Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurostimulators can be generally categorized as either external or fully implantable. The former has a longer history because external neurostimulators do not have the design and operational challenges of limited power sources, convenient size, hermeticity, and control interfaces among other details [ ]. Fully implantable neurostimulators, however, have inherited much from pacemaker technology development, though in recent years, as the neurostimulator market has seen higher rates of growth (generally due to the commodification of cardiac rhythm management devices), technologies custom to the needs of fully implantable neurostimulators have differentiated them from their cardiac precursors/cousins [ ]. This chapter will focus on implantable, clinical neurostimulators, with a high-level discussion on some of the circuit types responsible for generating and controlling the process of delivering electrical stimulation.
An overly simple analogy for an axon would be an electrical “wire”; indeed, the primary mathematical conceptualization of axon conduction is known as “cable theory” [ , ]. Clearly, the functions of any given neuron are more complex and diverse than mere conduction of electrical signals [ ]. Nonetheless, the transmission role of the axon, and the fact that the action potential (AP) is a key quantum of neural information provides a conceptual model for the design of neurostimulators. Computational modeling, as well as preclinical and clinical experimentation have generally confirmed that the most excitable structure of the neuron from the extracellular space is the axon [ , ]. Thus, “tapping into” the axon with an implanted neurostimulator becomes the tool or means by which neurostimulation can have a biological effect. The goal of most neurostimulators is to deliver a stimulation signal which can generate an AP in axons, and thereby “use” the nervous system to modulate physiologic function. This modulation, generally, is not electrical but chemical—the APs are a means to effect neurotransmitter release at the termini of the stimulated axon to a targeted circuit, and ideally achieve therapy. This concept has been called “the neurostimulator credo” by Mortimer [ ].
In order to achieve the controllable excitation of axons to generate APs, a neurostimulator is designed to deliver electrical signals which somewhat mimic how APs are conducted/generated in an axon. During normal physiologic AP generation, the regenerative current during the AP at the active node is a source/sink of current for adjacent nodes of Ranvier, driving extracellular and intracellular current flow in the surrounding region ( Fig. 9.1 ) [ ]. In the standard cable model of an axon, these flows form current “loops” that reach to and pass through adjacent nodes, where the strength of the current at any given inactive node is inversely proportional to its distance along the axon from the AP; nodes adjacent to the active node will experience strong depolarizing currents, while nodes further will see only weaker current [ ]. In the cable model, the depolarizing currents can be viewed as a “discharging” of the membrane capacitance at that node, and thus an increase in the nodal membrane potential [ ]. When the membrane potential reaches a certain “threshold” value (e.g., approximately −45 mV in mammalian neurons), the number of voltage-gated sodium channels activated at that node reaches a critical number, allowing significant sodium current to flow into the cell, thus depolarizing it toward the equilibrium potential for sodium (∼+50 mV), and generating an AP at that node [ ]. Effectively, the AP has then “conducted” to the next node, and that now-active node becomes the “source” of current for the next set of distal nodes.
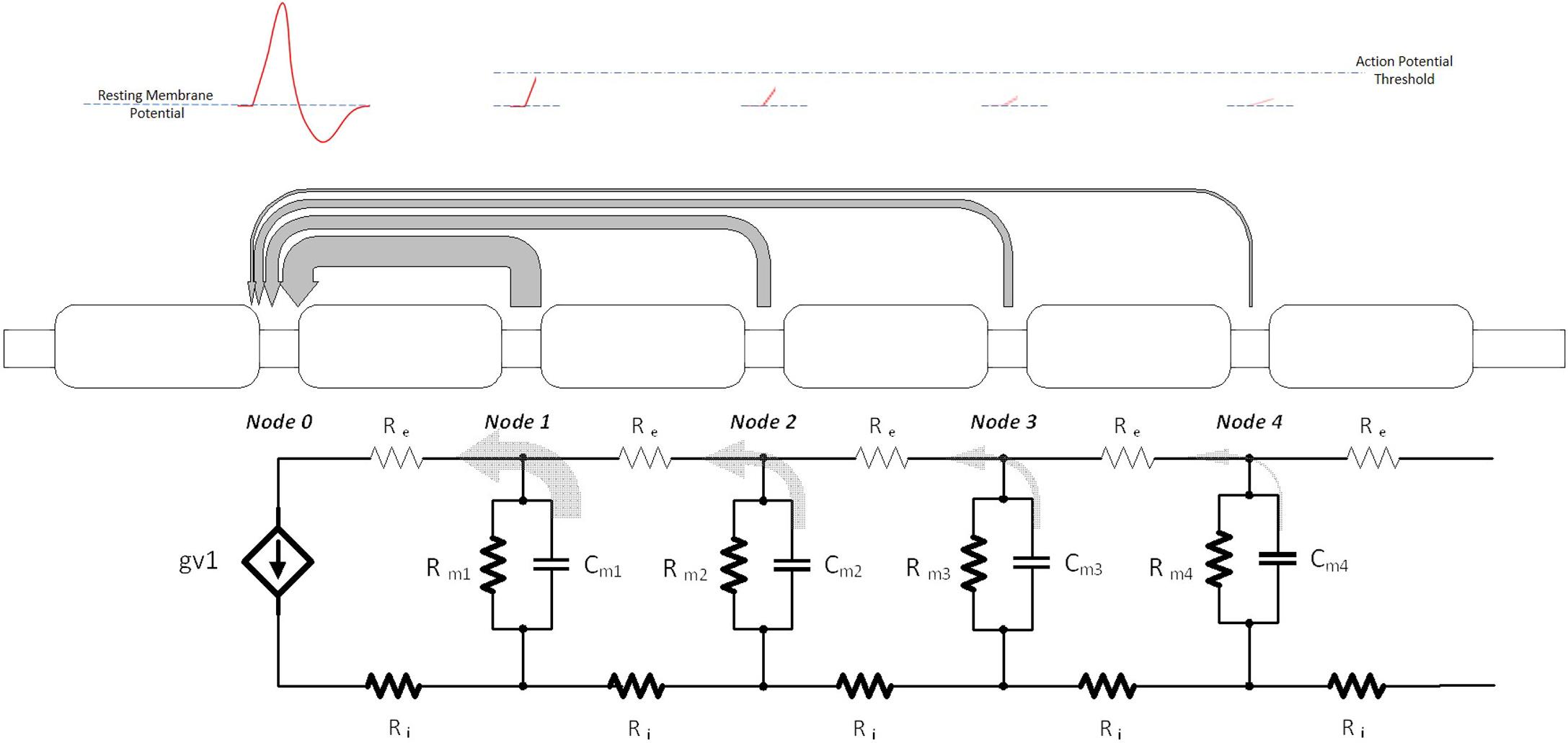
How a neurostimulator might artificially drive this process can be assessed by looking at the electrical activity of a quiescent node as a propagating AP approaches. (Since clinical neurostimulators provide only extracellular current delivery, the nodal activation process is similar, though not exactly the same). Typically, the neurostimulator delivers a brief pulse that manifests as a “sinking” current flow (i.e., the flow of positive current will be “into” the cathodic electrode), with a relatively large amplitude through a large metal electrode positioned near to an axon ( Fig. 9.2 ). The nearby nodes of Ranvier will then experience a temporary change in extracellular flow of ions through the membrane; this flow may be thought of as each node delivering an amount of current to the electrode and therefore a discharging of the membrane capacitance at each node. As discussed with Fig. 9.1 , this somewhat mimics the extracellular current flow that is seen during the presence of an AP on a nearby node, where the strength of the nodal flow is inversely proportional to the distance from the electrode to each node (While an extracellular electrode cannot explicitly control the intracellular current that also flows within the axon during AP generation, the change in extracellular current flow will, through the membrane capacitance and resistance, force intracellular current to also flow [ ].) The flow of this current changes the transmembrane voltage in the closest node (e.g., Node 0) to the point where enough voltage-gated sodium channels open to allow for a significant inrush of sodium current. Once this has occurred, the regenerative process of an AP begins, and the extracellular pulse from the neurostimulator is no longer needed—the generated AP will propagate down the axon (in both directions) in the standard natural manner.
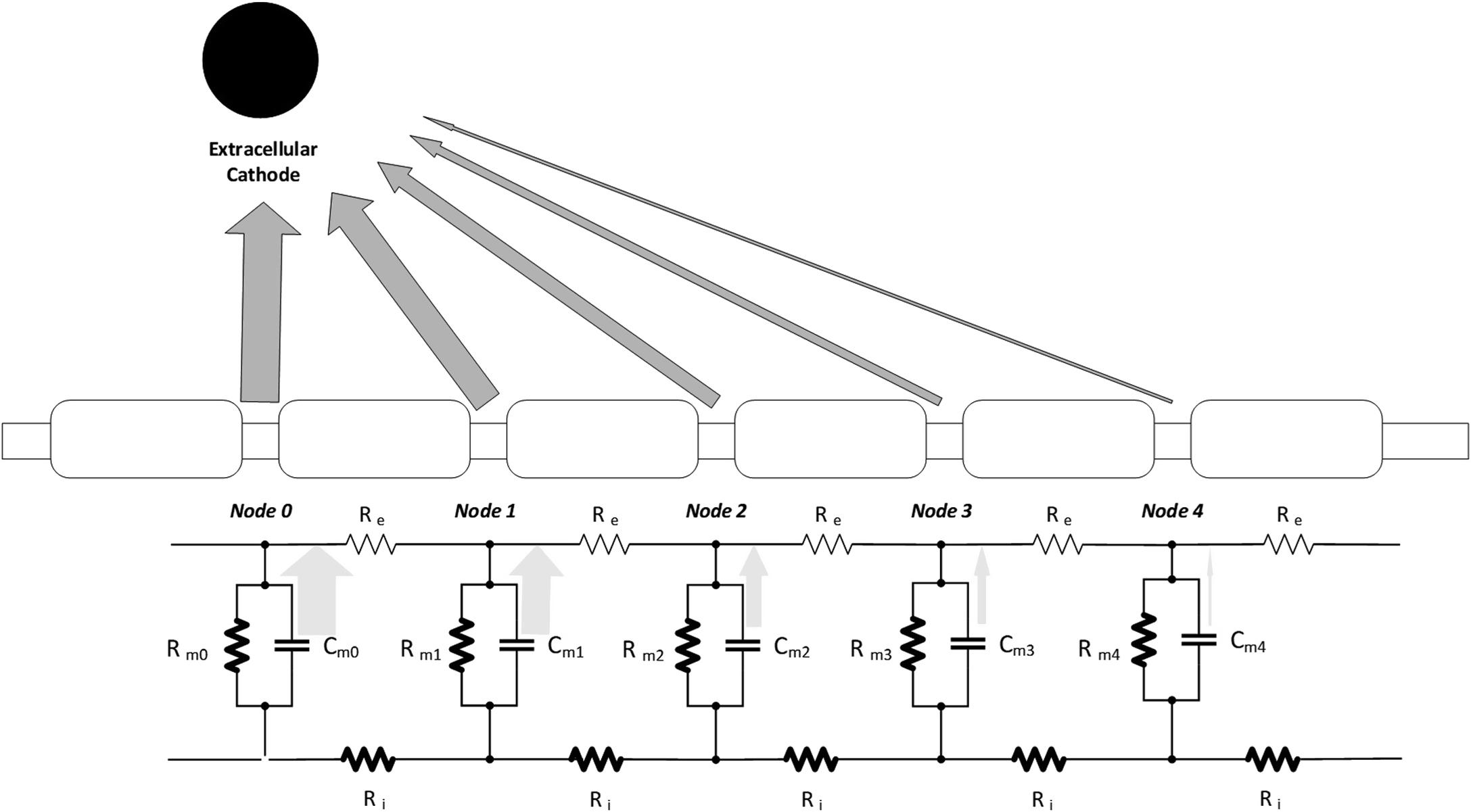
Typically, only narrow pulses (e.g., ≤ 100 s of microseconds or μs) are needed to start the AP generation process. Thus, at a high level, the primary design concept of a fully implantable neurostimulator is to convert a “constant” source of power (e.g., a battery) into an intermittent one (a pulse generator) [ ]. Sometimes the specifics of the stimulating signal are informed by a rudimentary understanding of neural mechanisms, but often it is based on empirical (read: clinical) application and design iteration [ , ]. In general, most neurostimulators deliver brief “square” pulses, primarily because they are simple to generate with basic electrical switches. More complex waveforms, while theoretically can improve neural targeting and device efficiency, have not yet shown significant clinical utility [ , ]. The intent of each pulse is to generate a single AP in each of the targeted axons. The AP generated then travels to an often distant target, where neurotransmitter is released. While there may be subsequent APs generated by the postsynaptic neurons, the most degree of control by the neurostimulator should occur if the first synapse encountered is part of the primary targeted circuit to be modulated [ ]. Thus, the neurotransmitter released may be thought of as the “drug” portion of the therapy resulting from neurostimulation.
The earliest electronic designs for neurostimulators were essentially passive pulse generators. An external radiofrequency electromagnetic field was applied to an implanted circuit, which, in turn, had two basic functions: demodulation and pulse generation. The stimulation pulse train was formed into a modulation signal for an RF transmission signal. This was transmitted transcutaneously via electromagnetic coupling to an implanted (hermetic) receiver. The receiver circuit was excited by the external field, which passed the signal to a demodulator and then a pulse smoothing circuit [ ]. The positive aspects of this system were that the timing and shape of the pulse train could be flexibly controlled in the external circuitry and enabled a relatively simple implanted circuit design [ ]. The limitations, however, were that the stimulation train was present only when the transmitter was over the receiver, and the integrity of the stimulation train could be highly dependent upon the integrity of the electromagnetic coupling. Such systems were used in the 1960s through 1990s for experimental and clinical applications [ ] ( Fig. 9.3 ) .
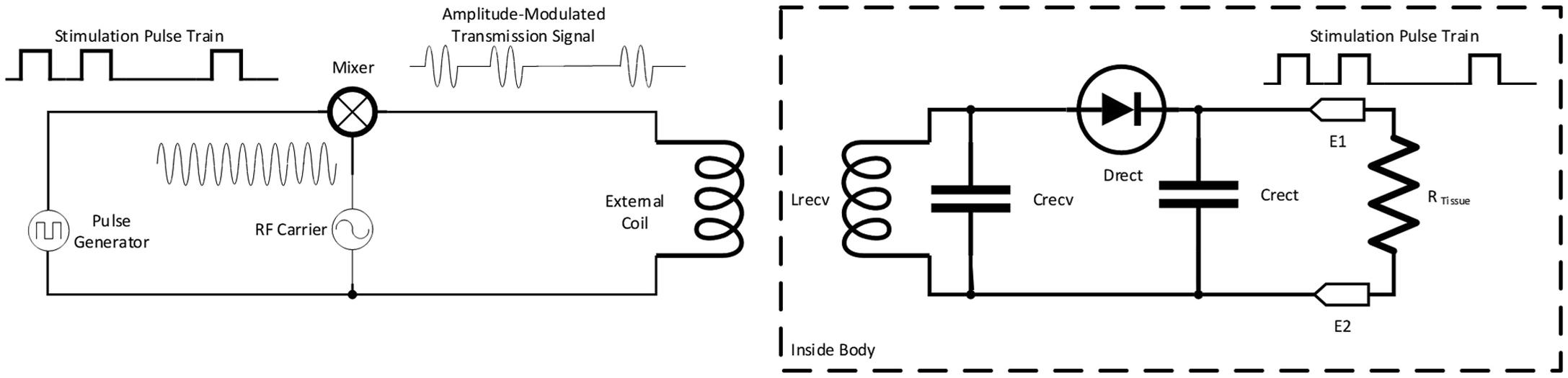
In this same era, simple early pacemakers provided some technology transfer for some neurostimulator designs. Early bradycardia pacemakers used a battery-powered relaxation oscillator, coupled to a transistor output circuit which could generate narrow stimulation pulses [ ]. These devices were not highly controllable, but served the very basic need of providing fixed-rate cardiac support often for several years. These devices still could not really be used for effective clinical neurostimulation, however, because the stimulation of the nervous system usually required pulse rates much greater than ∼1 Hz to be effective [ , ]. When pacemaker technology advanced in the 1970 and 1980s to allow for cardiac devices to have Lithium-based power sources, custom LSIs for system and stimulation control, neurostimulator designs also benefitted [ , ]. While still usually externally powered by RF fields (again, neurostimulators typically run at rates >> 1 Hz), the advances in system and stimulation control circuitry could be put to use for neurostimulation.
These electronic technology developments were used differently in the two device classes, because neurostimulators have different requirements than implantable cardiac devices. Stimulation of the heart is essentially an all-or-none proposition; activation of a few local endocardial cells around the tip of the pacemaker lead nearly always results in a full contraction of the entire heart. So, once the threshold of stimulation is found, the amplitude and configuration of the stimulation output (to only one or two implanted electrodes) in a cardiac pacemaker is fairly fixed, often set at a multiple of the stimulation threshold. The critical functions in a pacemaker are the sensing of native heart rhythm, and the timing of stimulation pulse delivery; to both save power (e.g., avoid delivering stimulation pulses while the heart is already contracting) and to assure physiologic rhythms (e.g., stimulate at certain times, allowing appropriate sequence of chamber activation and chamber filling), the pacemaker must intelligently sense and respond at certain times [ ].
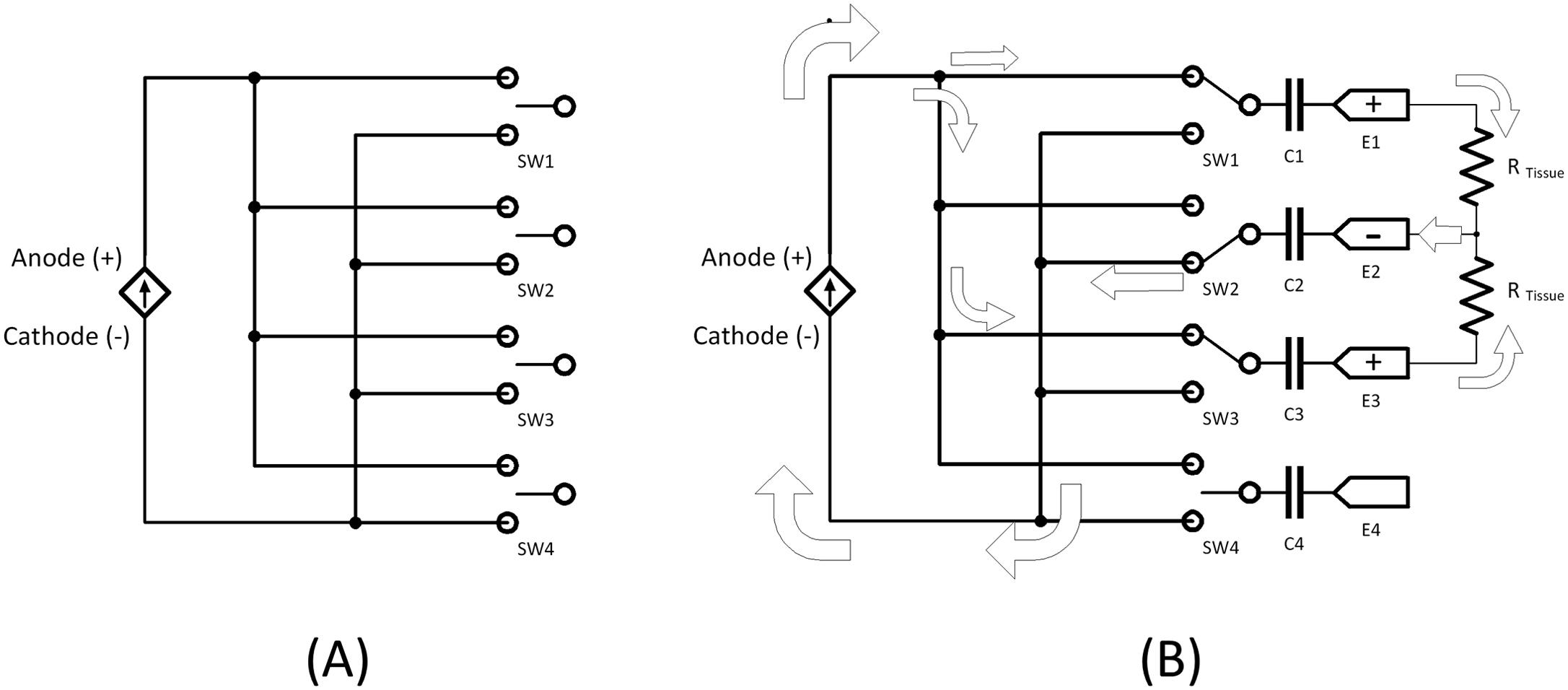
In a neurostimulator, however, the situation is somewhat opposite: the timing is generally “dumb” and the stimulation amplitude and field distribution are sophisticated. This is because the main concept in stimulating neurons is selectivity—the goal is to activate fibers which yield efficacy, while not activating fibers that can lead to side effects. Clinical neurostimulators often have multiple identical stimulation electrode contacts, distributed in linear arrays or grids; these contacts can be defined as anodes (where positive current is sourced into tissue, designated by a “+”) and cathodes (where positive current flows into the electrode; designated by a “–”) in myriad configurations. This is done to allow the clinician to select and activate those contacts which are closest to the targeted axons, as well as to “shape” the stimulation field by selecting which contacts will activate axons (typically cathodes) and which will avoid activating axons (typically anodes). Also in clinical neurostimulators, the stimulation amplitude is not only variable during therapeutic application, it is often controlled by the patient using an external remote control, adapting to patient daily activities. But the timing of neurostimulator pulse delivery is typically uncomplicated and often not highly programmable; usually just a continuous pulse train of a fixed frequency (e.g.,) is delivered, sometimes made intermittent (brief ON and OFF periods, usually done to save power) [ , ].
Thus, the internal circuits for pacemakers and neurostimulators may share the commonality of having a microprocessor/microcontrollers (these terms used interchangeably in this chapter), but the neurostimulator tends to have more sophisticated stimulation pulse delivery electronics. For example, complicated switching networks are often employed in neurostimulators, allowing for demultiplexing of cathodic and anodic current to various contacts. A hypothetical circuit is shown in Fig. 9.4 . The anodic current (in the case of a current-controlled stimulator) is routed to specific contacts which were programmed by the clinician to be anodes. A similar circuit is used for cathodic current. This switching allows for different field shapes to be generated by the stimulator—the shape of the field is determined by: the relative position and orientations of the contacts, the amount of anode or cathode (or none) current at each contact.
Critical to the safety of neurostimulator design is the limiting of direct charge transfer to the tissue. It has been demonstrated in animal studies that direct current delivered to tissue through metal electrodes can result in both tissue damage and electrode material loss [ ]. Given the nonlinear nature of the electrode-electrolyte interface, perfect charge balance can be challenging [ ]. Device designers often use multiple strategies to minimize direct charge transfer. These include (at least) biphasic stimulation waveforms, capacitive coupling from stimulation circuits to implanted metal electrodes, high impedance “bleed” resistors, among others [ ]. Biphasic stimulation implies that current flow is in one direction for a first phase of the stimulation pulse complex, and then reversed for a second, subsequent phase of the stimulation pulse complex. There are costs to these strategies, from both a battery-drain standpoint as well as a neural response standpoint. From the device side, the need to deliver charge in two directions implies that, at a minimum, extra switching circuitry must be implemented and controlled by the microcontroller in the device. Also, depending upon the particular charge recovery strategy, the stimulator circuit may need to deliver an identical-though-opposite-polarity second pulse that follows the first; in many applications of neurostimulation, this second pulse is not intended to activate neural tissue. Thus, the second pulse does not carry full therapeutic utility (i.e., the pulse is used only to recover the charge from essentially the surface of the electrode, not to affect neural activity). From the neuron side, in most neurostimulation applications, the first pulse (typically of cathodic polarity) is intended to generate at least one AP in nearby axons. In early pacemakers and stimulators (and in some preclinical work), the use of a monophasic stimulation pulse complex was deemed optimal from a neural activation standpoint. However, the damage thresholds for such pulse types were quite low. A second, opposite-polarity pulse was delivered very soon after the primary pulse to avoid this damage [ ]. The timing and shape of this second pulse introduced new challenges. First, if the charge recovery pulse was delivered too soon after the end of the first pulse, any neurons just barely beginning their regenerative process of generating an AP would then experience an externally applied field of the opposite polarity, which might drive hyperpolarizing processes and stop those neurons from firing [ ]. The net effect was a reduction in the neural response to the first pulse.
In many clinical circumstances, though, this effect can be avoided. Since most clinical neurostimulators deliver pulses at relatively low frequencies, for example, 30–60 Hz (ostensibly because most axons can only consistently generate APs at rates no greater than ∼200 Hz), the time between primary pulse phases that are intended to stimulate is relatively long compared to typical pulse durations [ , ]. For example, a 50 Hz stimulation pulse delivery rate has a 20 ms period. With neurostimulator pulses ranging between 50 and 1000 μs, this means that 95% of the pulse period is available for delivery of an electrode charge recovery pulse. Additionally, since only the primary pulse is intended to stimulate, then the shape of the second pulse, again intended only for charge recovery, can be designed with power minimization in mind.
A common second phase pulse, as shown in Fig. 9.5A , is simply the capacitive discharge of the stimulation output circuit: a low impedance (often a switching field effect transistor [FET] with a low Rds ON) is switched between the anodic and cathodic contacts. This allows for the stimulation charge that has built up on the coupling capacitors as well as the charge stored within the electrode-electrolyte interface (“Helmholtz capacitance”) from the first stimulation pulse to flow in a reverse direction [ ]. Reversal of the polarity of the electrodes during the second phase yields opposite electrode potentials, which can reverse many of the electrochemical reactions that occurred near the electrode surfaces during the first phase. The shape of this second charge recovery phase is a decaying exponential. Judicious choice of the coupling capacitor values at the anodes and cathodes and magnitude of the switched-in resistance allow for this phase to be of relatively low amplitude, ideally far below rheobase of the nearby neurons. This solution is simple, requiring merely appropriate switch timing and circuit parameter selection by the designer. The challenge is that the residual charge built up on the coupling capacitor is dependent upon the impedance properties of the tissue and the programmed electrodes, which can be different from patient to patient and electrode to electrode.
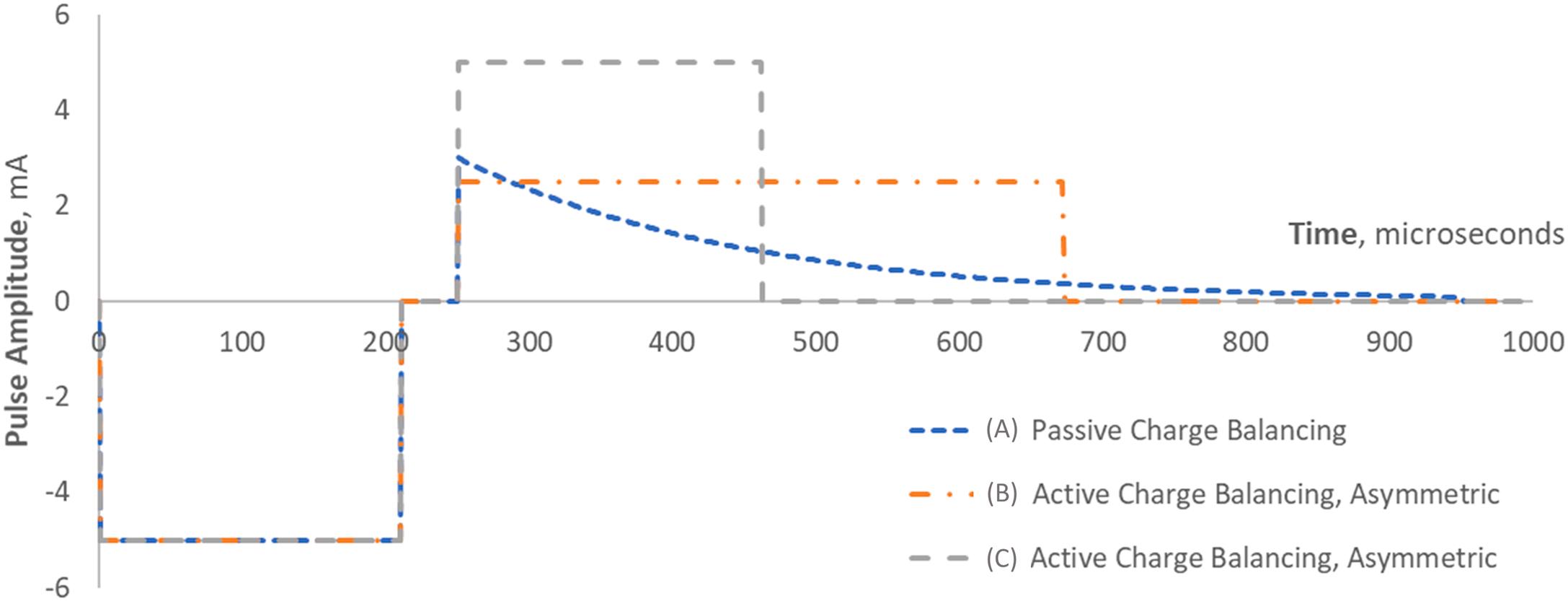
A similar strategy, one that uses more stimulation energy, but can address the requirements of more precise charge balance, is to use an “active” second phase. To balance the charge delivered during the first phase, the amplitude and duration of this charge recovery phase are chosen to have the same product as the amplitude and phase of the primary stimulating pulse [ ]. To minimize effects on the neuronal response, the second phase can be designed to have a longer duration and a lower amplitude, below the rheobase of nearby neurons, as shown in Fig. 9.5B . An advantage of this design is that the second phase can be programmed by the system for more controlled charge balance, depending upon the selection of the stimulation parameters chosen for therapy. For example, at a higher rate of stimulation, say 500 Hz, the time between primary stimulating pulses becomes 2 ms. If the primary stimulation pulse is 500 μs, then only 1.5 ms remains for charge recovery. Passive strategies might not allow for adequate “emptying” of the interface capacitances (compared to lower frequencies of stimulation, where there was more time for capacitive discharge). In this condition, the charge buildup on the output capacitances may drive the steady state stimulation voltage to significant levels, such that the “compliance voltage” of the device's output circuits would need to be raised to guarantee the voltage or current is delivered as the user programmed. The higher compliance voltage may then result in a higher battery drain because of the added circuitry needed to “pump” the battery voltage using boost circuitry (see the “Stimulation Output” section). With the active charge recovery strategy, the amount of compliance voltage necessary can likely be limited, since the charge recovery process is controlled to result in zero net charge delivered with each pulse complex.
Finally, a variant of this “active second phase” strategy shown in Fig. 9.5C , the “symmetric biphasic” waveform is simpler, but can have a higher battery drain than the asymmetric active pulsing. In symmetric biphasic pulse complexes, the charge recovery second phase is a reverse-polarity mimic of the first phase pulse. This makes for slightly simpler, more consistent pulse delivery strategy, at the cost of delivering two active pulses, where only the former is intended to stimulate the neural tissue. And, as discussed before, this second phase may compromise neural recruitment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here