junctions (see Chapter 6 ). Gap junctions are rather nonselective in their permeability to ions, and they create a low electrical (intercellular) resistance pathway that allows ionic current to pass from one cell to the next. Therefore, areas of the heart that have a greater number of gap junctions connecting adjacent cells can propagate impulses faster. For example, gap junction density is higher in Purkinje fibers and lower in certain regions of the AV node.
Where gap junctions are positioned in a cell can also affect the direction of impulse propagation. For example, in ventricular myocytes, which are long cylindrical cells, gap junctions are preferentially located in the intercalated disks that connect cells end-to-end, rather than side-to-side. This facilitates the (isotropic) propagation of impulses more readily in the direction of the long axis of the muscle cells, which are arranged to form fibers that wrap the chambers of the ventricles. This ensures that impulses propagate in an organized and orderly fashion, resulting in coordinated contraction of the heart.
The conduction velocity along a fiber also varies directly with the action potential amplitude and the rate of change of the membrane potential (dV M /dt) during phase 0. The action potential amplitude is the potential difference between fully depolarized and fully polarized regions of the cell. The magnitude of the local current generated is proportional to this potential difference (see Chapter 5 ). These local currents are responsible for depolarizing the adjacent resting portion of the cell or fiber to its threshold potential. The greater the potential difference between the polarized and depolarized regions (i.e., the greater the action potential amplitude), the more effectively local stimuli can depolarize adjacent parts of the membrane and the more rapidly the wave of depolarization is propagated down the fiber. The rate of change in membrane potential during phase 0 is also an important determinant of conduction velocity. If the active portion of a fiber depolarizes gradually, the local currents generated between resting and neighboring depolarized regions are small. The resting region adjacent to the active zone is depolarized gradually, and more time is therefore required for each new section of the fiber to reach threshold. For these reasons, fast-response action potentials propagate more quickly than slow-response action potentials.
Conduction velocity can also be influenced by changes in the resting membrane potential, especially in fast-response tissues. Depolarization of the resting potential inactivates the voltage-dependent Na + channels, decreasing both action potential amplitude and dV M /dt, which results in a slowing of conduction velocity. This effect can be observed when the resting potential is depolarized by an increase in extracellular [K + ] (see Fig. 16.10 ). It is also reflected in the response to premature excitation of a cell during the relative refractory period. Both situations can lead to slowed impulse propagation, which can contribute to the development of arrhythmias.
Initiation of each heartbeat is a characteristic intrinsic to the heart itself. Specific cell types within the heart possess the property of automaticity or the ability to spontaneously depolarize during diastole and initiate a propagated impulse. This behavior is most commonly associated with slow-response action potentials found in cells that make up the SA node, as described earlier. However, it is also observed in cells whose primary function is normally associated with conducting impulses from one region of the heart to another. This includes cells in the AV node, which elicit slow-response action potentials, as well as cells in the Purkinje network, which elicit fast-response action potentials.
Although multiple cell types have the potential to act as pacemakers for the heart, initiation of the heartbeat normally starts in the SA node because the cells there exhibit an intrinsic rate of firing (60–100 beats/minute) that is faster than that observed in the AV node (40–55 beats/minute) or Purkinje fibers (25–50 beats/minute). So, an impulse that originates in the SA node will reach these other latent pacemakers, triggering them to fire an action potential before they can do so on their own.
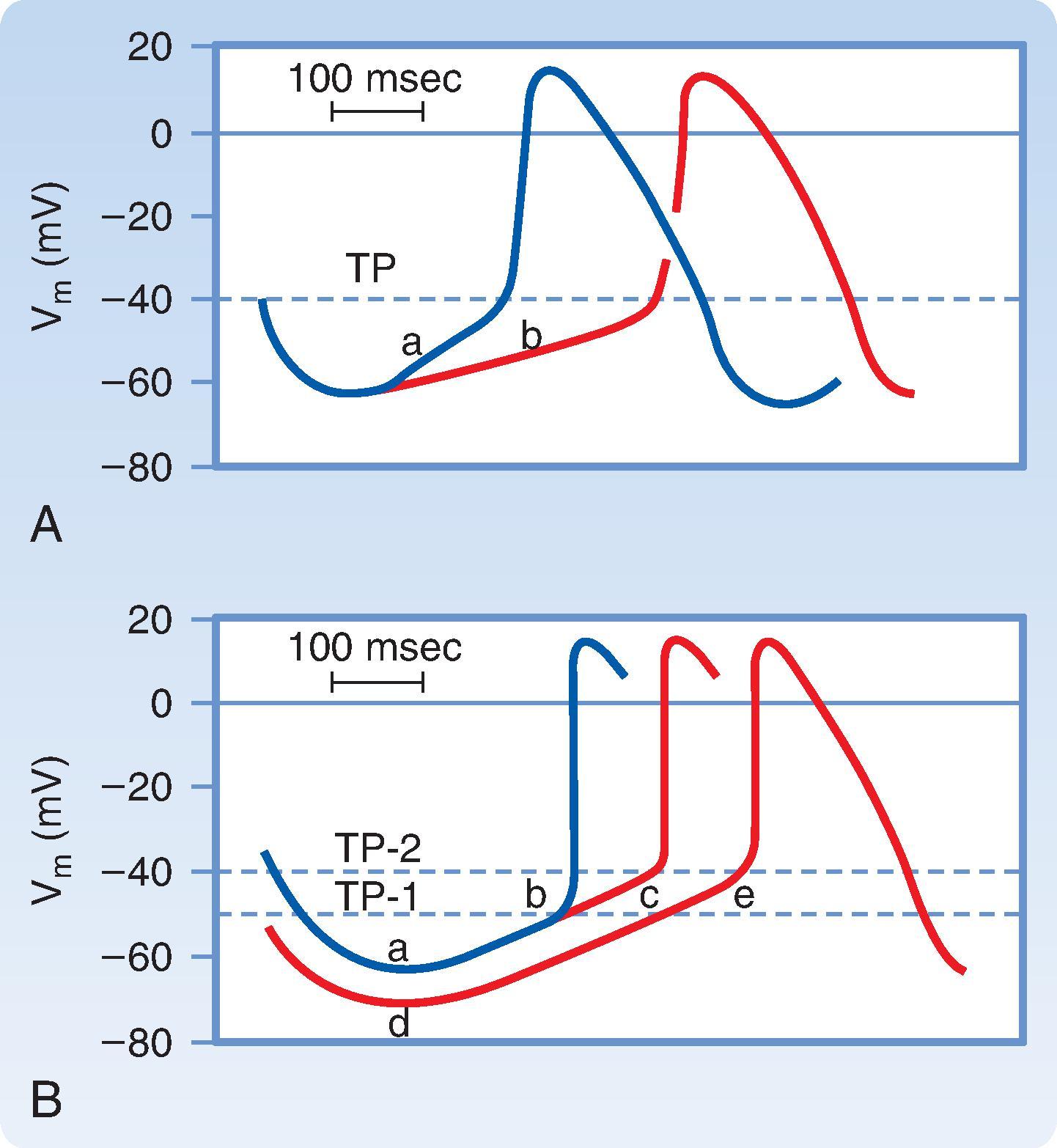
There are three main factors that affect the rate of spontaneous firing ( Fig. 16.17 ). They include: (1) the rate of spontaneous depolarization during phase 4, (2) the maximal maximum diastolic potential during phase 4, and (3) the threshold potential. Assuming two cells have the same maximum diastolic potential, the cell with the fastest rate of spontaneous depolarization will reach the threshold for initiating an action potential first, and thus fire at a faster rate. Alternatively, if these two cells spontaneously depolarize at the same rate during phase 4, the cell that starts from a more depolarized maximum diastolic potential is likely to reach threshold sooner and fire at a faster intrinsic rate. Finally, if these two cells have the same maximum diastolic potential and the same rate of phase 4 depolarization, then the cell that has a lower threshold potential is likely to initiate an upstroke first and exhibit the fastest intrinsic rate of firing.
The frequency of pacemaker firing is controlled by the activity of both divisions of the autonomic nervous system. Sympathetic stimulation increases heart rate by enhancing the rate of spontaneous depolarization during phase 4 of the slow-response action potential in SA node cells. This is due to due to β-adrenergic receptor production of cAMP, which can then directly act on I f pacemaker channels to increase their activity. The production of cAMP can also activate protein kinase A, enhancing the spontaneous release of Ca ++ from the SR and increasing the contribution of I NCX to phase 4 depolarization. These effects contribute to the increase in heart rate associated with stress and exercise.
Parasympathetic stimulation decreases heart rate by at least two mechanisms. First, it reduces the slope of diastolic depolarization during phase 4 of the slow-response action potential through muscarinic receptor inhibition of cAMP production, reversing the effects it has on I f pacemaker channel activity and spontaneous Ca ++ release. Second, muscarinic receptor activation shifts the maximum diastolic potential in a hyperpolarizing direction by activating I K,ACh channels and increasing g K . All of these effects contribute to the slowing of heart rate and subsequent decrease in cardiac output associated with vagal nerve stimulation. An extreme example is vasovagal syncope, a brief period of lightheadedness or loss of consciousness caused by an intense burst of vagal activity. This type of syncope is a reflex response to pain or to certain psychological stimuli. The autonomic neural effects on cardiac cells are described in greater detail in Chapter 18 .
Effects of the autonomic nervous system on pacemaking activity are not necessarily associated with changes the threshold level of pacemaker cells. However, drugs that directly inhibit L-type Ca ++ channels can slow heart rate by reducing the number of Ca ++ channels available to contribute to the upstroke. As a result, it takes pacemaker cells longer to depolarize to threshold, or the point where enough Ca ++ channels are activated to initiate an action potential.
As noted, the region of the mammalian heart that ordinarily generates impulses at the greatest frequency is the sinoatrial (SA) node; it is the main cardiac pacemaker. Detailed mapping of the electrical potentials on the surface of the right atrium has revealed that two or three sites of automaticity, located 1 or 2 cm from the SA node itself, serve along with the SA node as an atrial pacemaker complex. Sometimes these loci initiate impulses simultaneously. Other times the site of earliest excitation shifts from locus to locus, depending on certain conditions, such as the level of autonomic neural activity.
In humans, the SA node is approximately 8 mm long and 2 mm thick, and it lies posteriorly in the groove at the junction between the superior vena cava and the right atrium. The sinus node artery runs lengthwise through the center of the node. The SA node contains two principal cell types: (1) small, round cells that have few organelles and myofibrils; and (2) slender, elongated cells that are intermediate in appearance between the round and “ordinary” atrial myocardial cells. The round cells are probably the pacemaker cells; the slender, elongated cells probably conduct the impulses within the node and to the nodal margins.
The so-called funny current (I f ) in cardiac SA node cells is activated by h yperpolarization and gated by c yclic n ucleotides, and its channel is designated HCN. There are four members of the HCN gene family, and such channels are also found in central nervous system neurons that generate action potentials repetitively. Transmembrane segment 4 (S 4 ) of HCN has many positively charged amino acids that act as voltage sensors, which are also present in voltage-gated Na + , K + , and Ca ++ channels. The dominant channel expressed in the heart is derived from the HCN4 gene. Mutations in amino acids in S 4 and in the S 4 -to-S 5 linker cause marked changes in the voltage dependence of activation in such a way that greater hyperpolarization is needed to open the channel. This effect is like that of acetylcholine, and it has been predicted that the occurrence of such mutations in the human heart could underlie sinus bradycardia and sick sinus syndrome.
Regions of the heart other than the SA node may initiate beats in special circumstances. Such sites are called ectopic foci or ectopic pacemakers. Ectopic foci may become pacemakers when (1) their own rhythmicity becomes enhanced, (2) the rhythmicity of the higher order pacemakers becomes depressed, or (3) all conduction pathways between the ectopic focus and regions with greater rhythmicity become blocked. Ectopic pacemakers may act as a safety mechanism when normal pacemaking centers fail. However, if an ectopic center fires while the normal pacemaking center still functions, the ectopic activity may induce either sporadic rhythm disturbances, such as premature depolarizations, or continuous rhythm disturbances, such as paroxysmal tachycardias (see the section “Ectopic Tachycardias”).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here